Abstract
Background
The concept of specifying positional information in the adult cardiovascular system is largely unexplored. While the Hox transcriptional regulators have to be viewed as excellent candidates for assuming such a role, little is known about their presumptive cardiovascular control functions and in vivo expression patterns.
Results
We demonstrate that conventional reporter gene analysis in transgenic mice is a useful approach for defining highly complex Hox expression patterns in the adult vascular network as exemplified by our lacZ reporter gene models for Hoxa3 and Hoxc11. These mice revealed expression in subsets of vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) located in distinct regions of the vasculature that roughly correspond to the embryonic expression domains of the two genes. These reporter gene patterns were validated as authentic indicators of endogenous gene expression by immunolabeling and PCR analysis. Furthermore, we show that persistent reporter gene expression in cultured cells derived from vessel explants facilitates in vitro characterization of phenotypic properties as exemplified by the differential response of Hoxc11-lacZ-positive versus-negative cells in migration assays and to serum.
Conclusion
The data support a conceptual model of Hox-specified positional identities in adult blood vessels, which is of likely relevance for understanding the mechanisms underlying regional physiological diversities in the cardiovascular system. The data also demonstrate that conventional Hox reporter gene mice are useful tools for visualizing complex Hox expression patterns in the vascular network that might be unattainable otherwise. Finally, these mice are a resource for the isolation and phenotypic characterization of specific subpopulations of vascular cells marked by distinct Hox expression profiles.
Background
The Hox transcriptional regulators are known to play a critical role in establishing positional identities during embryonic patterning [1], whereas in postnatal and adult tissues, their functions are largely subject to speculation [2]. This also pertains to the adult cardiovascular system, although Hox genes are considered prime candidates for determining phenotypic characteristics of vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) during vasculogenesis and vascular remodeling both under normal (e.g. wound healing, menstrual cycle) and pathologic conditions (e.g. cancer-related angiogenesis, atherosclerosis) [3]. A prerequisite for defining these roles and underlying molecular mechanisms is information about Hox expression patterns in adult vasculature in vivo, which is currently scarcely available.
Considering the documented expression of various members of this gene family in ECs and VSMCs [3], the near-void of data concerning phenotypic changes in the cardiovascular system of the numerous Hox gene-targeted and transgenic mice is surprising. A notable exception is the Hoxa3 gene-targeted mice, which exhibit a range of cardiovascular defects [4-6], although a definition of the molecular mechanisms underlying these defects was essentially precluded due to lack of information about the cardiovascular Hoxa3 expression pattern, both in the embryo and the adult. Perhaps of equal relevance is that in the fruit fly Drosophila melanogaster the Hox gene abdA is required for specifying cell identity in a posterior section of the Drosophila dorsal vessel, which is functionally equivalent to the vertebrate heart [7].
Since these seminal observations with Hoxa3 and abdA in mice and flies, respectively, a series of studies has shown differential expression of Hox genes in ECs of different origin with respect to species and vessel type. For example, 8 of the 10 human HOXB genes were found expressed in cultured umbilical vein ECs and this expression could be modulated by vascular signaling molecules including tissue plasminogen activator (TPA), and vascular endothelial growth factor (VEGF) [8]. Most of the studies involving endothelial Hox expression suggest a role in the regulation of angiogenesis, i.e. the formation of new blood vessels and microvasculature associated either with normal developmental and physiological processes such as mammary gland development and wound healing or with pathological conditions such as tumorigenesis [8-16].
Evidence for Hox expression in VSMCs was initially provided by showing Hoxa2 expression in VSMCs of embryonic vessels leading from the heart, in embryonic cardiomyocytes, and in adult aortic VSMCs [17]; this was facilitated by the isolation of a Hoxa2-specific cDNA from a rat aorta-derived cDNA library. Additional VSMC-specific Hox cDNAs (HOXA5, HOXA11, HOXB1, HOXB7, and HOXC9) were isolated from fetal and adult human VSMC cDNA libraries by using degenerate primers corresponding to a highly conserved subregion of the homeobox for screening [18]. Although providing more direct evidence for in vivo expression of any of the corresponding genes in fetal or adult aorta remained elusive in that study, the potential relevance of these data was underscored in a separate study showing scattered expression of HOXB7 in media and neointima adjacent to calcification in human atherosclerotic plaques [19]. To assess whether this might reflect a role in directing immature cells towards either osteoblastic or VSMC differentiation, HOXB7 was overexpressed in multipotent C3H10T1/2 cells that are capable of differentiating into VSMCs, as well as osteogenic and chondrogenic lineages. The results showed 3.5-fold increase in proliferation and induction of VSMC-like morphology, thus suggesting a role for HOXB7 in the expansion of immature cell populations or dedifferentiation of mature cells [19]. Of relevance in this context is the apparent VSMC phenotype-dependent expression of HOXB7 and HOXC9 as evidenced by preferential expression in cultured fetal versus adult VSMCs, a result that might indicate a role for Hox genes in the control of VSMC-diversity [18].
Although the activity patterns in different cardiovascular lineages suggest potentially significant roles for Hox genes in cardiovascular patterning and remodeling, nearly none of these have been defined in vivo. Perhaps one of the most confounding factors is an inherent difficulty in defining gene expression patterns in a structurally and physiologically diverse organ system branched throughout the entire body. To overcome this obstacle, we used lacZ reporter gene analysis in transgenic mice to obtain an approximate global view of the postnatal and adult vascular expression patterns of two Hox genes, Hoxa3 and Hoxc11. The data indicate distinct regionally restricted zones of expression that roughly correspond to the disparate embryonic expression domains of the two genes. These results support a role for Hox genes in specifying and maintaining positional identities of VSMCs and ECs in the adult vasculature.
Materials and methods
Cloning of Hoxa3-lacZ
A segment of genomic DNA of 11,473 base pairs (bp) located directly upstream of the Hoxa3 translational start codon was fused to E.coli lacZ derived from plasmid pCH110 (Amersham Pharmacia). The Hoxa3 fragment was derived from mouse genomic BAC clone RP24-353A14 (Children's Research Institute, Oakland, CA) and extended from the NruI restriction half-site located just 2 bp upstream of the Hoxa3 translational start codon [20] to the BamHI site at position -11,473 relative to the translational start. Hoxa3-lacZ was cloned in a pBluescript (Promega) -derived vector termed pSafyre [21] and released by Spe1 and Not1 restriction enzymes for the preparation of transgenic mice. Transgenic lacZ reporter studies showed previously that sequences located upstream of the Hoxa3 coding region contain both transcriptional promoter and enhancer functions capable of reconstructing the main aspects of endogenous Hoxa3 expression in E8.5-E9.5 mouse embryos (Hoxa3 reporter gene analysis: [22]; endogenous Hoxa3 expression: [22-24]).
Transgenic mice
A Hoxc11-lacZ transgenic line carrying the LZc11-S construct was reported previously [25]. LZc11-S contained a 10 kb genomic fragment including the Hoxc11 transcription unit in addition to 2 kb and 5 kb of Hoxc11 upstream and downstream flanking sequences, respectively, as well as E.coli lacZ fused in frame to first exon coding sequences [25]; LZc11-S consistently reproduced endogenous Hoxc11 expression pattern in E11.5 – E13.5 embryos (n = 3 founders, including 2 transgenic lines). One of the LZc11-S lines has been designated TG(Hoxc11/lacZ)62D9Awg [26] and kept on FVB/NTac strain background. Mice from this line were used for lacZ expression analysis in blood vessels at postnatal and adult stages up to 1 year of age.
Hoxa3-lacZ mice were made according to standard transgenic procedures using single-cell FVB/NTac embryos. Transient transgenic mice (n = 6) and mice from one transgenic line designated TG(Hoxa3-lacZ)184H3Awg [26] were analyzed for reporter gene expression patterns at embryonic stage E14.5 (2 transient) and at 11 days post natum (p.n.) (2 transient), as well as at adult age of ≥ 6 weeks (line TG(Hoxa3-lacZ)184H3Awg, plus 2 transient transgenic mice). Patterns observed at E14.5 were consistent with the endogenous Hoxa3 expression patterns previously reported, albeit at earlier stages of embryonic development [22-24]. Among the postnatal and adult transient transgenic mice and the F1 mice of the TG(Hoxa3-lacZ)184H3Awg line, the vascular expression patterns observed at 11 days p.n. and at ≥ 6 weeks were consistent.
X-Gal staining of lacZ reporter mouse tissue
Vascular tissues were fixed in a solution containing 0.2% glutaraldehyde, 2% paraformaldehyde, 2 mM MgCl2 in PBS at room temperature for periods ranging from 20 minutes to 1 hr depending on tissue. After rinsing for several hours in detergent solution containing 2 mM MCl2, 0.02% Nonidet P-40, 0.0001% Na-deoxycholate in PBS, the tissues were stained overnight at 32°C in a solution containing 20 mM Tris-HCl (pH 7.3), 2 mM MgCl2, 0.02% Nonidet P-40, 0.0001% Na-deoxycholate, 20 mM potassium-ferrocyanide, 20 mM potassium-ferricyanide, and 0.625 mg/ml X-Gal in PBS. After rinsing in PBS, stained tissues were fixed in 4% paraformaldehyde. For the preparation of frozen sections, stained tissues were infiltrated with 30% sucrose/PBS overnight at 4°C prior to embedding in OCT compound; tissues were cryosectioned and imaged using differential interference contrast (DIC) imaging.
Reverse transcriptase (RT) – PCR
Under isoflurane anesthesia by inhalation, mouse tissues were prepared by transcardial perfusion with diluted (80%) RNAlater (Ambion, Austin, TX) to stabilize vascular tissue prior to excision followed by overnight incubation in undiluted RNAlater at 4°C. Vascular tissues were homogenized, and RNA was isolated with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to manufacturers instructions and subsequently treated with RQ1 RNAse-free DNase (Promega, Madison WI). First-strand cDNA was prepared from total RNA using Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Gene-specific cDNA fragments were amplified with AmpliTaq Gold DNA Polymerase (Applied Biosystems, Foster City, CA) using the following primers: Hoxa3 (218 bp), F-GGGCACCGATGGCGTTGAGT and R-GCTGTGGTGGGGGCTGTGGA; Hoxc11 (311 bp), F-CCGGAGGAGGCAGGAGAAGA and R-CCGCCGCATAACAAGACGA. Gapdh primers were used for positive control reaction.
Immunofluorescence (IF) labeling/Immunohistochemistry (IHC)
Under isoflurane anesthesia by inhalation, 8 week old normal FVB and TG(Hoxa3-lacZ)184H3Awg mouse tissues were prepared by transcardial perfusion with 0.1 M PIPES-buffered (pH 7.0) 2% paraformaldehyde followed by overnight immersion of excised tissues in PIPES-buffered 0.2% paraformaldehyde at 4°C. Carotid and dorsal aorta tissues were subsequently processed for cryosectioning (7 μm). For IF, rabbit anti-mouse primary antibodies for Hoxa3 (Santa Cruz Biotechnology, Santa Cruz, CA) and smooth muscle-specific Cy3-conjugated anti-Acta2 (Smooth muscle alpha actin [SMαA] Sigma, St. Louis, MO) were applied to carotid cross-sections at 10 μg/ml at room temperature for 2 hours following antigen retrieval with 10 mM Sodium Citrate (pH 6.0) buffer and brief microwave heating. For immunolocalization, fluorochrome-conjugated (Cy3 or Cy5) donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used. Cell nuclei were stained with Hoechst 33342. Fluorescence imaging at 400× was conducted using a Leica DMRB HC microscope, supported by a digital imaging workstation that includes a real time color digital camera (SPOT-RT) and a Gateway1400 PC.
For immunocytofluorescence (ICF), smooth muscle cells from dissociated vessel (see below) were immunolabeled with primary antibodies specific for either Hoxc11, β-gal, or Transgelin (SM22a; Abcam, Cambridge, MA) and detected with appropriate fluorochrome secondary antibodies followed by Hoechst 33342 nuclear labeling as described above. ICF/X-Gal double-labeling was accomplished by labeling the fixed cells with X-Gal prior to incubation with Cy3-conjugated anti-Acta2 antibodies and Hoechst 33342.
For IHC, rabbit anti-mouse primary antibodies for Hoxa3 were applied to dorsal aorta cross-sections at 10 μg/ml at room temperature for 2 hours following antigen retrieval with 10 mM Sodium Citrate (pH 6.0) buffer and brief microwave heating. Immunolocalization was detected with Vectastain ABC Kit utilizing peroxidase-conjugated anti-rabbit IgG antibodies and color detection with DAB, perioxidase substrate kit (Vector Labs, Burlingame, CA) according to manufacturers instructions. Micrographs of DAB-labeled sections were taken with differential interference contrast (DIC) imaging at 630×.
Vessel explant cultures – in vitro assays
Small segments (2 mm length) of the lateral marginal vein and the femoral artery (mid-femoral region) of 6 weeks old Hoxc11-lacZ mice were dissected and placed into collagen-coated culture chambers. Explants were cultured in M199 medium supplemented with 20% FBS and pen-strep-glutamine. Outgrowing cells could be observed initially after ≈ 4 days, and cell density increased with prolonged culture.
To assess possible differential migration rates of transgene (Hoxc11-lacZ) expressing versus non-expressing cells, VSMCs from primary cultures were collected subsequent to the 5th passage and plated in multi-well (4 wells) chambered slides at a concentration of approximately 500 cells/cm2 in M199 media supplemented with 20% fetal calf serum (FCS). Cultures were allowed to grow to near confluence then "scratched" with a 200 μL pipette tip producing a 0.8 mm wound traversing the length of each chamber (2 cm). Under DIC imaging the wounded area was defined and marked for subsequent orientation at 100×. Cultures were allowed to grow for 24 hrs prior to processing for X-Gal labeling as described above with minor alterations: cells were fixed for 8 min prior to labeling. Using DIC imaging randomly selected fields were chosen from scratched areas of the four experimental wells and photographed at 100×; randomly selected fields from "normal" un-scratched areas were likewise imaged and photographed and used as negative controls. β-gal-positive (blue) and -negative cells of the experimental and control groups were scored and expressed as proportions of total number of cells and differences expressed as -fold change between groups. Data were expressed as mean ± standard deviation, and statistical significance (defined as P < 0.05) was determined using Student's t-test. For ICF experiments, cells were dissociated by enzymatic digestion (M199 media supplemented with 0.3 mg/ml elastase type III [Sigma, St. Louis MO], 1.8 mg/ml collagenase type I [Sigma, St. Louis MO] and 0.44 mg/ml soybean trypsin inhibitor [Sigma, St. Louis MO]) at 37°C for 1 hr prior to plating under the conditions as described above.
Hoxc11 Antibody Production
Custom-made, affinity-purified, chicken-anti-mouse Hoxc11 antibodies were synthesized (Sigma Genosys, St. Louis MO) against an epitope of the Hoxc11 protein with the peptide sequence PPSTVTEILMKNEGS that comprises amino acid residues 106 – 120 of the variable region of the Hoxc11 protein; the human equivalent of this epitope was previously used successfully for raising HOXC11 antibodies [27]. Please note that the antibodies directed against this peptide are unable to recognize the Hoxc11-βgal fusion protein produced by the TG(Hoxc11/lacZ)62D9Awg mice as the lacZ coding region displaces Hoxc11 amino acids 96 – 304 in the transgene protein product [25]. Keyhole limpet hemocyanin (KLH) was selected as the peptide carrier protein and conjugation was achieved through attachment to the thiol-group of an additional cysteine residue placed at the N-terminus of the peptide sequence. The specificity of the affinity-purified antisera was determined by ELISA, as well as immunolabeling experiments with sagittal and cross sections of E12.5 mouse embryos that yielded detection patterns very similar to the familiar Hoxc11 RNA expression patterns ([28,29]; data not shown).
Results
Distinct Hoxc11- and Hoxa3-lacZ expression patterns in adult blood vessels
We previously reported that transgenic mice (n = 4 founders) carrying a Hoxc11-lacZ reporter gene construct in which E.coli lacZ with SV40 RNA processing signals was fused in-frame to Hoxc11 exon 1 coding sequences exhibited a conspicuous and reproducible β-gal expression pattern in mid-gestation embryos at ≈ E12 [25]. This pattern (Fig. 1A) was consistent with the posteriorly restricted pattern of endogenous Hoxc11 expression in par-axial and hindlimb mesenchyme as determined by ISH [25,28,29]. Accordingly, we concluded that this Hoxc11-lacZ construct included most of the control elements required for establishing the native Hoxc11 expression pattern in mid-gestation embryos [25,28,29]. Here, we used one of these Hoxc11-lacZ transgenic lines as a tool for gaining insight into global aspects of Hoxc11 expression during fetal and postnatal development, as well as in adulthood. This revealed β-gal activity in the hindlimb vasculature that had previously escaped our attention. Examination of young adult transgenic mice showed lacZ expression (X-Gal labeling) in all major blood vessels of the hindlimb, including arteries and veins (Fig. 1D–F) with strongest expression at the level of the zeugopod and lower stylopod (femur). In several vessels, including the femoral artery, X-Gal labeling was rather uniform in the lower limb and became progressively mosaic in upper femoral limb regions near the expression boundary (Fig. 1E, F). However, vessels showing mosaic β-gal expression patterns could also be seen next to vessels that appeared to be uniformly stained at a given proximal-distal level of the zeugopod. Persistent vascular expression was observed in animals of 1 year of age (Fig. 1G), the latest stage examined.
Figure 1.
Analysis of Hoxc11-lacZ reporter gene expression in hindlimb vasculature. (A) E12 embryo of the transgenic shows posteriorly restricted lacZ reporter gene expression (blue) in most of the hindlimb region and tailbud, as well as in the posterior zeugopodal forelimb region. (B) X-Gal staining of posterior trunk and hindlimbs of 0.5 d newborn Hoxc11-lacZ transgenic mouse after skinning reveals expression in skeletal muscles and blood vessels (yellow arrow). (C) Lateral view of stained hindlimb of 42 d Hoxc11-lacZ transgenic mouse shows conspicuous β-gal activity in sciatic artery. (D-F) Medial view of same hindlimb as shown in panel C is presented in panel E and reveals distally restricted expression in both arteries and veins; close-up views of upper and lower sections of femoral artery (red arrows) and vein (blue arrows) are shown in panels D and F, respectively. (G) Lateral view of stained hindlimb from 1 yr old Hoxc11-lacZ transgenic mouse shows persistent reporter gene expression in blood vessels, most prominently in sciatic artery. All mice were derived from transgenic line TG(Hoxc11-lacZ)62D9Awg; fl: forelimb; hl: hindlimb; tlb: tailbud.
In addition to expression in blood vessels, we observed strong and specific Hoxc11-lacZ activity in kidney, ureter, urinary bladder, and uterus (data not shown). This activity in both the male and female urogenital system is consistent with previously reported endogenous Hoxc11 expression in the metanephric mesenchyme during mid-gestation, and in cortical regions of the developing kidney during later development [28], as well as HOXC11 expression in human uterine endometrium [30]. Furthermore, we also observed diffuse β-gal activity in hindlimb cartilage and skeletal muscle of postnatal and adult mice (Fig. 1B, C, E); although this activity apparently became gradually weaker and increasingly restricted to distal limb regions with age, it was still clearly detectable in 1 year old mice (Fig. 1G). This may indicate that endogenous Hoxc11 expression in skeletal muscle, first observed in embryonic myotomes and developing muscle during fetal development [28], persists in adulthood.
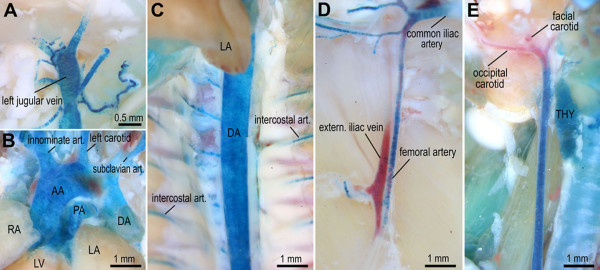
To gain further support for the emerging concept of distinct zones of Hox activity in the adult vascular network, we prepared a Hoxa3-lacZ transgene construct that contained 11.5 kb of genomic sequences located directly upstream of the Hoxa3 translational start site (see Materials and Methods). Analysis of independent Hoxa3-lacZ transgenic mice (n = 4) at 11 d, 6 wks, and ≥ 8 wks post natum revealed a reproducible pattern of lacZ expression in most major blood vessels (common carotid arteries, aortic arch, dorsal aorta and inter-costal arteries, pulmonary and renal arteries, cardinal and jugular veins, proximal femoral arteries and veins) (Fig. 2A–E). Remarkably, the distinct anterior expression boundary near the cranial branch point of the common carotid arteries (Fig. 2E) was approximately at the same anterior-posterior level as the expression boundary in the jugular veins (Fig. 2A), and these expression boundaries roughly correspond to the anterior Hoxa3 expression limit at the somite s4/5 boundary during embryonic development [22]. Hoxa3-lacZ expression in the dorsal aorta and inferior vena cava extended beyond the iliac branching point into the most caudal blood vessels of the tail, as well as the proximal limb vessels, including expression in the proximal segments of the femoral artery and vein that reached just to the branch of the epigastric artery (Fig. 2D).
Figure 2.
Hoxa3-lacZ expression in major blood vessels. (A-E) Prominent expression (blue) was detected by whole-mount X-Gal staining of 6 wk old Hoxa3-lacZ transgenic mice in external jugular vein (panel A), aortic arch (AA) and all major vessels in the aortic arch region including innominate and left carotid artery, pulmonary vein (PA), and subclavian artery (panel B); strong expression was also detected in the descending thoracic aorta (DA) and intercostal arteries (panel C), as well as the common iliac artery and proximal femoral artery (panel D); Hoxa3-lacZ expression in the common carotid artery exhibits a distinct anterior boundary near the branch point into facial and occipital carotid arteries (panel E); anterior points to the top in panels A-C, and E, and to the right in panel D; please note the distal expression boundary in the femoral artery just below the branch point of the internal iliac. LA: left atrium; LV: left ventricle; RA: right atrium; thy: thyroid gland.
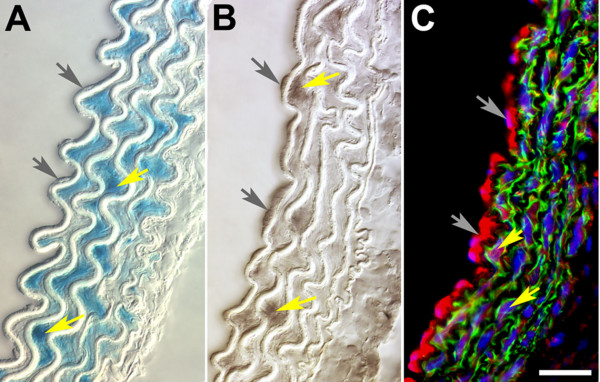
To determine reporter gene expression patterns in vessel walls, we analyzed sections of X-Gal-stained blood vessels from adult Hoxc11-lacZ and Hoxa3-lacZ mice (≥ 6 wks). As exemplified by data shown in Figure 3, Hoxc11-lacZ expression was observed predominantly, if not exclusively, in subsets of VSMCs of the major blood vessels of the lower hindlimb, including arteries (Fig. 3A) and veins (Fig. 3B). Overall, the Hoxa3-lacZ-expressing vessel walls of the iliac artery, the descending thoracic aorta, the intercostal arteries, and the common carotid arteries (Fig. 3C–F) exhibited similar patterns as the Hoxc11-lacZ-expressing walls of the lower limb, including prominent expression in VSMCs. Due to their flat morphology, expression in epithelial cells lining the intimal lamina was difficult to discern based on X-Gal staining in either case (i.e. Hoxc11-lacZ and Hoxa3-lacZ transgenic mice). This was resolved by performing immunofluorescent labeling studies using β-gal-specific antibodies, which detected endothelial β-gal expression in the common carotids of Hoxa3-lacZ mice, but not in the tibial/femoral arteries of Hoxc11-lacZ mice (data not shown).
Figure 3.
Hoxc11- and Hoxa3-lacZ expression patterns in blood vessel sections of 6 wk old transgenic mice. (A, B) Cross sections of the distal femoral artery (panel A) and femoral vein (B) show restriction of Hoxc11-lacZ expression in subsets of VSMCs in arterial and venous vessel walls as indicated by X-Gal staining (blue). (C-F) Cross sections of X-Gal-stained iliac artery (C), descending thoracic aorta (D), intercostal artery (E), and common carotid artery (F) of Hoxa3-lacZ transgenic mice indicate strong β-gal expression (blue) in the VSMCs of the vessel walls, whereas X-Gal staining in the flattened endothelial cells lining the intimal laminae is difficult to discern. adv: adventitia eem: external elastic membrane, iem: internal elastic membrane, in: intima, m: media. Space bars: 25 μm in all panels.
Validation of Hoxc11 and Hoxa3 reporter gene expression patterns
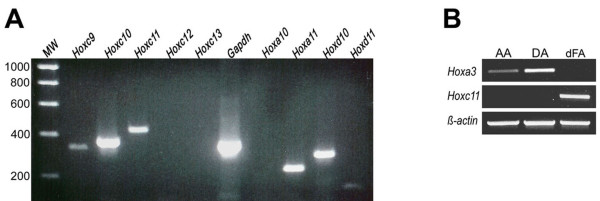
To determine whether the vascular Hoxc11- and Hoxa3-lacZ reporter gene expression reflected corresponding endogenous gene activities, we performed semi-quantitative reverse transcriptase (RT)-PCR analysis of total RNA isolated from defined vessel segments of 6 wk old mice. For a first set of reactions, we used RNA isolated from femoral artery and vein segments ranging from the mid-femoral region just proximal to the Hoxc11-lacZ expression boundary to the distal femoral artery branch point approximately at knee-level. In addition to examining this RNA sample for the presence of Hoxc11-specific transcripts, we determined whether there was evidence for expression of the remaining AbdB-type Hoxc genes (Hoxc9, Hoxc10, Hoxc12, and Hoxc13), as well as for the Hoxc11 and Hoxc10 paralogous genes (Hoxa11, Hoxd11, and Hoxa10, Hoxd10). The results indicate strong expression of Hoxc11 and Hoxc10, whereas Hoxc9 expression appeared less abundant, and expression of Hoxc12 and Hoxc13 was not detectable (Fig. 4A). Strong expression was also indicated for two of the paralogous genes, Hoxa11 and Hoxd10, whereas no RT-PCR amplification product was detected for Hoxa10, and the band corresponding to Hoxd11 was weak (Fig. 4A). These data suggest selective activity of AbdB-type Hoxc genes in major blood vessels of the mid-femoral hindlimb region. Specifically, the detection of Hoxc11-specific amplification products suggests that the vascular Hoxc11-lacZ reporter gene expression observed in this region reflects authentic Hoxc11 transcriptional activity. The detection of Hoxa11- and Hoxc9-specific amplification products is consistent with the isolation of HOXA11 and HOXC9 cDNAs from human smooth muscle cell cDNA libraries reported previously [18].
Figure 4.
RT-PCR analysis of Hox gene expression in adult blood vessels. (A) Total RNA derived from hindlimb blood vessels (6 wk old FVB/NTac mice) as defined in the text was used for cDNA synthesis, and Hox-specific cDNA fragments were amplified by using primers specific for the AbdB-type Hoxc genes; this resulted in PCR products in the expected size range for Hoxc9 (323 bp), Hoxc10 (350 bp), and Hoxc11 (435 bp); PCR reactions with primers specific for the Hoxc11 and Hoxc10 paralogous genes (Hoxa11 and Hoxd11, as well as Hoxa10 and Hoxd10) resulted in amplification products in the expected size range for Hoxa11 (220 bp), Hoxd11 (131 bp), and Hoxd10 (286 bp), whereas no product was detected for Hoxa10; positive control reaction: Gapdh-specific primers; MW: molecular weight standards in base pairs (bp). (B) RT-PCR analysis of Hoxa3 and Hoxc11 expression in adult mouse (6 wk) blood vessel segments including aortic arch (AA), descending thoracic aorta (DA), and distal femoral artery (dFA); positive control reaction was performed with β-actin-specific primers.
To obtain supporting evidence for regionally restricted expression of endogenous Hoxa3 and Hoxc11 within the vascular network, we isolated RNA from the aortic arch, the descending thoracic aorta, and the distal femoral artery (the distal femoral artery segment used was as defined above) for RT-PCR analysis. In agreement with the Hoxa3- and Hoxc11-lacZ reporter gene analysis, the data indicate Hoxa3 expression in the aortic arch and the descending thoracic aorta but not in the distal femoral artery, whereas Hoxc11 expression is detectable exclusively in the latter (Fig. 4B).
Evidence that the anterior Hoxa3-lacZ boundary in carotid arteries (Fig. 2E) reflects the endogenous Hoxa3 pattern in these vessels was provided at the protein level by immunolabeling studies with Hoxa3-specific antibodies that detected Hoxa3 protein exclusively in sections of carotid vessel segments located posterior of this boundary (Fig. 5). Furthermore, while Hoxa3-lacZ expression in the endothelial layer was difficult to discern by X-Gal staining as exemplified in Figure 6A, endogenous Hoxa3 protein expression in both ECs and VSMCs of the aortic arch was confirmed by immunolabeling (Fig. 6B,C). Combined, these data strongly suggest that Hoxa3-lacZ mimics the authentic Hoxa3 expression pattern in adult blood vessels. The Hoxa3-lacZ patterns correlate remarkably well with the broad spectrum of vascular defects previously observed in Hoxa3 null mice, which included abnormalities of the carotid system, thinning of the aorta and enlargement of major veins [4,6]. Lack of information about the Hoxa3 vascular expression pattern, however, largely precluded interpretation of these data with regard to potential underlying mechanisms.
Figure 5.
Validation of endogenous Hoxa3 expression boundary in adult carotid artery of FVB/NTac mouse. (A) Diagram of right carotid segment indicating reporter gene expression domain (blue shading) with anterior boundary at level of lingual artery (la) as observed in Hoxa3-lacZ transgenic mouse (see Fig. 2E for comparison); yellow arrow points to carotid bifurcation into internal (ica) and external carotid arteries (eca), respectively; sections corresponding to levels L1 and L2 as indicated in the cartoon were used for detection of Hoxa3 and Acta2 by immunofluorescence as shown in the three upper (B,B'B") and lower (C,C',C") panels to the right. Both L1 and L2 sections were incubated with Hoxa3-specific and Cy3-conjugated Acta2-specific antibodies; using Cy5-conjugated secondary antibodies, red-fluoresent-labeled Hoxa3 protein was detected only in section L2 (panel C'), whereas green-fluorescent-labeled Acta2 expressed in VSMCs was detected in both L1 and L2 sections (panels B" and C"); accordingly, multichannel composite micrographs (panels B and C) for detecting red (Cy5), green (Cy3) and blue (Hoechst 33342 for nuclear labeling; this is not shown individually) indicate co-localization of Hoxa3 and Acta2 (yellow) in VSMCs only in section L2 (panel C). fa: facial artery; oa: occipital artery.
Figure 6.
Comparison of Hoxa3-lacZ reporter and endogenous Hoxa3 protein expression in VSMCs and ECs of aortic arch. (A) Cross section of aortic arch from adult Hoxa3-lacZ mouse after X-Gal-staining indicates reporter gene expression (blue) in VSMCs (yellow arrows) whereas expression in cells of the endothelial layer (gray arrows) is difficult to discern. (B) Immunohistochemical detection of Hoxa3 in comparable section of adult (8 wk) dorsal aorta; immunolabeling of section with anti-Hoxa3 antibodies and subsequent colorimetric detection (DAB-Ni) reveals expression in ECs (gray arrows) and vascular VSMC (yellow arrows). (C) Multichannel composite micrograph of adult aortic arch section after incubation with immunoreagents specific for Hoxa3 (Cy5-red) and Acta2 (Cy3- green), and Hoechst 33342 for nuclear labeling. The bright red labeling in ECs (gray arrows) confirms Hoxa3 expression. The purple labeling in the media indicates Hoxa3 expression in certain nuclei of VSMCs (yellow arrows). Green label indicates that Acta2 expression is restricted to the media. Lumen is to the left in all panels; space bar: 25 μm.
Hoxc11-lacZ expression ex vivo
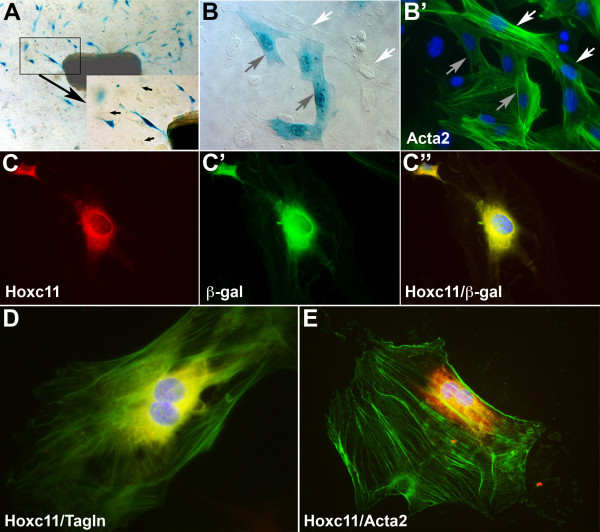
Blood vessel explants (segments of ≈ 2 mm length) of the lateral marginal vein and the distal femoral artery of 6 wk old Hoxc11-lacZ mice were cultured to determine whether reporter gene activity was retained in outgrowing cells. Initial outgrowth was observed after ≈ 4 days under standard conditions (M199 medium supplemented with 20% FBS and pen-strep glutamine; see Materials and Methods), and X-Gal staining after 11 days of culture consistently showed β-gal activity in a fraction of the outgrowing cells (Fig. 7A, B), while control explants from corresponding regions of non-transgenic mice did not produce β-gal-positive cells (not shown). Co-labeling with antibodies directed against a common marker for smooth muscle cells (SMCs), i.e. Acta2 (previously known as SMαA) showed that the β-gal-positive cells expressed Acta2, whereas not all of the Acta2-expressing cells were β-gal-positive (compare panels B and B' in Fig. 7). Using Hoxc11- and β-gal-specific antibodies (see Materials and Methods for preparation and testing of Hoxc11-specific antibodies) in double-immunolabeling studies, we determined that these β-gal-positive cells do express endogenous Hoxc11 (Fig. 7C,C',C"). Furthermore, using the Hoxc11 antibodies in double-immunolabeling assays with Acta2 antibodies, as well as antibodies against a second SMC marker, Transgelin (Tagln; previously known as SM22) showed co-labeling in the perinuclear region of individual cultured VSMCs from the femoral artery of TG(Hoxc11/lacZ)62D9Awg mice in both cases (Fig. 7D,E).
Figure 7.
Hoxc11-lacZ expression is restricted to a subset of smooth muscle cells in vessel explant culture. (A) Cellular outgrowth of explanted femoral artery/lateral marginal vein vessel segments (~2 mm; central dark ovoid structure in panel A) from young adult Hoxc11-lacZ transgenic mice [TG(Hoxc11/lacZ)62D9Awg] is comprised of β-gal-positive (blue) and -negative cells (black arrows in panel A, inset). (B) X-Gal labeling of cultured, femoral artery SMCs of Hoxc11-lacZ reporter mice reveals β-gal-positive (blue) and β-gal-negative cells as indicated by grey and white arrows, respectively. (B') Subsequent to X-Gal labeling, the cells shown in panel B were immunolabeled with antibodies specific for SMC marker Acta2 (formerly known as SMαA) as indicated by green fluorescence; note that both β-gal-positive and -negative cells as marked by grey and white arrows, respectively (grey and white arrows correspond to the same cells in panels B and B'), are labeled by anti-Acta2; nuclei were labeled with Hoechst 33342 (blue). (C/C'/C") Double-labeling of explant-derived, cultured femoral artery SMCs of TG(Hoxc11/lacZ)62D9Awg mice with antibodies against Hoxc11 (red signal, panel C) and anti-β-gal (green signal, panel C') results in a merged yellow signal in panel C". (D/E) Double-immunolabeling of cultured mitotic femoral artery SMCs with antibodies against Hoxc11 (red signal) and two standard SMC markers, including Transgelin (Tagln, formerly known as Smooth muscle protein 22-alpha; green signal, panel D), and Acta2 (green signal, panel E); note, overlap between Hoxc11 and Tagln signals in panel D results in yellow fluorescence, whereas the lesser degree of overlap between Hoxc11 and Acta2 signals in panel E results in discernible Hoxc11 (red) signal in the peri-nuclear region. Nuclei (blue) were labeled with Hoechst 33342.
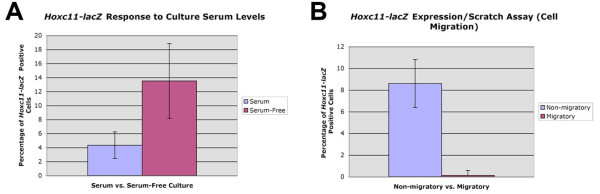
Continued Hoxc11-lacZ expression in only a subpopulation of cultured VSMCs raised the question whether these cells might be functionally distinct compared to β-gal-negative cells. This was addressed in a preliminary manner by performing two simple and straightforward in vitro assays that tested response to culture serum levels and migration behavior. The results show that the proportion of Hoxc11-lacZ-expressing cells increased approximately 3-fold to 13.5% under serum-free culture conditions compared to about 4.3% under conditions including 20% FBS (Fig. 8A). In scratch assays, the proportion of β-gal-positive cells was dramatically reduced (71-fold) among the migratory cells found in the scratch area compared to the cells residing in the undisturbed monolayer (Fig. 8B). These differential reporter gene expression patterns in cultured VSMCs undergoing phenotypic modulation suggest that Hoxc11 expression is associated with distinct VSMC phenotypes.
Figure 8.
Functional assays of Hoxc11-lacZ expression in cell culture. (A/B) Explant cultures. (A) The proportion of β-gal-positve cells derived from explant cultures of lateral marginal veins and femoral arteries from 6 weeks old Hoxc11-lacZ mice is approximately 4.3% (± 1.92%) under culture conditions that include 20% fetal bovine serum; this increases to approximately 3-fold to 13.5% (± 5.34%) under serum-free conditions; P < 0.001. (B) Cell migration/scratch assay indicates a dramatic (71-fold) reduction in transgene expression in the group of migratory cells found in the scratch area, (8.6% ± 2.2 blue cells in normal (unwounded) area compared to 0.11% ± 0.46 in wounded area); difference of approximately 71-fold, P < 0.001.
VSMCs are capable of exhibiting a wide range of different phenotypes in response to changes in local environmental cues, a phenomenon known as phenotypic modulation or phenotype switching [31,32]. The morphologically and functionally distinct synthetic and contractile VSMCs are considered to mark the boundaries at the opposite ends of a spectrum within which VSMCs can modulate their phenotypic properties [33]. The decreased Hoxc11-lacZ expression in migrating cells is consistent with the lower level of reporter gene activity in response to serum – in both cases the phenotypic equilibrium is shifted towards a proliferative and synthetic phenotype [32]. Accordingly, these data suggest preferential Hoxc11 activity in contractile VSMCs of spindle-like morphology.
Discussion
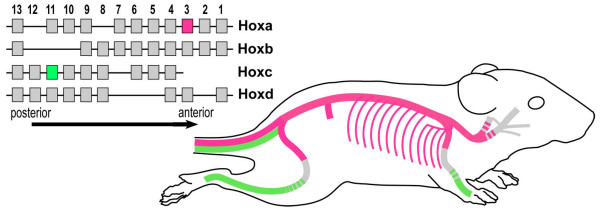
During development, a primary role of Hox genes is thought to "translate" positional information established within a spatial coordinate system into positional identities, which is essential for the differentiation of defined body structures at their appropriate anatomic sites [34]. Once adult structures and organs have formed, a fundamental question arises regarding how their organo-typic shape and physiological properties are being maintained. According to a recently proposed concept this may be achieved by retaining positional identities specified by a Hox code [35,36], which is initially set up during embryonic patterning [37]. This positional identity model was supported by gene expression profiling of human fibroblast populations derived from distinct adult anatomic sites, which revealed that the site-specific Hox profiles mirrored the embryonic Hox patterns relative to the main developmental axes. This was interpreted as evidence for a Hox code-based positional memory underlying the control of topographic fibroblast differentiation [35,36]. The results presented here show complex, yet distinct, spatially restricted Hoxa3 and Hoxc11 patterns in adult blood vessels (see Fig. 9 for a schematic summary) that largely correspond to the embryonic Hoxa3 [23,24] and Hoxc11 [25,28] activity domains. These data are consistent with the positional identity model and support the idea of discrete Hox-specified identities in the vascular network. Validation of this concept in future studies will require a comprehensive analysis of the spatial expression patterns of all 39 Hox genes in the developing and adult cardiovascular system.
Figure 9.
Summary of Hoxa3 and Hoxc11 expression domains in major blood vessels as indicated by reporter gene analysis. Left, diagram of the four Hox clusters shown in parallel alignment with arrow at bottom indicating transcriptional orientation and spatial co-linearity of Hox map positions with distinct expression domains along the longitudinal body axis during embryonic development. Right, cartoon of mouse depicting Hoxa3-lacZ (red) and Hoxc11-lacZ (green) zones of expression in major blood vessels.
In keeping with the positional identity model, recent global gene expression profiling revealed that similar to fibroblasts, ECs and SMCs exhibit considerable diversity depending on function and anatomic site [38,39]. Within the cardiovascular system, this is likely to reflect diverse physiological requirements, including regional variations in vessel tone, blood flow, blood pressure, oxygen content, nutrient content, etc., and Hox genes are considered excellent candidates for orchestrating the corresponding regional gene expression profiles [3,38,39]. Data demonstrating that genes encoding SMC-restricted proteins are direct targets of Hox transcription factors [40] are consistent with this model. Furthermore, several Hox genes (including Hoxa3) have been implicated in controlling the conversion of ECs to the angiogenic phenotype [41], which is of great relevance for tumor-angiogenesis, as well as for neo-vascularization during wound healing and other normal adult physiological activities. Likewise, phenotype switching of VSMCs is known to be associated with a wide range of diseases of the cardiovascular system including atherosclerosis, stroke, high blood pressure, aneurisms, etc. [32]. Our data showing Hoxc11 expression to be restricted to a subset of VSMCs derived from the same vessel and anatomic site as VSMCs not expressing Hoxc11 may indicate that Hox expression profiles are associated with distinct VSMC phenotypes. This proposition is further underscored by the preliminary results from our functional assays suggesting a role for Hoxc11 in defining phenotypic properties of VSMCs. Taken together, elucidating the role of Hox genes in adult blood vessels is likely to be critically important for understanding vascular disease mechanisms.
Conclusion
Our results support a conceptual model of Hox-specified positional identities in adult blood vessels, which is of likely relevance for understanding the mechanisms underlying regional physiological diversities in the cardiovascular system. The data also demonstrate that conventional Hox reporter gene mice are useful tools for visualizing complex Hox expression patterns in the vascular network that might be unattainable otherwise. Importantly, these mice are a valuable resource for the isolation and phenotypic characterization of specific subpopulations of vascular cells marked by distinct Hox expression profiles.
Authors' contributions
NDP performed the reporter gene and immunolabeling studies, RT-PCR analysis, and functional assays with cultured cells and contributed to the data analysis and the preparation of the manuscript. RPV participated in the design and interpretation of the immunolabeling experiments and the interpretation of pertinent results. DFJ made the Hoxa3-lacZ transgenic mice and helped with finalizing the manuscript. DM contributed to the histological analysis of X-Gal-stained blood vessels and participated in preparing the manuscript. TM participated in planning the experiments and discussing the results. JPS helped with interpreting the lacZ staining and contributed to the final version of the manuscript. AA designed this study, cloned the Hoxa3 reporter gene construct, and drafted the original manuscript. All authors have read and approved the manuscript.
Acknowledgments
Acknowledgements
We thank Dr. R. Markwald for advice and critical reading of the manuscript. This work was supported by NIAMS grants AR047204 and AR 053639 to A.A., and NHLBI training grant HL07260 to N.D.P. Transgenic mice were created in a facility constructed with support from NIH grant C06 RR015455 of the Extramural Research Facilities Program of the National Center for Research Resources.
Contributor Information
Nathanael D Pruett, Email: pruettnd@musc.edu.
Richard P Visconti, Email: visconrp@musc.edu.
Donna F Jacobs, Email: jacobsdf@musc.edu.
Dimitri Scholz, Email: dimitri.scholz@ucd.ie.
Tim McQuinn, Email: mcquinnt@musc.edu.
John P Sundberg, Email: john.sundberg@jax.org.
Alexander Awgulewitsch, Email: awgulewa@musc.edu.
References
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- Morgan R. Hox genes: a continuation of embryonic patterning? Trends Genet. 2006;22:67–69. doi: 10.1016/j.tig.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Gorski DH, Walsh K. Control of vascular cell differentiation by homeobox transcription factors. Trends Cardiovasc Med. 2003;13:213–220. doi: 10.1016/S1050-1738(03)00081-1. [DOI] [PubMed] [Google Scholar]
- Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Kameda Y, Nishimaki T, Takeichi M, Chisaka O. Homeobox gene hoxa3 is essential for the formation of the carotid body in the mouse embryos. Dev Biol. 2002;247:197–209. doi: 10.1006/dbio.2002.0689. [DOI] [PubMed] [Google Scholar]
- Kameda Y, Watari-Goshima N, Nishimaki T, Chisaka O. Disruption of the Hoxa3 homeobox gene results in anomalies of the carotid artery system and the arterial baroreceptors. Cell Tissue Res. 2003;311:343–352. doi: 10.1007/s00441-002-0681-1. [DOI] [PubMed] [Google Scholar]
- Lovato TL, Nguyen TP, Molina MR, Cripps RM. The Hox gene abdominal-A specifies heart cell fate in the Drosophila dorsal vessel. Development. 2002;129:5019–5027. doi: 10.1242/dev.129.21.5019. [DOI] [PubMed] [Google Scholar]
- Belotti D, Clausse N, Flagiello D, Alami Y, Daukandt M, Deroanne C, Malfoy B, Boncinelli E, Faiella A, Castronovo V. Expression and modulation of homeobox genes from cluster B in endothelial cells. Lab Invest. 1998;78:1291–1299. [PubMed] [Google Scholar]
- Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau NJ, Varner JA. The homeobox transcription factor Hox D3 promotes integrin alpha5beta1 expression and function during angiogenesis. J Biol Chem. 2004;279:4862–4868. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- Myers C, Charboneau A, Boudreau N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol. 2000;148:343–351. doi: 10.1083/jcb.148.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox D10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- Soncin F, Fafeur V, Vandenbunder B. [Transcription factors and angiogenesis] Pathol Biol (Paris) 1999;47:358–363. [PubMed] [Google Scholar]
- Uyeno LA, Newman-Keagle JA, Cheung I, Hunt TK, Young DM, Boudreau N. Hox D3 expression in normal and impaired wound healing. J Surg Res. 2001;100:46–56. doi: 10.1006/jsre.2001.6174. [DOI] [PubMed] [Google Scholar]
- Patel CV, Gorski DH, LePage DF, Lincecum J, Walsh K. Molecular cloning of a homeobox transcription factor from adult aortic smooth muscle. J Biol Chem. 1992;267:26085–26090. [PubMed] [Google Scholar]
- Miano JM, Firulli AB, Olson EN, Hara P, Giachelli CM, Schwartz SM. Restricted expression of homeobox genes distinguishes fetal from adult human smooth muscle cells. Proc Natl Acad Sci USA. 1996;93:900–905. doi: 10.1073/pnas.93.2.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom K, Tintut Y, Kao SC, Stanford WP, Demer LL. HOXB7 overexpression promotes differentiation of C3H10T1/2 cells to smooth muscle cells. J Cell Biochem. 2000;78:210–221. doi: 10.1002/(SICI)1097-4644(20000801)78:2<210::AID-JCB4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vega Mouse http://vega.sanger.ac.uk/Mus_musculus
- Bieberich CJ, Utset MF, Awgulewitsch A, Ruddle FH. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci USA. 1990;87:8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares M, Cordes S, Ariza-McNaughton L, Sadl V, Maruthainar K, Barsh G, Krumlauf R. Conserved and distinct roles of kreisler in regulation of the paralogous Hoxa3 and Hoxb3 genes. Development. 1999;126:759–769. doi: 10.1242/dev.126.4.759. [DOI] [PubMed] [Google Scholar]
- Gaunt SJ. Homoeobox gene Hox-1.5 expression in mouse embryos: earliest detection by in situ hybridization is during gastrulation. Development. 1987;101:51–60. [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- Papenbrock T, Visconti RP, Awgulewitsch A. Loss of fibula in mice overexpressing Hoxc11. Mech Dev. 2000;92:113–123. doi: 10.1016/S0925-4773(99)00344-5. [DOI] [PubMed] [Google Scholar]
- International Mouse Strain Resource (IMSR) http://www.informatics.jax.org/imsr
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Hostikka SL, Capecchi MR. The mouse Hoxc11 gene: genomic structure and expression pattern. Mech Dev. 1998;70:133–145. doi: 10.1016/S0925-4773(97)00182-2. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Papenbrock T, Davda MM, Awgulewitsch A. The murine Hoxc cluster contains five neighboring AbdB-related Hox genes that show unique spatially coordinated expression in posterior embryonic subregions. Mech Dev. 1994;47:253–260. doi: 10.1016/0925-4773(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Akbas GE, Taylor HS. HOXC and HOXD gene expression in human endometrium: lack of redundancy with HOXA paralogs. Biol Reprod. 2004;70:39–45. doi: 10.1095/biolreprod.102.014969. [DOI] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. One hundred years of positional information. Trends Genet. 1996;12:359–364. doi: 10.1016/S0168-9525(96)80019-9. [DOI] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, Rijn M van de, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-I. [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, Rijn M van de, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JT, Rodriguez EH, Wang Z, Nuyten DS, Mukherjee S, Rijn M van de, Vijver MJ van de, Hastie T, Brown PO. Gene expression programs of human smooth muscle cells: tissue-specific differentiation and prognostic significance in breast cancers. PLoS Genet. 2007;3:1770–1784. doi: 10.1371/journal.pgen.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mounayri O, Triplett JW, Yates CW, Herring BP. Regulation of smooth muscle-specific gene expression by homeodomain proteins, Hoxa10 and Hoxb8. J Biol Chem. 2005;280:25854–25863. doi: 10.1074/jbc.M501044200. [DOI] [PubMed] [Google Scholar]
- Mace KA, Hansen SL, Myers C, Young DM, Boudreau N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J Cell Sci. 2005;118:2567–2577. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]