Abstract
Vasoactive intestinal polypeptide (VIP), acting via the VPAC2 receptor, is a key signaling pathway in the suprachiasmatic nuclei (SCN), the master clock controlling daily rhythms in mammals. Most mice lacking functional VPAC2 receptors are unable to sustain behavioral rhythms and lack detectable SCN electrical rhythms in vitro. Adult mice that do not produce VIP (VIP/PHI-/-) exhibit less severe alterations in wheel-running rhythms, but the effects of this deficiency on the amplitude, phasing, or periodicity of their SCN cellular rhythms are unknown. To investigate this, we used suction electrodes to extracellularly record multiple- and single-unit electrical activity in SCN brain slices from mice with varying degrees of VIP deficiency, ranging from wild-type (VIP/PHI+/+) to heterozygous (VIP/PHI+/-) and VIP/PHI-/- animals. We found decreasing proportions of rhythmic cells in SCN slices from VIP/PHI+/+ (∼91%, n = 23) through VIP/PHI-/+ (∼71%, n = 28) to VIP/PHI-/- mice (62%; n = 37) and a parallel trend toward decreasing amplitude in the remaining rhythmic cells. SCN neurons from VIP/PHI-/- mice exhibited a broad range in the period and phasing of electrical rhythms, concordant with the known alterations in their behavioral rhythms. Further, treatment of VIP/PHI-/- slices with a VPAC2 receptor antagonist significantly reduced the proportion of oscillating neurons, suggesting that VPAC2 receptors still become activated in the SCN of these mice. The results establish that VIP is important for appropriate periodicity and phasing of SCN neuronal rhythms and suggest that residual VPAC2 receptor signaling promotes rhythmicity in adult VIP/PHI-/- mice.
INTRODUCTION
The suprachiasmatic nuclei (SCN) function as the master pacemaker controlling mammalian circadian behavior. Individual SCN neurons can act as autonomous clocks, but when isolated in cell culture, they are unable to synchronize their rhythms (Herzog et al. 2004; Welsh et al. 1995). In brain slice preparations in which the SCN network is preserved, wild-type rodent SCN neurons have synchronized electrical rhythms. Manipulations that impair intercellular communication not only desynchronize these neurons but also render many cells apparently arrhythmic (Brown et al. 2005; Maywood et al. 2006; Yamaguchi et al. 2003). These findings indicate that intercellular communication is vital for the SCN to function as an effective clock at the tissue level.
Recent studies highlight vasoactive intestinal polypeptide (VIP), acting via the VPAC2 receptor, as a key pathway in the processes enabling SCN cells to produce the coordinated rhythmic output required to drive behavioral rhythms: mice with disrupted genes encoding VIP (VIP/PHI-/-) or the VPAC2 receptor (Vipr2-/-) exhibit impaired wheel-running rhythms (Aton et al. 2005; Colwell et al. 2003; Harmar et al. 2002; Hughes et al. 2004); SCN neurons in brain slices from adult Vipr2-/- mice are arrhythmic or show low-amplitude electrical rhythms with the degree of impairment observed in vitro correlating with the behavioral disruption exhibited by the donor animal (Brown et al. 2005); SCN slices from Vipr2-/- mice show dramatic reductions in the amplitude of rhythmic gene expression and asynchrony among individual cellular oscillators (Maywood et al. 2006); and amplitude and synchrony of electrical rhythms are reduced in neonatal Vipr2-/- and VIP/PHI-/- SCN neurons cultured at high density (Aton et al. 2005).
This last study demonstrates essentially identical impairments in neuronal rhythms in Vipr2-/- and VIP/PHI-/- cells, indicating that in high-density cultures, VIP-VPAC2 receptor signaling is the main pathway by which neonatal SCN clock cells synchronize and sustain rhythms. The proportion of rhythmic SCN cells detected in this study are consistent with observations of cellular rhythms in adult Vipr2-/- slices (Brown et al. 2005) and behaving animals (Harmar et al. 2002; Hughes et al. 2004). VIP/PHI-/- mice typically display less behavioral disruption than Vipr2-/- mice (Colwell et al. 2003). Unfortunately, the long-term activity patterns of adult neurons in adult VIP/PHI-/- SCN slices are unknown.
Here we used a sensitive extracellular recording technique to show that SCN neuronal discharge rhythms in slices from adult VIP/PHI-/- mice are altered in a manner consistent with the known behavioral impairments of these animals. Additionally, using a receptor antagonist, we provide evidence that residual signaling via the VPAC2 receptor promotes electrical rhythmicity in VIP/PHI-/- SCN neurons.
METHODS
Animals
The University of Manchester colony of VIP/PHI+/+, VIP/PHI+/-, and VIP/PHI-/- mice were derived from breeding pairs of heterozygous VIP/PHI+/- mice provided by Jim Waschek (UCLA). Initial breeding pairs were the offspring of C57BL/6 × sv129 VIP/PHI+/- mice, backcrossed with C57BL/6 mice for seven generations (Colwell et al. 2003). Therefore VIP/PHI+/+ mice are genetically very similar to C57BL/6 mice. Adult male and female mice were housed under a 12 h:12 h light:dark cycle (LD), at an ambient temperature of 22 ± 1°C. Food and water were available ad libitum. Zeitgeber time (ZT) 0 was defined as lights on and ZT12 as lights off. Animals were maintained under these conditions for >2 wk prior to experimental procedures. All scientific procedures were carried out in accordance with the UK Animal (Scientific Procedures) Act 1986. Prior to experimentation, all mice were genotyped as previously described (Colwell et al. 2003).
Slice preparation
Slices were prepared during the early subjective day (ZT 1-3) and maintained using methods previously described (Brown et al. 2005). Mice were killed by cervical dislocation and decapitation, then the brain was removed and placed in 4°C artificial cerebrospinal fluid (ACSF: pH 7.4) containing (in mM) 124 NaCl, 2.2 KCl, 1.2 KH2PO4, 2.5 CaCl2, 1.0 MgSO4, 25.5 NaHCO3, 10 d-glucose, and 1.14 ascorbic acid. Coronal brain sections (350 μm thick) were cut using a vibroslicer (Campden Instruments, Leicester, UK) and transferred to an interface style brain slice chamber continuously perfused (∼1.5 ml/min) with oxygenated (95% O2-5% CO2) ACSF supplemented with 0.0005% gentamicin (Sigma, UK) and warmed to 36 ± 1°C.
Electrophysiological recordings
Extracellular multiunit activity (MUA) was recorded from the SCN for >48 h, using ACSF-filled suction electrodes as previously described (Brown et al. 2006). The SCN multiunit signal was differentially amplified (×20,000) and band-pass filtered (300 -3,000 Hz) via a Neurolog system (Digitimer, Welwyn Garden City, UK), digitized (25,000 Hz) using a micro 1401 mkII interface [Cambridge Electronic Design (CED), Cambridge, UK] and recorded on a PC running Spike2 version 5 software (CED). In some experiments, the VPAC2 receptor antagonist PG 99 - 465 (Moreno et al. 2000) was dissolved in ACSF (10 nM) and applied by the perfusion line for 48 h starting at ZT 4.5 on day one of the experiment.
Data analysis
The total neural activity recorded with a signal-to-noise ratio of >2:1 is reported as MUA (Hz). Single-unit activity was discriminated from these recordings off-line using Spike2 software and analyzed as described previously (Brown et al. 2005, 2006). Briefly, single units were discriminated on the basis of waveform shape and validated by measurement-based clustering and the presence of a clear refractory period in an interspike interval histogram. Using these criteria we were able to successfully isolate up to four single units from each MUA recording. Single cell and MUA rhythms were analyzed by curve-fitting, using the equation y = Asin(Bx) where A equals the amplitude of the rhythm, and B equals the frequency in radians/h. A neuron/slice was judged arrhythmic when the best fit curve had zero amplitude (i.e., a straight line) or had a period of <12 or >36 h.
Acute drug effects were assessed as the mean single unit firing rate in the 30-min period after drug perfusion compared with the mean discharge in the 30-min period immediately before drug application. Changes in single-unit discharge >20% were considered significant (Reed et al. 2002).
Firing rate traces were moderately smoothed using a 1-h running average. Data are presented as means ± SE. Proportions of rhythmic neurons were compared by χ2 test, acute drug-induced changes in firing rate by paired t-test, and amplitudes of single-cell rhythms by one-way ANOVA followed by Tukey tests. Significance was set at P = 0.05. All statistical tests were carried out using GraphPad Prism 3.0 (San Diego, CA).
RESULTS
Consistent with previous findings (Bouskila and Dudek 1993; Brown et al. 2005, 2006; Gribkoff et al. 1998; Mrugala et al. 2000), all wild-type (VIP/PHI+/+) slices (n = 7) exhibited clear rhythms in SCN MUA (Fig. 1A; amplitude: 38 ± 16 Hz) with peaks during the mid projected day (ZT: 5.8 ± 1.0 h) and a mean period of 23.6 ± 0.9 h. Nine of 10 SCN slices from VIP/PHI± mice exhibited MUA rhythms similar to wild type (Fig. 1B; mean period: 23.4 ± 0.5 h; peak ZT: 6.9 ± 0.5 h) with the exception that these tended to oscillate more weakly (18 ± 4 Hz). The remaining VIP/PHI+/- slice was judged arrhythmic. In contrast, rhythmicity, period and phasing of MUA recorded in SCN slices from VIP/PHI-/- mice was highly variable (Fig. 1, C and D). Overall, 4 of 12 SCN slices from these VIP/PHI-/- mice exhibited arrhythmic MUA patterns (Fig. 1D) with the remaining rhythmic slices (Fig. 1C) exhibiting weak oscillations (16 ± 4 Hz) with variable periodicity (mean period: 22.9 ± 1.9 h; n = 8) and, often, peaks during the projected night (ZT: 13.9 ± 2.2 h).
FIG. 1.
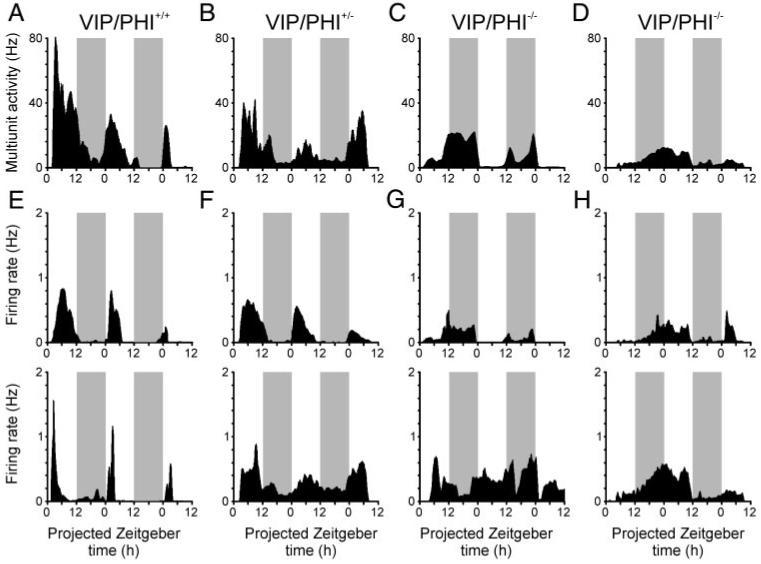
Neuronal firing rate rhythms are disrupted in the suprachiasmatic nucleus (SCN) of VIP/PHI-/- mice. SCN multiunit activity recordings from VIP/PHI+/+ (A) and VIP/PHI+/- (B) mice show clear rhythms with peak firing during the projected day, whereas recordings from VIP/PHI-/- mice exhibit abnormal rhythms (C) or are arrhythmic (D). Most individual SCN neurons in VIP/PHI+/+ (E) and VIP/PHI+/- (F) mice also show firing rate rhythms that peak during the projected day, whereas cells from VIP/PHI-/- mice (G and H) express rhythms with variable phasing (top) or are arrhythmic (bottom). All graphs show average firing rate (Hz) each minute from 1 representative slice, moderately smoothed using a 1-h moving average.  . periods of projected night.
. periods of projected night.
To determine whether the impaired MUA rhythms observed in SCN slices from VIP/PHI-/- mice were due to disrupted and/or asynchronous rhythms in individual neurons, we next examined the activity of single units discriminated from these MUA recordings. Interestingly, the majority of SCN neurons discriminated from MUA recordings in all genotypes were rhythmic; however, the proportion of rhythmic cells varied significantly with genotype (χ2 test, P < 0.05), from 21/23 neurons (∼91%) in wild-type VIP/PHI+/+ mice to 20/28 cells (∼71%) in VIP/PHI+/- mice and 23 of 37 neurons (∼62%) in VIP/PHI-/- mice (Fig. 1, E-H). There was also a parallel trend toward decreased amplitude of firing rate rhythms across these three genotypes with VIP/PHI-/- exhibiting significantly reduced amplitude firing rate rhythms compared with wild-type animals (Fig. 2A; Tukey test, P < 0.05). Accompanying these changes in rhythmicity and firing rate amplitude, the distribution of the estimated period of single-unit rhythms in VIP/PHI-/- was broader than in VIP/PHI+/+ mice (Fig. 2B). Interestingly, although most rhythmic VIP/PHI-/- SCN neurons exhibited accelerated periods (<23 h; 14/23 rhythmic cells; ∼61%), some had very long periods (26 -30 h; 6/23 rhythmic cells; ∼27%); consistent with the range of periodicities seen in the behavioral rhythms of these mice (22-29 h) (Colwell et al. 2003).
FIG. 2.
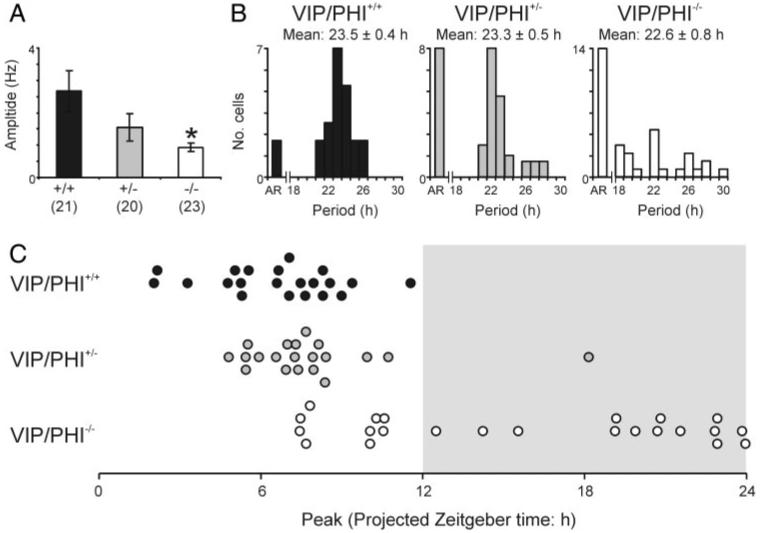
Vasoactive intestinal polypeptide (VIP) signaling regulates the amplitude, period, and phase of SCN neuronal rhythms. A: amplitude of SCN neuronal rhythms is significantly reduced in VIP/PHI-/- mice, compared with wildtype animals (Tukey test). Data are means ± SE; numbers in parentheses indicate number of rhythmic animals; * = P < 0.05. B: number of arrhythmic SCN neurons increases in VIP/PHI+/- and VIP/PHI-/- mice compared with wild ype, while the remaining rhythmic cells show altered free running periods. C: time of peak firing in rhythmic SCN neurons from VIP/PHI+/+ and VIP/PHI+/- mice tends to occur around the mid projected day, whereas oscillating cells from VIP/PHI-/- mice peak throughout the late day and projected night.
We also observed abnormalities in the phasing of single-cell rhythms in VIP/PHI-/- mice (Fig. 2C). SCN neurons from wild-type VIP/PHI+/+ or VIP/PHI+/- mice clustered their times of peak firing around a phase during the middle of the projected day (mean ZT: 6.6 ± 0.5 and 7.8 ± 0.6 h, respectively), whereas many cells from VIP/PHI-/- mice exhibited peak discharge during the projected night (mean ZT: 15.7 ± 1.3 h). Indeed, there was no significant clustering (Rayleigh test, P > 0.05; data not shown) in the peak times of these rhythmic VIP/PHI-/- SCN neurons, demonstrating an impaired ability of SCN neurons from adult VIP/PHI-/- mice to synchronize their activity patterns to environmental lighting conditions or one another.
We observed a higher proportion of rhythmic SCN cells in VIP/PHI-/- slices (∼62%) compared with those prepared from Vipr2-/- mice maintained under similar conditions (30%) (Brown et al. 2005). Because VIP/PHI-/- mice express functional VPAC2 receptors, it is possible that non-VIP-dependent activation of this pathway enables a greater proportion of cells to sustain rhythms in these animals compared with mice lacking VPAC2 receptors. To investigate this, we chronically treated SCN slices (n = 6) from VIP/PHI-/- mice with an antagonist of the VPAC2 receptor, PG-99465 (10 nM; 48 h starting ZT 4.5). On average, this treatment suppressed SCN single-unit firing so that discharge during the first 30 min of application was significantly lower than predrug values (∼75%; paired t-test, P < 0.01; Fig. 3A). In contrast, SCN neurons in control untreated VIP/PHI-/-slices tended to increase their firing rate over this time period (∼150% predrug values; paired t-test, P < 0.05; Fig. 3A). Using a previously established criterion (Reed et al. 2002), we found 8 of 17 VIP/PHI-/- SCN neurons tested (∼47%) showed decreases (Fig. 3B) and only 1 cell an increase in firing considered significant (see methods: average suppression: 52.1 ± 9.0%) in the first 30 min after VPAC2 receptor blockade. In contrast, most control untreated VIP/PHI-/- SCN neurons increased their firing rate (15/37; ∼41%) or did not show significant changes in action potential discharge rate (16/37; 43%) over the same time period. Therefore as observed in mice with intact VIP-VPAC2 receptor signaling (Brown et al. 2005; Cutler et al. 2003), endogenous activation of the VPAC2 receptor seems important for VIP/PHI-/- SCN cells to maintain high firing rates.
FIG. 3.

VPAC2 receptor blockade suppresses SCN neuronal firing rate and impairs cellular rhythmicity in VIP/PHI-/- mice. A: average firing rate (±SE) of VIP/PHI-/- SCN cells is significantly decreased in the 30-min period after application of the VPAC2 receptor antagonist PG 994 - 65 (ZT4.5-5) compared with the 30-min period before application (ZT4-4.5), whereas in untreated slices, cellular discharge significantly increase over the same time period (paired t-test). * and **, P < 0.05 and P < 0.01, respectively. B: examples of VIP/PHI-/- SCN cells exhibiting suppressions in firing rate after PG 99 - 465 application. C: representative multiunit activity recording from a VIP/PHI-/- SCN slice that does not show rhythmic discharge after treatment with PG 99 - 465. D: most VIP/PHI-/- SCN cells lack rhythms after VPAC2 receptor blockade (left), whereas a minority express low-amplitude, short-period, rhythms (right).  , periods of projected night.
, periods of projected night.
Subsequently, we determined the ability of SCN neurons in these VPAC2 receptor antagonist treated VIP/PHI-/- slices to sustain rhythms in action potential discharge. Five of these six PG 99 - 465 treated slices lacked detectable rhythms in MUA (Fig. 3C) and, overall, the proportion of VIP/PHI-/- SCN neurons sustaining rhythms (Fig. 3D) in the presence of this antagonist (4/17; ∼24%) was significantly decreased compared with untreated slices (∼62%; χ2-test, P < 0.01). Our observations here in VIP/PHI-/- slices are similar to the proportion of SCN neurons we have previously observed in wild-type SCN slices after VPAC2 receptor antagonism (∼27%) (Brown et al. 2005). Interestingly, all remaining rhythmic cells in these VPAC2 receptor antagonist treated VIP/PHI-/- slices showed extremely accelerated rhythms (Fig. 3D; mean period: 20.1 ± 0.3 h) in contrast to control untreated VIP/PHI-/- slices.
DISCUSSION
Here we show, for the first time, impairments in the amplitude, period, and phasing of neuronal activity rhythms in the SCN of adult VIP/PHI-/- mice. These alterations in cellular rhythms are consistent with the behavioral disruptions observed in VIP/PHI-/- mice (Aton et al. 2005; Colwell et al. 2003). Further, we demonstrate that SCN firing rate rhythms in VIP/PHI-/- slices can be additionally perturbed by treatment with a VPAC2 receptor antagonist, suggesting that this receptor still signals in these VIP-lacking mice. Neurons in VIP/PHI+/- mice express an intermediate phenotype between wild-type and homozygous mutants, suggesting that a reduced level of VIP in these animals results in subtle impairments in the ability of the SCN to sustain rhythms in vitro. Taken together with our findings in SCN slices where VPAC2 receptors are absent or pharmacologically blocked (Brown et al. 2005), these data indicate that the majority of adult SCN neurons require VPAC2 receptor signaling to express detectable rhythms and that the proportion of oscillating cells decreases in parallel with the levels of endogenous receptor stimulation.
Disruptions in VIP-VPAC2 receptor signaling reduce both the amplitude and synchrony of SCN cellular rhythms (Aton et al. 2005; Brown et al. 2005; Maywood et al. 2006; present study). It is not yet clear, however, if both phenomena result directly from the loss of this signaling pathway. For example, loss of VIP or its receptor may simply disrupt the timing of SCN cellular rhythms with the reduction in amplitude resulting from asynchronous release of other neurochemicals that adjust levels of SCN gene expression or neuronal discharge. Regardless of the exact mechanism involved, it is apparent that loss of VIP results in a population of SCN neurons with low-amplitude rhythms and variable periods and phasing. Presumably, an individual animal’s behavioral activity reflects the summed output of this mixed neuronal population.
Our finding that VPAC2 receptor blockade reduced the firing rate of VIP/PHI-/- neurons is consistent with previous observations in wild-type slices (Brown et al. 2005; Culter et al. 2003) and the recent demonstration that SCN cells in mice lacking these receptors are hyperpolarized with respect to wild-type neurons (Pakhotin et al. 2006). These data suggest that residual VPAC2 receptor signaling in VIP-deficient animals may provide some excitatory drive to SCN neurons, promoting action potential discharge.
It remains to be determined how VPAC2 receptors are activated in the absence of VIP. Several lines of evidence suggest pituitary adenylyl cyclase activating peptide (PACAP), which is present in retinohypothalamic tract (RHT) terminals innervating the SCN (for review, see Hannibal 2006), as a likely candidate: PACAP binds to the VPAC2 receptor with approximately equal affinity to VIP, as well as binding a selective receptor subtype, PAC1 (Harmar et al. 1998); PACAP induces both PAC1- and VPAC2-receptor-dependent alterations in neuronal firing rate when applied to rat SCN slices (Reed et al. 2002); studies in transgenic mice indicate that PACAP-PAC1 receptor signaling is involved in the response of the circadian system to light, but the findings are difficult to interpret (Colwell et al. 2004; Hannibal et al. 2001; Kawaguchi et al. 2003). Mice lacking PAC1 expression show enhanced resetting to early night light pulses (Hannibal et al. 2001), whereas mice lacking PACAP show reduced phase shifts to night time light pulses (Colwell et al. 2004). These apparently contradictory findings could imply that PACAP acts on more than one receptor to reset the clock, PAC1 and perhaps VPAC2.
SCN neurons do not produce PACAP but RHT terminals are present in adult SCN brain slices, and these could function as a source of PACAP to activate VPAC2 receptors. Because sources of PACAP are absent in dispersed neuron preparations, this may explain why neonatal VIP/PHI-/- and Vipr2-/- SCN cultures show similarly profound disruptions in neuronal rhythms (Aton et al. 2005). However, we cannot exclude the possibility that another unidentified ligand activates VPAC2 receptors in the adult VIP/PHI-/- SCN, or that VPAC2 receptors somehow become constitutively active in these animals. An additional possibility is that these effects of PG 99 - 465 arise through actions on a pathway separate from the VPAC2 receptor. This seems unlikely because wild-type or VIP/PHI-/- SCN slices treated with PG 99 - 465 are very similar to Vipr2-/- slices in terms of the proportion of rhythmic neurons and the periods and amplitudes of cellular rhythms (Brown et al. 2005; present study). Therefore these data suggest potentially important actions of the VPAC2 receptor that do not require VIP. Future studies will have to determine exactly how, or whether, non-VIP-dependent activation of the VPAC2 receptor contributes to the maintenance of rhythms in the wildtype SCN.
Wheel-running studies indicate that VIP/PHI-/- mice sustaining behavioral rhythms entrain to LD cycles but with an altered phase angle because when released into constant darkness, they start to run ∼8.5 h earlier than predicted by their previous light exposure (Colwell et al. 2003). Our experiments revealed the lack of phase clustering among VIP/PHI-/- SCN neurons, consistent with previous research implicating VIP-VPAC2 receptor signaling in photic entrainment (see Piggins and Cutler 2003 for review) and the maintenance of cellular synchrony within the SCN (Aton et al. 2005; Maywood et al. 2006). In our analysis, we pooled data from behaviorally unscreened VIP/PHI-/- mice, which would be predicted to exhibit varying degrees of behavioral disruption. Therefore it is possible that some VIP/PHI-/- SCN cells entrain to the LD cycle with an abnormal phase angle. In support of this idea, we observed many VIP/PHI-/- cells that exhibited peak firing during the projected night, whereas, in the present study only one VIP/PHI+/- and no WT VIP/PHI+/+ SCN neurons expressed their peak firing during this phase of the circadian cycle.
We previously demonstrated that the mechanisms that enable SCN neurons to sustain oscillations in electrical activity in the absence of functional VPAC2 receptors tend to result in short-period rhythms (Brown et al. 2005). Here we show that when VIP is absent but VPAC2 receptors are still functional, some SCN cells show accelerated periods and others exhibit long-period oscillations. Consistent with our earlier findings, when VPAC2 receptors were blocked the remaining rhythmic VIP/PHI-/- neurons exhibited accelerated periods. Regardless of the process by which VPAC2 receptors become activated in the SCN of VIP/PHI-/- mice, these data suggest that signaling by this pathway in the absence of VIP tends to lengthen the circadian period of SCN neurons.
In conclusion, these data support recent views that VPAC2 receptor signaling is important for high-amplitude, near-24-h rhythms in the majority of SCN neurons and for entrainment of these rhythms to environmental lighting conditions. Further, our findings suggest that VIP-deficient mice retain some degree of residual VPAC2 receptor signaling, enabling them to sustain a greater degree of cellular rhythmicity than animals lacking these receptors.
ACKNOWLEDGMENTS
We thank R. E. Samuels for assistance with genotyping mice and P. Robberecht for supplying the VPAC2 receptor antagonist.
GRANTS
This research was supported by funding to H. D. Piggins from the Biotechnology and Biological Sciences Research Council (UK) and National Institute of Health Grants NS-43169 to C. S. Colwell and MH-65497 to J. A. Washeck.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. Neuronal synchronization without calcium-dependent synaptic transmission in the hypothalamus. Proc Nat Acad Sci USA. 1993;90:3207–3210. doi: 10.1073/pnas.90.8.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Banks JR, Piggins HD. A novel suction electrode recording technique for monitoring circadian rhythms in single and multiunit discharge from brain slices. J Neurosci Methods. 2006;156:173–181. doi: 10.1016/j.jneumeth.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regulatory Integrative Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regulatory Integrative Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Roles of PACAP-containing retinal ganglion cells in circadian timing. Int Rev Cytol. 2006;251:1–39. doi: 10.1016/S0074-7696(06)51001-0. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci. 2001;21:4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–70. [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi C, Tanaka K, Isojima Y, Shintani N, Hashimoto H, Baba A, Nagai K. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem Biophys Res Commun. 2003;310:169–175. doi: 10.1016/j.bbrc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC(2) receptor. Peptides. 2000;21:1543–1549. doi: 10.1016/s0196-9781(00)00309-0. [DOI] [PubMed] [Google Scholar]
- Mrugala M, Zlomanczuk P, Jagota A, Schwartz WJ. Rhythmic multi-unit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am J Physiol Regulatory Integrative Comp Physiol. 2000;278:R987–R994. doi: 10.1152/ajpregu.2000.278.4.R987. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pfluegers. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ. The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. J Endocrinol. 2003;177:7–15. doi: 10.1677/joe.0.1770007. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed HE, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002;14:639–46. doi: 10.1046/j.1365-2826.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- Schaap J, Albus H, VanderLeest HT, Eilers PH, Detari L, Meijer JH. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: implications for circadian waveform and photoperiodic encoding. Proc Nat Acad Sci USA. 2003;100:15994–15999. doi: 10.1073/pnas.2436298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons from dissociated rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]


