Abstract
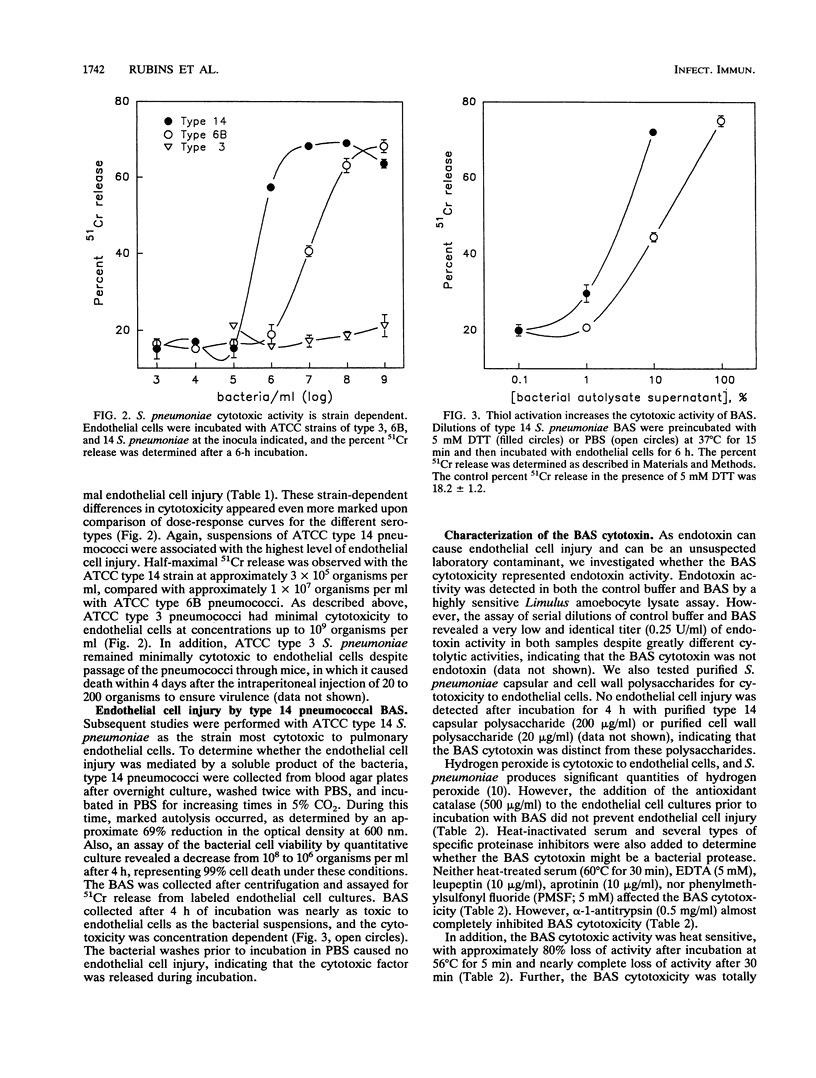
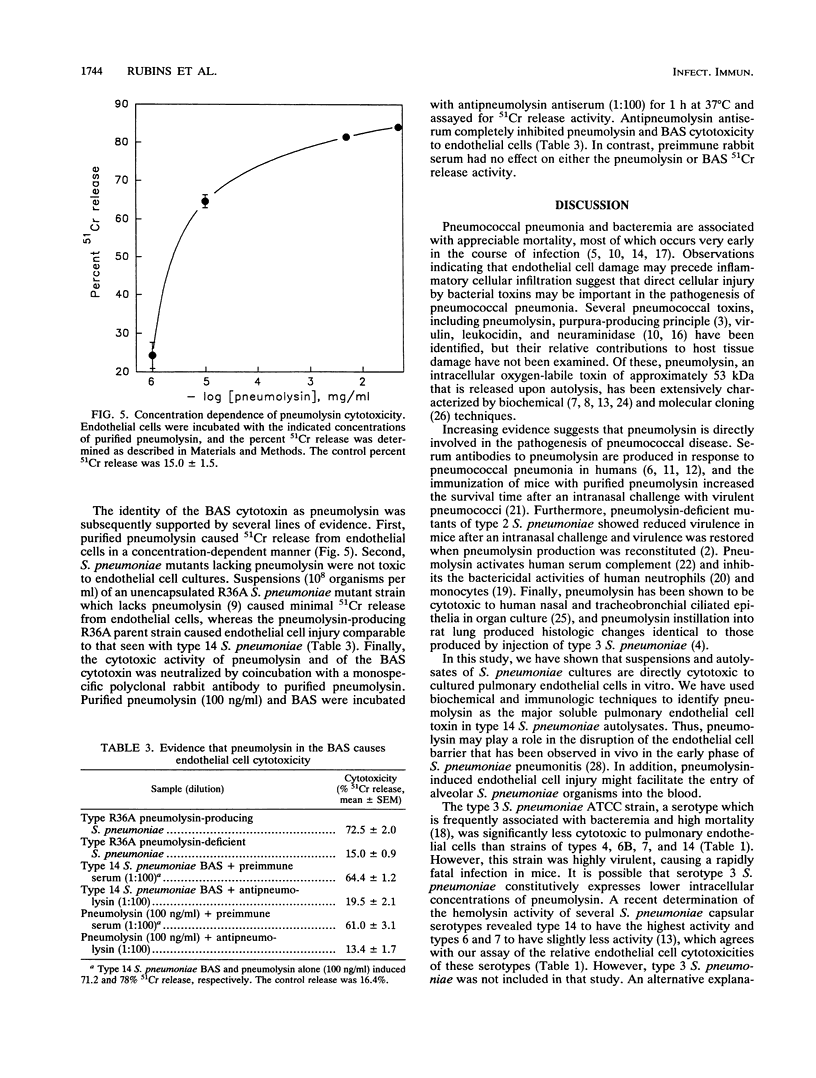
Pneumococcal pneumonia and bacteremia are associated with appreciable mortality, most of which occurs very early in the course of infection. An initial step in the pathogenesis of pneumococcal pneumonia may include disruption of the pulmonary endothelial barrier with subsequent alveolar hemorrhage. We sought to determine whether soluble factors from Streptococcus pneumoniae can directly injure pulmonary endothelial cells in vitro and to identify pneumococcal toxins that may be involved in endothelial cell injury. Suspensions of S. pneumoniae (10(8) organisms per ml) caused significant injury to cultured bovine pulmonary artery endothelial cells in a time-dependent manner. The degree of endothelial cell cytotoxicity differed among S. pneumoniae strains; among the strains tested, a type 14 strain was the most cytotoxic and a type 3 strain was the least cytotoxic. During autolysis, type 14 S. pneumoniae released a soluble endothelial cell cytotoxin that was distinct from S. pneumoniae capsular and cell wall polysaccharides. The soluble cytotoxin was further characterized as a thiol-activated, heat-sensitive protein that coeluted with purified pneumolysin during gel filtration. The identity of the S. pneumoniae endothelial cell cytotoxin as pneumolysin was further supported by the ability of purified pneumolysin and the inability of S. pneumoniae mutants which lack pneumolysin to injure endothelial cells, as well as by the inhibition of the soluble S. pneumoniae cytotoxin by a neutralizing antibody to pneumolysin. Pneumolysin appears to be the major S. pneumoniae soluble cytotoxin for pulmonary endothelial cells in vitro and may be an important factor in the pathogenesis of alveolar hemorrhages in S. pneumoniae infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty C., Kreger A. Characterization of pneumococcal purpura-producing principle. Infect Immun. 1980 Jul;29(1):158–164. doi: 10.1128/iai.29.1.158-164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman C., Munro N. C., Jeffery P. K., Mitchell T. J., Andrew P. W., Boulnois G. J., Guerreiro D., Rohde J. A., Todd H. C., Cole P. J. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am J Respir Cell Mol Biol. 1991 Nov;5(5):416–423. doi: 10.1165/ajrcmb/5.5.416. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Horton C. A., Schaberg D. R. Failure of intensive care unit support to influence mortality from pneumococcal bacteremia. JAMA. 1983 Feb 25;249(8):1055–1057. [PubMed] [Google Scholar]
- Jalonen E., Paton J. C., Koskela M., Kerttula Y., Leinonen M. Measurement of antibody responses to pneumolysin--a promising method for the presumptive aetiological diagnosis of pneumococcal pneumonia. J Infect. 1989 Sep;19(2):127–134. doi: 10.1016/s0163-4453(89)91864-1. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Geoffroy C., Alouf J. E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980 Jan;27(1):97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Hamon D., Drew G. K. Isolation and characterization of pneumolysin-negative mutants of Streptococcus pneumoniae. Infect Immun. 1982 Aug;37(2):837–839. doi: 10.1128/iai.37.2.837-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Pathogenesis of pneumococcal pneumonia. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S509–S517. doi: 10.1093/clinids/13.supplement_6.s509. [DOI] [PubMed] [Google Scholar]
- Kalin M., Kanclerski K., Granström M., Möllby R. Diagnosis of pneumococcal pneumonia by enzyme-linked immunosorbent assay of antibodies to pneumococcal hemolysin (pneumolysin). J Clin Microbiol. 1987 Feb;25(2):226–229. doi: 10.1128/jcm.25.2.226-229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanclerski K., Blomquist S., Granström M., Möllby R. Serum antibodies to pneumolysin in patients with pneumonia. J Clin Microbiol. 1988 Jan;26(1):96–100. doi: 10.1128/jcm.26.1.96-100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanclerski K., Möllby R. Production and purification of Streptococcus pneumoniae hemolysin (pneumolysin). J Clin Microbiol. 1987 Feb;25(2):222–225. doi: 10.1128/jcm.25.2.222-225.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. R., Rudensky B., Hadas-Halperin I., Isacsohn M., Melzer E. Pneumococcal bacteremia--no change in mortality in 30 years: analysis of 104 cases and review of the literature. Isr J Med Sci. 1987 Mar;23(3):174–180. [PubMed] [Google Scholar]
- Kreger A. S., Bernheimer A. W. Physical behavior of pneumolysin. J Bacteriol. 1969 Apr;98(1):306–307. doi: 10.1128/jb.98.1.306-307.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog. 1988 Jan;4(1):33–43. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Kruss D. M., Wasil R. E., Metzger W. I. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974 Sep;134(3):505–510. [PubMed] [Google Scholar]
- Nandoskar M., Ferrante A., Bates E. J., Hurst N., Paton J. C. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986 Dec;59(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Hansman D. J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Rowan-Kelly B., Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway C. N., Klebanoff S. J. Purification of pneumolysin. Infect Immun. 1971 Oct;4(4):388–392. doi: 10.1128/iai.4.4.388-392.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfort C., Wilson R., Mitchell T., Feldman C., Rutman A., Todd H., Sykes D., Walker J., Saunders K., Andrew P. W. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989 Jul;57(7):2006–2013. doi: 10.1128/iai.57.7.2006-2013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Coonrod J. D. Experimental type 25 pneumococcal pneumonia in rats: an electron-microscopic study. Am J Pathol. 1980 Apr;99(1):231–242. [PMC free article] [PubMed] [Google Scholar]


