Abstract
The interaction of purified human fibrinogen with Aspergillus fumigatus conidia was investigated by immunofluorescence and electron microscopy and binding assays with radiolabeled proteins. We described the localization of the binding sites on the A. fumigatus conidia and on the fibrinogen molecule and determined the binding characteristics. Immunofluorescence revealed that the fixation of purified fibrinogen was selectively associated with conidia and suggested a role for the D domains of the fibrinogen molecule. Binding assays performed with 125I-radiolabeled proteins confirmed that binding sites were located specifically in the D domains. No reaction could be detected with fragment E. The binding of 125I-fragment D to conidia was time dependent, saturable, and specific. Scatchard analysis of the data revealed an average of 1,200 binding sites per conidium, and an apparent dissociation constant (Kd) of 2.2 x 10(-9) M was estimated. Pretreatment of the cells with proteolytic enzymes or heat abolished binding, demonstrating the protein nature of the binding sites. Ultrastructural localization of the fungal receptors was determined by transmission electron microscopy. Labeling appeared to be associated with the outer electron-dense layer of the conidial wall and progressively decreased during the germination process. Labeling of thin sections with fragment D and an antifibrinogen immune serum revealed that binding sites also lay in the inner part of the wall and in vacuoles. These results indicate the presence at the conidial surface of specific receptors for fibrinogen which could act as mediators of conidial adherence to host tissues.
Full text
PDF




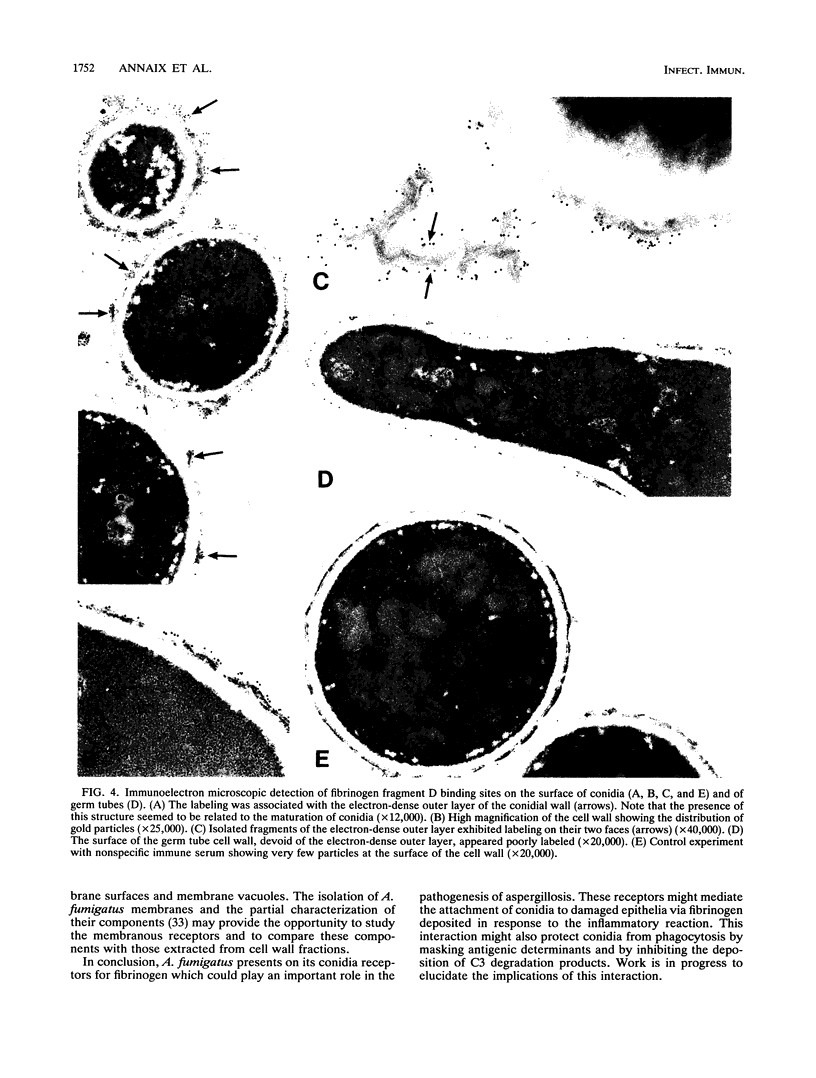



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annaix V., Bouchara J. P., Tronchin G., Senet J. M., Robert R. Structures involved in the binding of human fibrinogen to Candida albicans germ tubes. FEMS Microbiol Immunol. 1990 Oct;2(3):147–153. doi: 10.1111/j.1574-6968.1990.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988 Feb;11 (Suppl A):411–426. doi: 10.1016/0195-6701(88)90220-4. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Binding of human fibrinogen to Candida albicans in vitro: a preliminary study. J Med Vet Mycol. 1986 Aug;24(4):345–348. doi: 10.1080/02681218680000511. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Bouali A., Tronchin G., Robert R., Chabasse D., Senet J. M. Binding of fibrinogen to the pathogenic Aspergillus species. J Med Vet Mycol. 1988;26(6):327–334. [PubMed] [Google Scholar]
- Bénichou J. C., Fréhel C., Ryter A. Improved sectioning and ultrastructure of bacteria and animal cells embedded in Lowicryl. J Electron Microsc Tech. 1990 Apr;14(4):289–297. doi: 10.1002/jemt.1060140402. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Dutra I. S., Blobel H. Fibrinogen binding inhibits the fixation of the third component of human complement on surface of groups A, B, C, and G streptococci. Microbiol Immunol. 1985;29(10):973–980. doi: 10.1111/j.1348-0421.1985.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Polentarutti N., Balconi G., Ryckewaert J. J., Larrieu M. J., Donati M. B., Mantovani A., Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985 Jan;75(1):11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Vergara-Dauden M., Balconi G., Pietra A., Cherel G., Donati M. B., Larrieu M. J., Marguerie G. Specific binding of human fibrinogen to cultured human fibroblasts. Evidence for the involvement of the E domain. Eur J Biochem. 1984 Mar 15;139(3):657–662. doi: 10.1111/j.1432-1033.1984.tb08054.x. [DOI] [PubMed] [Google Scholar]
- Del Rosso M., Cappelletti R., Viti M., Vannucchi S., Chiarugi V. Binding of the basement-membrane glycoprotein laminin to glycosaminoglycans. An affinity-chromatography study. Biochem J. 1981 Dec 1;199(3):699–704. doi: 10.1042/bj1990699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fanti F., Conti S., Campani L., Morace G., Dettori G., Polonelli L. Studies on the epidemiology of Aspergillus fumigatus infections in a university hospital. Eur J Epidemiol. 1989 Mar;5(1):8–14. doi: 10.1007/BF00145038. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Talbot G. H., Hurwitz S., Strom B. L., Lusk E. J., Cassileth P. A. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984 Mar;100(3):345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Timmons S., Strong D. D., Cottrell B. A., Riley M., Doolittle R. F. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982 Mar 16;21(6):1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E. Beta 1,4-oligoglucosides inhibit the binding of Aspergillus fumigatus conidia to human monocytes. J Infect Dis. 1991 May;163(5):1154–1156. doi: 10.1093/infdis/163.5.1154. [DOI] [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E. Lectin-like attachment sites on murine pulmonary alveolar macrophages bind Aspergillus fumigatus conidia. J Infect Dis. 1988 Aug;158(2):407–414. doi: 10.1093/infdis/158.2.407. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Farrell T. P., Levitz S. M. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect Immun. 1989 Nov;57(11):3412–3417. doi: 10.1128/iai.57.11.3412-3417.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- Kühnemund O., Havlicek J., Knöll H., Sjöquist J., Köhler W. Interaction of group A type 1 streptococcal M protein with fibrinogen. Acta Pathol Microbiol Immunol Scand B. 1985 Jun;93(3):201–209. doi: 10.1111/j.1699-0463.1985.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Bounelis P., Switalski L. M., Hook M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990 Feb;172(2):716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Rowland R. W., Switalski L. M., Hök M. Interactions of Bacteroides gingivalis with fibrinogen. Infect Immun. 1986 Dec;54(3):654–658. doi: 10.1128/iai.54.3.654-658.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Switalski L. M., Kornman K. S., Hök M. Bacteroides intermedius binds fibrinogen. J Bacteriol. 1985 Aug;163(2):623–628. doi: 10.1128/jb.163.2.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J. P., Hatzfeld A., Hatzfeld J. Fibrinogen mitogenic effect on hemopoietic cell lines: control via receptor modulation. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6494–6498. doi: 10.1073/pnas.83.17.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J. P., Hatzfeld A., Hudry-Clergeon G., Wilner G. D., Hatzfeld J. Evidence for two functionally different fibrinogen receptors on hemopoietic cells: the glycoprotein IIb-IIIa and the mitogenic fibrinogen receptor. J Cell Physiol. 1987 Aug;132(2):303–310. doi: 10.1002/jcp.1041320215. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Chhatwal G. S., Blobel H. Binding of human fibrinogen and its polypeptide chains to group B streptococci. Med Microbiol Immunol. 1983;172(3):149–153. doi: 10.1007/BF02123799. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Piechura J. E., Riefel R. S., Daft L. J. Biochemical and immunochemical analyses of detergent solubilized antigens from membrane vesicles of Aspergillus fumigatus. Can J Microbiol. 1987 Nov;33(11):955–962. doi: 10.1139/m87-168. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi M. G. Invasive aspergillosis. Rev Infect Dis. 1983 Nov-Dec;5(6):1061–1077. doi: 10.1093/clinids/5.6.1061. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Tokuyasu K. T. Contrasting of Lowicryl K4M thin sections. Histochemistry. 1990;95(2):123–136. doi: 10.1007/BF00266584. [DOI] [PubMed] [Google Scholar]
- Schmidt K. H., Gerlach D., Kühnemund O., Köhler W. Quantitative differences in specific binding of fibrinogen fragment D by M-positive and M-negative group-A streptococci. Med Microbiol Immunol. 1984;173(3):145–153. doi: 10.1007/BF02123763. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Laudano A. P., Hawiger J., Doolittle R. F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982 Mar 16;21(6):1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Annaix V., Robert R., Senet J. M. Fungal cell adhesion molecules in Candida albicans. Eur J Epidemiol. 1991 Jan;7(1):23–33. doi: 10.1007/BF00221338. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Robert R., Bouali A., Senet J. M. Immunocytochemical localization of in vitro binding of human fibrinogen to Candida albicans germ tube and mycelium. Ann Inst Pasteur Microbiol. 1987 Mar-Apr;138(2):177–187. doi: 10.1016/0769-2609(87)90194-3. [DOI] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Biochemical and biological properties of the binding of human fibrinogen to M protein in group A streptococci. J Bacteriol. 1985 Oct;164(1):350–358. doi: 10.1128/jb.164.1.350-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]





