Abstract
Passivity experiences are hallmark symptoms of schizophrenia that can be characterized by the belief that one's thoughts or actions are controlled by an external agent. It has recently been suggested that these psychotic experiences result from defective monitoring of one's own actions, i.e. disturbed comparison of actions and perceived outcomes. In this study, we examined the function of the previously characterized action monitoring network of the inferior parietal lobule (IPL), medial (mPFC) and lateral prefrontal cortices in patients with different levels of passivity symptoms with an fMRI task. The visuomotor fMRI task demanded control of visually perceived object movements by alternating button presses with the left and the right index finger. In the monitoring condition of this task subjects stopped their actions whenever they detected visuomotor incongruence. fMRI and behavioural data from 15 patients were tested for correlation with passivity symptoms using standardized Scale for Assessment of Positive Symptoms (SAPS)- and AMDP- passivity symptom ratings. Both types of data were tested for differences between the patients group and 15 healthy controls. In the patient group we found the expected correlation of passivity symptoms and visuomotor monitoring performance. There was a significant positive correlation of passivity symptoms with increased latency of incongruence detection and a negative correlation of SAPS-passivity with the number of detected events. fMRI data revealed correlations of passivity symptoms with activation in bilateral IPL, primary motor and sensory cortices in the action monitoring condition. A correlation of passivity symptoms with the main experimental effect (actions with – actions without monitoring) was found in the posterior cingulate cortex (PCC) and in the left IPL. No group differences or group by task interactions were found within the visuomotor-action-monitoring network. Our results demonstrate the association between passivity symptoms and the dysfunction of visuomotor action monitoring and support the idea that psychotic passivity experiences result from dysfunctions of central action monitoring mechanisms: According to pre-existing concepts of parietal cortex function, IPL-hyperactivation may represent an increase in false detections of visuomotor incongruence while the correlation between passivity and the differential effect of monitoring on PCC-activation assumedly represents greater self-monitoring effort in passivity experiences.
Keywords: action monitoring, visuomotor, schizophrenia, functional magnetic resonance imaging, parietal lobe
Introduction
Passivity experiences are hallmark symptoms of schizophrenia. They are characterized by the belief that one's thoughts or actions are influenced or controlled by an external agent, for example when a psychotic patient is experiencing the movement of his own limbs like a passive observer (Frith, 2005). From this point of view psychotic passivity experiences can be generally understood as a failure of the causal association between internal representations of action programs and the perception of external changes resulting from those actions. The understanding of the neural underpinnings of this dysfunction promises deeper insights into the pathophysiology of psychotic perceptions.
Traditional concepts of psychopathology highlight the cognitive features of passivity experiences by categorizing psychotic passivity symptoms as delusions or within the discrete category ‘ego-disturbances’ (Schneider, 1962). In contrast, recent psychopathological models underline the perceptual features of passivity experiences. These models suggest that passivity experiences in schizophrenia generally arise from dysfunctional processing of sensory perceptions resulting from own actions (Blakemore et al., 2000; Fourneret et al., 2001; Franck et al., 2001; Frith, 2005). This concept implies that the prediction of a perceptual action consequence depends on a forward model of the motor program for the intended action (Blakemore et al., 1998b). In contemporary theories of motor control, the reafferent sensory control of actions by their outcomes is generally referred to as action monitoring. The crucial question is, if the impairment of causal association between an own intention and an external event in patients with passivity experiences is demonstrably associated with behavioural and neurophysiological disturbances of action monitoring when these patients compare their original action with the perceived outcome of that action.
If so, the action monitoring approach might provide a pathophysiological model of the self-monitoring failure involved not only in passivity experiences but also in other schizophrenic symptoms like, for example, acoustic hallucinations which are possibly linked to defective monitoring of speech production (Frith and Done, 1989; McGuire et al., 1996; Frith et al., 2000a, b). From the motor systems perspective passivity experiences promise an exemplary insight into the neural foundation of coordination between (efferent) action planning and the perception of (afferent) action consequences.
Behavioural observations in healthy subjects have demonstrated that central monitoring of own actions utilizes sensory feedback resulting from own actions for comparison with the original motor programs (Wolpert, 1997; Blakemore et al., 1998a; Fourneret and Jeannerod, 1998;). The action monitoring system automatically adjusts motor programs to changing target locations (Fourneret and Jeannerod, 1998) and directs attenuation of the sensory cortices' response to afferent sensory information resulting from own actions, like e.g. the sensation of a hand touching the own body (Chapman et al., 1987; Milne et al., 1988; Blakemore et al., 1999). Beyond these unconscious adjustments the central monitoring of actions is fundamental for the indication of errors (Ridderinkhof et al., 2003) and for the conscious determination if sensory input was generated by the acting subject itself or by another agent.
Schizophrenia most likely impairs the central monitoring functions in sensory processing, i.e. unconscious attenuation of self-produced sensory information (Blakemore et al., 2000) as well as the conscious discrimination of internal and external causes of sensory perceptions (Franck et al., 2001; Fourneret et al., 2002), while automatic motor adjustment functions remain unaffected (Knoblich et al., 2004). Disturbances of action monitoring are moreover assumed fundamental for cognitive symptoms of schizophrenia like defective executive error monitoring (Malenka et al., 1982; Turken et al., 2003).
From an anatomical perspective visuomotor action monitoring involves the activation of a network comprising posterior parietal and prefrontal cortices (Schnell et al., 2007). Cingulate and paracingulate divisions of the posterior medial prefrontal cortex (mPFC) are involved in the control of action execution (Goldberg et al., 1981) and error detection (Gehring and Knight, 2000), especially in probabilistic contexts (Ridderinkhof et al., 2004). The dorsolateral PFC accounts for a variety of attentional functions (Corbetta and Shulman, 1998). Neurons in the inferior parietal lobule (IPL) near the temporoparietal junction code for the goals of actions (Blakemore and Sirigu, 2003; Fogassi and Luppino, 2005). The IPL obviously represents visuomotor transformations (Goodale and Milner, 1992) especially in the processing of sensory data that is generated by own actions (Vaillancourt et al., 2006) and is activated by the detection of sensorimotor incongruence during action execution (Balslev et al., 2006).
In schizophrenic patients Farrer et al. (2004) have observed a correlation between inferior parietal cortex activation and schneiderian first rank symptoms of schizophrenia including acoustic hallucinations and passivity experiences when spatial incongruence between own hand movements and the movements of a virtual representation of the hand occurred in a PET experiment. This observation indicates the global importance of monitoring dysfunctions in the occurrence of sensory symptoms in schizophrenia. However, since passivity symptoms and auditory hallucinations are groups of symptoms with a low correlation in schizophrenic patients (Stuart et al., 1995), the crucial question if a distinct category of psychopathologic symptoms is specifically associated with the dysfunction of a pertinent neural network remains unsolved. The dysfunction within the visuomotor action monitoring network should thus be correlated with the occurrence of psychotic passivity experiences. Accordingly, hyperactivation of the IPL during the execution of simple movements has already been found to be associated with the occurrence of passivity experiences (Spence et al., 1997). However, the task of Spence et al. did not address action monitoring directly. On this background our experiment is the first to directly study the connection between passivity symptoms and the visuomotor capacity to differentiate own and external actions. The basic idea was to test for correlates of passivity symptoms in both fMRI and behavioural data acquired during action monitoring in a group of schizophrenic patients where some of the patients experience different individual degrees of passivity while other patients are completely free of passivity experiences.
For this purpose, we have previously designed an experiment to test the capability to monitor the congruence between own actions and visual feedback during automated sequences of simple actions. To increase the attendance and cooperation of the participants, especially of the patients with positive psychotic symptoms, the task was designed as a simple racing game. To directly address the causal attributions which are impaired in passivity experiences, subjects were instructed to judge if observed actions in this simple motor task, i.e. changes of the car's direction, were controlled by themselves or by the computer (Schnell et al., 2007). In a previous experiment with a group of healthy subjects the global maximum of differential activation in the action monitoring condition was observed in bilateral IPL in Brodmann area 40 (BA 40) near the temporoparietal junction, accompanied by activations in bilateral posterior mPFC (BA 8, 10) and lateral PFC (BA 9, 46). In the present study we examined patients diagnosed with schizophrenia who experienced different individual levels of passivity symptoms or were completely free of passivity experiences.
We hypothesized that these different individual levels of passivity symptoms would be correlated with different individual levels of behavioural deficits in the visuomotor monitoring of causal linkage between intended actions and observed events. Namely, we expected a correlation of passivity symptoms with a decreased ability to correctly detect visuomotor incongruence and with functional changes in the pertinent neural action monitoring network, i.e. IPL, mPFC and lateral PFC. According to the report of Spence et al., we especially expected increased activation of the parietal cortex.
Since psychotic disturbances in the perception of control over own thoughts and actions have been conceptualized differentially in different psychopathological traditions we used two measuring approaches simultaneously: The AMDP-rating (Arbeitsgemeinschaft für Methodik und Dokumentation in der Psychiatrie) system (AMDP, 2000), which is frequently trained and used for standardized clinical assessment of psychopathology in Germany and Switzerland, as well as the internationally established SAPS/SANS-rating scales (Scale for Assessment of Positive/Negative Symptoms) (Andreasen et al., 1995). Both systems comprise subscales for the rating of positive psychotic symptoms that include passivity experiences.
Experimental procedure
Subjects
Twenty patient volunteers diagnosed with paranoid type schizophrenia were recruited after admission to psychiatric inpatient treatment in the psychiatric department of the University Hospital of Cologne. Five of these patients were excluded from further analysis after scanning due to head movements exceeding one voxel (>3 mm). The study comprised only male participants to control for confounds from possible gender related differences of perceptual-motor performance (Kennedy and Raz, 2005). The patients were matched for age and years of education with 15 healthy controls without a history of neurological or psychiatric disorders. The mean age was 30.16 (SD 8.89) in the patients group and 30.80 (SD 7.71) years in the control group. The mean years of education were 11.47 (SD 1.46) in patients and 12.2 years (SD 1.37) in controls (Table 1). All subjects were right-handed [Edinburgh handedness inventory (Oldfield, 1971)]. All participants gave their written consent. The study was conducted in accordance to the regulations of the local ethics committee and the declaration of Helsinki (World Medical Association, 2004).
Table 1.
Demographic and clinical data of included patients
| Patient | Age | Years of education | Time of initial diagnosis | Antipsychotic medication | AMDP 53–58 passivity | SAPS 15–19 passivity | SAPS 1–7 hallucin. | SAPS all sum | SANS all sum |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 10 | At present | – | 1 | 3 | 16 | 25 | 1 |
| 2 | 35 | 13 | 2 years | – | 0 | 0 | 7 | 23 | 35 |
| 3 | 28 | 13 | At present | – | 0 | 0 | 3 | 24 | 9 |
| 4 | 22 | 10 | At present | Aripiprazole | 0 | 0 | 0 | 19 | 33 |
| 5 | 19 | 10 | At present | – | 0 | 0 | 0 | 25 | 0 |
| 6 | 53 | 12 | 20 years | Risperidone | 0 | 0 | 9 | 35 | 8 |
| 7 | 27 | 10 | 4 years | Risperidone | 4 | 10 | 0 | 41 | 67 |
| 8 | 26 | 13 | At present | – | 5 | 8 | 9 | 23 | 20 |
| 9 | 39 | 10 | At present | – | 2 | 3 | 4 | 21 | 4 |
| 10 | 36 | 13 | At present | – | 2 | 0 | 5 | 9 | 53 |
| 11 | 39 | 12 | At present | – | 1 | 3 | 3 | 19 | 8 |
| 12 | 26 | 13 | At present | – | 0 | 0 | 7 | 43 | 24 |
| 13 | 26 | 10 | 6 years | Quetiapine | 3 | 5 | 8 | 35 | 23 |
| 14 | 26 | 13 | At present | – | 1 | 2 | 0 | 10 | 6 |
| 15 | 33 | 10 | At present | – | 2 | 5 | 0 | 10 | 22 |
| Mean | 30.16 | 11.47 | 1 | 3 | 5 | 24 | 21 | ||
| SD | 8.89 | 1.46 | 1.6 | 3.2 | 4.7 | 10.6 | 19.5 |
Columns 6 and 7 indicate scores of passivity symptoms, column 8 scores for hallucinations and columns 9 and 10 list the sum of scores for positive (SAPS) and negative (SANS) psychotic symptoms.
Clinical information
Diagnosis of paranoid type schizophrenia (ICD 10: F20.2, DSM IV: 295.3x) was assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) by expert raters and confirmed by the course of at least 6 months of clinical treatment as in- or outpatients of the department following the fMRI examination. At the time of inclusion 11 of the 15 patients were diagnosed with schizophrenia for the first time. While 11 of the patients had never received any antipsychotic medication, four patients were stabilized on atypical antipsychotic treatment as indicated in Table 1. Extrapyramidal motor side effects of this medication were ruled out using the EPS Scale (EPS) (Simpson and Angus, 1970) and the Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976).
Assessment of psychopathology
While the recruitment was not symptom-specific we expected the patients group to contain individuals with different levels of passivity symptoms or even without passivity experiences. Two different rating systems were used to assess these differences and, moreover the full range of frequently reported psychotic symptoms. The AMDP (AMDP, 2000) and the SAPS (Andreasen et al., 1995) ratings were conducted 30 min before scanning to assess current symptoms. The AMDP-rating procedure renders 88 symptoms on a scale from 0 (absent) to 3 (severe). The category for passivity symptoms comprised the items: 53, derealization; 54, depersonalization; 55, thought broadcasting; 56, thought withdrawal; 57, thought insertion and 58, (other) experiences of alien control. In our study, item 58 coded for bodily experiences of alien (external) control. Moreover, the general degree of psychotic symptoms and the extent of passivity experiences were assessed with the SAPS and the SANS, levels 0 (none) to 5 (severe). The SAPS has been previously used in neuroimaging studies (Franck et al., 2002; Gaser et al., 2004). The SAPS rating of passivity comprises the following items: 15, delusions of being controlled; 16, delusions of mind reading; 17, thought broadcasting; 18, thought insertion and 19, thought withdrawal. To improve the coverage of passivity symptom occurrence we used the sum of symptom ratings and not the maximum of single symptom scores for correlational analyses. Thereafter, score intervals for passivity symptoms were expanded from 0–3 to 0–18 for the AMDP and from 0–5 to 0–25 for the SAPS. One subject could score differently for one item in both systems e.g. for ‘thought broadcasting’ with 1 in the SAPS (questionable) and with 0 (absent) in the AMDP rating, since the AMDP does not comprise the level ‘questionable’.
Action monitoring task
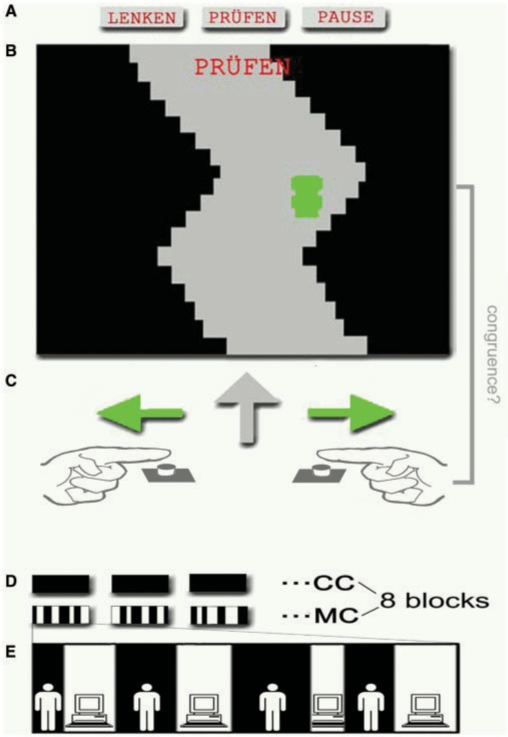
We used a previously reported task (Schnell et al., 2007), which was designed to analyse the monitoring of incongruence between the subjects’ own actions and resulting visual perceptions (Fig. 1). The monitoring condition (MC) and the control condition (CC) were based on the same motor task, comprising a simple video game: subjects had to keep a horizontal moving car within the boundaries of a vertically moving curved track. The horizontal motion of the car was controlled bimanually by pressing a button with either the right or the left index finger alternately. Participants were instructed to steer the car within the boundaries of the track as accurately as possible. On average this challenge demanded a button press every 366 ms. The automatic execution of this motor task was ensured by a preceding training and controlling for movement errors below 5% (percentage of time when the car exceeded the boundaries of the track) in the fMRI experiment.
Fig. 1.
Experimental Design: MC and CC of the epoch design (E) were based on the same motor task, comprising a simple video game (B). Subjects had to keep a horizontal moving car within the boundaries of a vertically moving curved track by changing its direction with either the right or the left index finger (C). Conditions were indicated by the words ‘Lenken’ (‘steer’) or ‘Prüfen’ (‘verify’) (A). In the MC the computer would take over control of the cars motion reversals a varying number of times during the 30 s MC epochs (E).
The CC was indicated by the word ‘Lenken’ (‘steer’). During the 30 s epochs of the CC the subjects’ control over the cars’ movement was uninterrupted. In the MC, by contrast, the computer would control the timing of the cars’ motion reversals for a variable number of times during the 30 s MC epochs. Participants had been instructed to monitor for the resulting incongruence between their own and the perceived actions and to abstain from their own motor actions as soon as they recognized such a transfer of control to the computer. The MC was indicated by the word ‘Prüfen’ (‘verify’) with visual presentation of track and car identical to the CC.
The baseline condition (BC) was indicated by the word ‘Stop’. Subjects were presented the same scene as in MC and CC, while the car remained fixed in the last position of the preceding trial for 10 s.
Eight blocks of each condition (CC and MC) separated by BC were presented in a pseudorandomized order in two sessions. The eight epochs of the MC contained 21 onsets of visuomotor incongruence (transfer of control from subject to computer). The number of predefined switches from subject to computer varied from three to five for each MC epoch. The interval between the onset of computer control (visuomotor incongruence) and the re-establishment of subjects’ control was alternated in a pseudorandomized manner between 2600 ms and 5200 ms.
The task was designed to explore the neural underpinnings of a predictive model for the timing of own actions: The boundaries of the track marked a restriction for the timing of both, the test subjects’ actions—i.e. reversals of car movement—and the reversals generated by the computer algorithm. Hence, both movement patterns were highly similar. External observers were actually not able to detect the changes in the control of reversals. The detection of visuomotor incongruence rather demanded the use of an efference copy of own actions, i.e. the active comparison of subjectively expected and actually presented reversals of object movement. After a behavioural pilot study the visuomotor load was reduced in order to detect symptom-specific effects rather than group differences caused by a floor effect in the patients group. Finally, the speed of the previously reported experiment with healthy subjects was reduced by 25% (Schnell et al., 2007). The experiment was presented with the Presentation software (Neurobehavioral Systems, Albany, California, USA) using overhead projection on a mirror mounted to the headcoil. The motor responses were recorded with Lumitouch fiberoptic response devices (Photon Control Inc., Burnaby, BC, Canada).
fMRI data acquisition
Imaging was performed at 1.5 T on a Philips Gyroscan NT Intera (Philips, Eindhoven, Netherlands) system with a standard birdcage head coil. For each subject 2 × 125 whole brain volumes were acquired using an echoplanar imaging (FEE-EPI) sequence (TR 2600 ms, TE 50 ms, flip angle 90°, interleaved scanning in ascending slice order, 22 slices, 64 × 64 matrix, field of view 192 mm × 192 mm, slice spacing 5 mm including 0.5 mm gap). The first six volumes were discarded to allow T1 equilibration. Individual anatomical data were acquired with a T1-FFE sequence (TR 30 ms, TE 4.5 ms, flip angle 30°, 70 slices, 256 × 256 matrix, field of view 256 mm × 256 mm, slice spacing 2 mm). Initial data analysis was carried out using Statistical Parametric Mapping with SPM2 (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk) implemented in Matlab 6.5 (Mathworks, Natick, Massachusetts, USA) for whole brain data analysis. In order to use the extended options for second level analysis the whole dataset was additionally processed with SPM5 in a subsequent analysis. Preprocessing of individual data started with a fourth degree B-spline realignment. For normalization, a transformation matrix between the mean image of realigned volumes and the SPM2-EPI (MNI) template was generated with a fourth degree B-spline algorithm and applied to reslice volumes with a voxel size of 3 mm × 3 mm × 3 mm. For spatial smoothing a Gaussian Kernel of 9 mm (i.e. 3 × in plane voxel dimension) full width at half maximum was chosen to increase sensitivity for primarily expected cortical activations in group inference. The standard haemodynamic response function (HRF) was used for convolution with the regressors of the experimental design.
Behavioural data analysis
Behavioural performance was analysed with SPSS (SPSS Inc., Chicago, Illinois, USA) by means of Pearson's correlation analyses and ANOVA analysis of intraindividual mean values.
fMRI data analysis
First-level analysis of fMRI data was performed according to the general linear model. The epoch model was generated with two blocked predictive regressors (MC, CC) to test for effects of sustained monitoring for incongruencies between the subjects’ own and perceived actions. To reduce the influence of varying numbers of actions per epoch, the number of actions was included as a parametric modulator for each single epoch of MC and CC. Realignment parameters of head movements were included as effects of no interest to reduce the influence of these movements.
Second-level analysis utilized the individual contrast images from the first-level analysis. To replicate the findings of our previous study, the main effect of the experimental task (MC–CC) was assessed separately for each group with repeated measures ANOVA (i.e. SPM5 ‘flexible factorial’ model). In the patients group this repeated measures ANOVA additionally included the individual AMDP scores of passivity (0–5) as a covariate (1: factor, subject; 2: factor, experimental condition; 3: covariate, AMDP scores of passivity). The confounding effect of passivity on the main effect was controlled in order to demonstrate that—despite of passivity related dysfunctions—schizophrenic patients generally activated the same IPL regions during action monitoring like healthy subjects. In addition, we wanted to demonstrate that the regional correlations between passivity and functional activations were actually located within the area of the IPL that is involved in action monitoring.
Group differences between healthy controls and patients for both experimental conditions [Pat(MC) – Cont(MC); Pat (CC) – Cont(CC)] were tested separately with an ANOVA model for each condition (i.e. ‘full factorial’ SPM 5 model comprising 1: factor group and 2: covariate individual number of actions in MC or CC. Group × condition interaction [Pat(MC–CC) – Cont(MC–CC)] was tested in both directions using a repeated measures ANOVA (‘flexible factorial’ SPM5 model) modelling repeated (MC–CC) measures within and independent measures between groups (1: factor, subject; 2: factor, group; 3: factor, experimental condition and 4: covariate- individual number of actions in MC/CC for each subject).
The main hypotheses, which predicted a correlation between passivity symptoms and the BOLD response within the monitoring network in the patients group, was tested with a simple regression analysis only in the patients group (i.e. SPM5 ‘Multiple Regression’ with just one regressor per model i.e. SAPS or SANS).
Significance levels applied in the analyses were P < 0.05 false discovery rate (FDR) corrected for whole brain analysis of the main effect of the experimental task, P < 0.001 uncorrected for whole brain analysis of differences between groups and regression analysis within the patient group. The regression analysis of correlation between passivity and BOLD response was additionally applied within an a priori region of interest (IPL) on a family wise error corrected level of P < 0.05.
Results
Participants
The data of five out of the 20 initially recruited patients were excluded from further analysis due to movements exceeding 3 mm during aquisition of fMRI EPI-volumes. The average SAPS score in the group of remaining patients was 24 (SD 10.6) and the mean SANS score 21 (SD 19.5) (Table 1). We assessed SAPS passivity scores between 2 and 10 in n = 8 patients, while the rest of the group was free of passivity (n = 7, SAPS = 0). AMDP passivity scores were rated between 1 and 5 in n = 9 subjects while n = 6 patients were free of passivity according to the AMDP (AMDP = 0). Remarkably there was a very low correlation (Pearson's r = –0.017, P = 0.951) between the SAPS scores for hallucinations and passivity symptoms.
Behavioural data
Differences between patients and control group
According to the experimental design the number of actions was smaller in the MC compared to the CC in both groups since participants had been instructed to abstain from own actions when the computer was in control in the MC. One-way ANOVA of behavioural data revealed a significantly higher number of actions in the control group during both, the monitoring condition [controls 45.16 (SD = 12.03) vs patients 32.96 (SD = 9.09), (F(1, 28) = 15.17, P = 0.001, Table 2)] and the control condition [controls 108.10 (SD = 23.03) vs patients 79.03 (SD = 17.01), F(1, 28) = 9.81, P = 0.004]. Additionally the interaction of group and condition had a significant effect [F(1, 28) = 10.865, P = 0.003] on the number of actions as well. This difference in the frequency of actions between patients and controls was not ascribable to performance differences in the motor task, since no significant group differences of motor error rates were detected in the CC [controls 1.51% (SD 1.15) vs patients 2.63% (SD 2.73), F(1, 28) = 2.12, P = 0.157]. There was no significant difference in the monitoring performance i.e. the latency and the number of incongruence detections between groups.
Table 2.
Behavioural data of schizophrenic patients and controls in the monitoring (MC) and control (CC) condition, tested for group differences with a one-way ANOVA
| Behavioural group differences | Patients | Controls | F | df | P |
|---|---|---|---|---|---|
| Number of detected incongruence events in MC | 36.73 (3.95) | 36.80 (5.54) | <0.01 | 28 | 0.970 |
| Latency of incongruence detection (ms) in MC | 537 (464) | 316 (417) | 1.87 | 28 | 0.181 |
| Number of actions/epoch MC | 32.96 (9.09) | 45.16 (12.03) | 15.17 | 28 | 0.001 |
| Number of actions/epoch CC | 79.03 (17.01) | 108.10 (23.03) | 9.81 | 28 | 0.004 |
| Number of actions/epoch MC–CC (interaction group × condition) | −46.07 (12.86) | −62.94 (15.93) | 10.86 | 28 | 0.003 |
Correlation between passivity symptoms and behavioural measures of action monitoring
In accordance with the hypothesis of impaired action monitoring in patients with passivity symptoms, the analysis of behavioural data revealed a significant correlation (AMDP Pearson's r = 0.483, P = 0.034, SAPS r = 0.515, P = 0.025) between the level of passivity symptoms and increased latency of the detection of visuomotor incongruence events (Table 3). Although we expected a reduction in the number of detected events to be adjunct to passivity phenomena, the analysis revealed a significant correlation between both measures only for the SAPS scores (Pearson's r = –0.511, P = 0.026) and only a trend for the AMDP [Pearson's r = –0.424, P = 0.058]. The number and the latency of incongruence detections were significantly correlated with each other [Pearson's r = –0.694, P = 0.002]. Since there was no significant correlation of passivity symptoms with errors in the underlying motor task, there was no indication that a general disturbance of motor functions outside the monitoring system was associated with passivity symptoms.
Table 3.
Pearson's correlation of AMDP- and SAPS- ratings with behavioural and fMRI data (correlation with β estimates of individual activations averaged from a sphere of 10 mm diameter centred at the given coordinates)
| Correlation between | Latency of incongruence detection |
Number of detected incongruence events |
Number of actions in MC |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |||
| AMDP passivity | 0.483 | 0.0341 | −0.424 | 0.0578 | 0.227 | 0.2077 | ||
| SAPS passivity | 0.515 | 0.0246 | −0.511 | 0.0258 | 0.184 | 0.2556 | ||
| Correlation between | Latency of incongruence detection |
Number of detected incongruence events |
AMDP passivity score |
SAPS passivity score |
||||
| r | P | r | P | r | P | r | P | |
| VOI with AMDP correlated activation | ||||||||
| l. IPL [−45, −51, 42] activation in MC | 0.627* | 0.0061 | −0.457* | 0.0435 | 0.793* | 0.0002 | ||
| r. IPL [51, −48, 48] activation in MC | 0.379 | 0.0815 | −0.182 | 0.2578 | 0.787* | 0.0003 | ||
| l. IPL [−45, −51, 42] activation MC–CC | 0.045 | 0.4373 | −0.087 | 0.3788 | 0.546* | 0.0177 | ||
| r. IPL [51, −48, 48] activation MC–CC | 0.457* | 0.0435 | −0.117 | 0.3386 | 0.292 | 0.1455 | ||
| VOI with SAPS correlated activation | ||||||||
| l. IPL [−39, −54, 40] activation in MC | 0.610* | 0.0079 | −0.568* | 0.0135 | 0.707* | 0.0016 | ||
| r. IPL [42, −54, 42] activation in MC | 0.423 | 0.0582 | −0.299 | 0.1398 | 0.743* | 0.0007 | ||
| l. IPL [−39, −54, 40] activation MC–CC | 0.030 | 0.4581 | −0.140 | 0.3093 | 0.508* | 0.0266 | ||
| r. IPL [42, −54, 42] activation MC–CC | 0.039 | 0.4454 | −0.249 | 0.1851 | 0.417 | 0.0611 | ||
Behavioural data was analysed by one-sided Pearson's test for correlation according to the hypotheses of decreased performance in patients with passivity symptoms.
Imaging data
Main effect of action monitoring
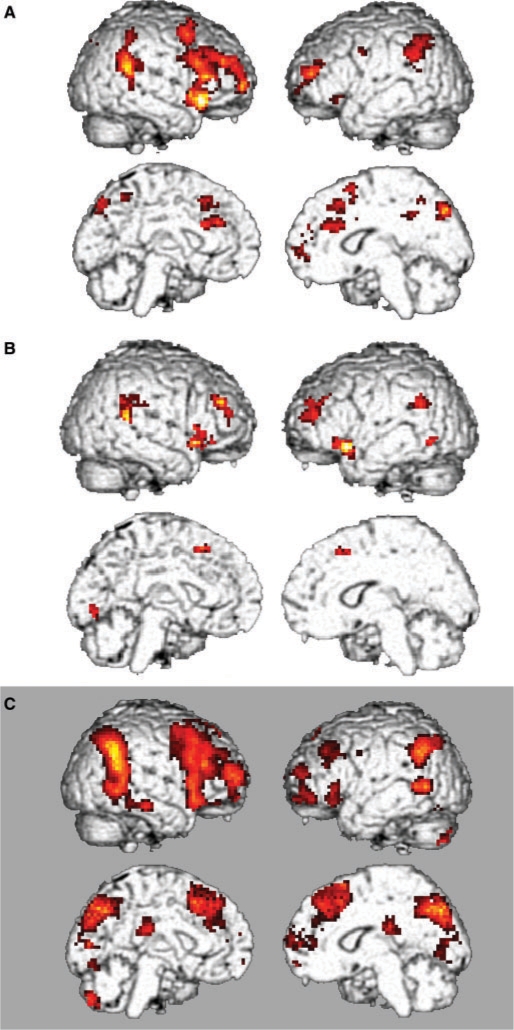
In the patient group the differential activation pattern in the action monitoring condition (MC–CC) resembled the pattern found in the previous study in healthy subjects after passivity was controlled for as a covariate of no interest. This pattern included bilateral IPL, the temporoparietal junction, the anterior cingulate cortex and dorsolateral PFC as well as right inferior PFC (P < 0.05, FDR-corrected, voxel level, k = 10, Table 4, Fig. 2). The control group displayed a similar pattern however at a lower significance level than expected in comparison to the healthy subjects in the previous experiment (P < 0.001 uncorr., voxel level, k = 10, Fig. 2).
Table 4.
Main effect of visuomotor monitoring
| Condition | FWE corr. cluster | Cluster size k | Z | x, y, z (mm) | BA | Region |
|---|---|---|---|---|---|---|
| MC–CC | ||||||
| Frontal | <0.001 | 1185 | 4.93 | 36, 24, −9 | 47 | R. Inf. Front. Gyr. |
| 4.56 | 36, 42, 27 | 10 | R. Middle Front. Gyr. | |||
| 4.47 | 39, 33, 33 | 9 | R. Middle Front. Gyr. | |||
| 0.011 | 57 | 3.65 | 3, 24, 48 | 8 | R. Medial Front. Gyr. | |
| 3.39 | 3, 30, 42 | 8 | R. Medial Front. Gyr. | |||
| <0.001 | 118 | 4.10 | −33, 54, 18 | 10 | L. Sup. Front. Gyr. | |
| 3.65 | −30, 63, −3 | 10 | L. Sup. Front. Gyr. | |||
| 3.37 | −27, 42, 27 | 10 | L. Middle Front. Gyr. | |||
| 0.819 | 13 | 3.41 | −33, 27, −9 | 47 | L. Inf. Front. Gyr. | |
| 3.03 | −33, 18, −6 | 47 | L. Inf. Front. Gyr. | |||
| <0.001 | 118 | 4.18 | −3, 33, 27 | 32 | L. Ant. Cingulate | |
| 3.94 | −6, 18, 24 | 33 | L. Ant. Cingulate | |||
| 3.64 | 12, 27, 21 | 24 | R. Ant. Cingulate | |||
| 0.864 | 12 | 3.39 | −42, 0 36 | 6 | L. Precentral Gyr. | |
| Temporoparietal | <0.001 | 284 | 4.65 | 63, −48, 21 | 22 | R. Sup. Temporal Gyr. |
| 3.61 | 51, −51, 33 | 40 | R. Supramarginal Gyr. | |||
| 3.55 | 51, −45, 54 | 40 | R. Inf. Parietal Lobule | |||
| <0.001 | 145 | 4.24 | −57, −51, 36 | 40 | L. Supramarginal Gyr. | |
| 3.54 | −54, −51, 48 | 40 | L. Inf. Parietal Lobule | |||
| 3.49 | −63, −54, 18 | 22 | L. Sup. Temporal Gyr. | |||
| Parietal | <0.001 | 116 | 3.72 | −3, −78, 48 | 7 | L. Precuneus |
| 3.57 | 15, −75, 39 | 7 | R. Precuneus | |||
| 3.52 | 6, −75, 42 | 7 | R. Precuneus | |||
| 0.513 | 19 | 3.36 | 12, −45, 36 | 31 | R. Cingulate Gyr. |
Differential activation in the patient group during visuomotor monitoring compared to the control condition (MC–CC, P < 0.05, FDR corr., coordinates according to the standard MNI template). Influence of passivity symptoms was controlled by using AMDP symptom scores as a covariate.
Fig. 2.
The pattern of differential activation for action monitoring (MC–CC) in the patient group (A, P < 0.05 FDR-corrected, k > 10 voxel, AMDP passivity as covariate of no interest) was not statistically different from the pattern in the control group (B, P < 0.001 uncorr. for display). Both patterns reassembled the network found in the previous study with the same design but higher visuomotor load in healthy subjects (C, P < 0.05 FDR-corrected, k > 10 voxel).
Functional group differences
Although interindividual differences of action frequency had been controlled, no significant functional effect of group × condition interaction was found after implicit masking with the effect of monitoring. While we had primarily expected increased monitoring dependent activation in patients [Pat(MC–CC) – Cont(MC–CC)]—no such effect was detected on a significance level of P < 0.001. In single conditions the only significant functional difference between groups was a higher activation in the right parahippocampal gyrus (x, y, z = 30, –54, 0, z = 3.46, MNI coordinates) in patients in the CC [Pat(CC) – Cont(CC)].
Correlation between passivity symptoms and functional data in patients
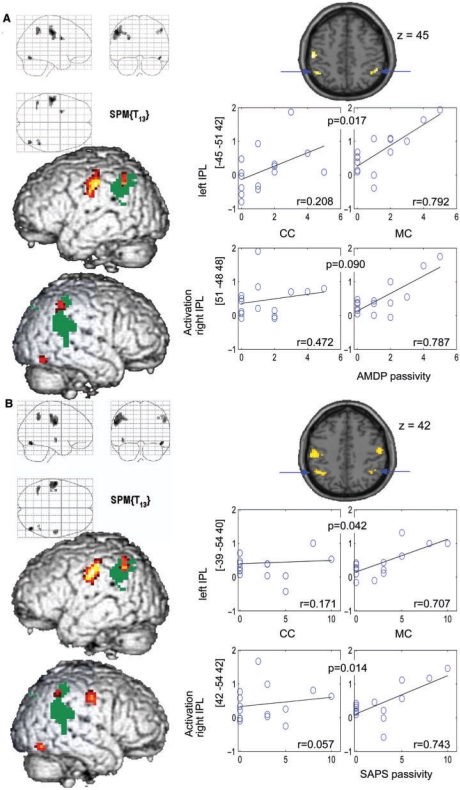
SPM correlation analysis with small volume corrections in bilateral IPL defined by a mask for BA 40—a key component of the action monitoring network identified in the preceding study (Schnell et al., 2007)—revealed a significant positive correlation between AMDP passivity symptom scores and BOLD-signal in the MC bilaterally [right IPL (x, y, z = 51, –48, 48, z = 3.79), left IPL (x, y, z = –45, –51, 42, z = 3.73), P < 0.05, FWE corrected for BA 40, Table 5)]. There was also a significant correlation between AMPD passivity scores and individual beta values in the MC extracted from spheres (diameter = 10 mm) centred in the given correlation maxima in the right IPL (Pearson's r = 0.787, P = 0.0002) and in the left IPL (Pearson's r = 0.793, P = 0.0003) (Table 3, Fig. 3). Remarkably the beta values from the left IPL were also correlated with latency and the number of incongruence detections.
Table 5.
Correlations of passivity ratings (AMDP and SAPS) and functional activations in the patient group detected by SPM whole brain analysis
| Condition | FWE corr. cluster | Cluster size k | Z | x, y, z (mm) | BA | Region |
|---|---|---|---|---|---|---|
| MC | ||||||
| Correlation AMDP-passivity symptoms | ||||||
| Front./parietal | 0.010 | 121 | 3.99 | −51, −18, 42 | 3 | L. Postcentral Gyr. |
| 3.62 | −57, −15, 30 | 4 | L. Precentral Gyr. | |||
| 3.51 | −42, −21, 57 | 3 | L. Postcentral Gyr. | |||
| Parietal | 0.050 | 24 | 3.79* | 51, −48, 48 | 40 | R. inf. Parietal Lobule |
| 3.30 | 42, −54, 42 | 40 | R. inf. Parietal Lobule | |||
| 0.010 | 33 | 3.73* | −45, −51, 42 | 40 | L. inf. Parietal Lobule | |
| 3.59 | −33, −54, 39 | 40 | L. inf. Parietal Lobule | |||
| Occipital | 0.350 | 13 | 3.78 | 45, −69, −18 | 19 | R. Fusiform Gyr. |
| Correlation SAPS-passivity symptoms | ||||||
| Front./parietal | <0.001 | 131 | 4.11 | −57, −12, 33 | 4 | L. Precentral G. |
| 4.02 | −54, −24, 48 | 2 | L. Postcentral G. | |||
| 4.01 | −51, −15, 39 | 4 | L. Precentral G. | |||
| 0.002 | 45 | 3.71 | 57, −15, 39 | 4 | R. Precentral G. | |
| 0.406 | 12 | 3.80 | −42, −12, −6 | 13 | L. Insula | |
| Parietal | 0.002 | 43 | 3.87* | −39, −54, 40 | 40 | L. inf. Parietal Lobule |
| 3.78 | −45, −54, 48 | 40 | L. inf. Parietal Lobule | |||
| 0.406 | 12 | 3.42 | 42, −54, 42 | 40 | R. inf. Parietal Lobule | |
| 3.39 | 51, −48, 48 | 40 | R. inf. Parietal Lobule | |||
| Occipital | 0.117 | 19 | 4.48 | 48, −69, −15 | 19 | R. Fusiform Gyr. |
| MC–CC | ||||||
| Correlation AMDP-passivity symptoms | ||||||
| Parietal | 0.160 | 18 | 3.82 | −6, −27, 39 | 31 | L. post. Cing. Gyr. |
| Correlation SAPS-passivity symptoms | ||||||
| Parietal | 0.222 | 16 | 4.05 | −3, −30, 39 | 31 | L. post. Cing. Gyr. |
Asterisks indicate activations that were FWE corrected (P < 0.05) for region of interest (BA 40) according to a global activation maximum during visuomotor action monitoring in this region in the previous study. All coordinates are indicated according to the standard MNI template.
Fig. 3.
LEFT: Whole brain analysis of correlation of haemodynamic activation in the visuomotor MC and ratings of passivity symptoms from the AMDP (A) and SAPS (B) (P = 0.001 uncorr. for display; k > 10 voxel) overlayed on parietal clusters of main effects (MC–CC) of the monitoring task in the parietal cortex (dark green, P = 0.05 FDR corr.) for illustration. RIGHT: Scatter plots of passivity and beta estimates of activation in CC and MC extracted from spheres (10 mm) centred in indicated IPL coordinates. Correlations (Pearson's; r) in MC and CC are indicated as well as P-values specifying the significance of differences between these correlations.
The maxima of whole brain correlation analysis of SAPS scores for passivity symptoms with activation in the MC were actually located in similar coordinates of the IPL as correlations with AMDP scores, i.e. right IPL (x, y, z = 42, −54, 42, z = 3.42, P < 0.001, uncorr.), left IPL (x, y, z = –39, −54, 40, z = 3.87, P < 0.05, FWE corrected for BA 40). Accordingly, analysis of extracted beta estimates showed significant correlations with passivity in the right IPL (Pearson's r = 0.743, P = 0.0007) and in the left IPL (Pearson's r = 0.707, P = 0.0016) as well. Noticeably the maxima of correlations with both passivity scores were located within the IPL region activated by the main effect of the task (projection of the parietal main effect in Fig. 3). Outside those predefined regions of interest whole brain analysis of the monitoring condition (P < 0.001 uncorr.) revealed additional positive correlation of functional activation with the AMDP passivity ratings in left primary motor (x, y, z = −57, −15, 30, z = 3.62) and sensory cortices (x, y, z = −51, −18, 42, z = 3.99) and in the right fusiform gyrus (x, y, z = 45, –69, –18, z = 3.78). SAPS passivity scores were additionally correlated with activation of right primary motor cortex (x, y, z = 57, –15, 39, z = 3.71) and the left insula (x, y, z = –42, –12, –6, z = 3.80). Activations in the MC were not correlated with the number of actions of patients in this condition.
Similar to the analysis in the MC the functional effect of the correlation between passivity symptoms and the experimental main effect, i.e. the differential effect of monitoring (MC–CC) was analysed with whole brain analysis and beta values extracted from volumes of interest simultaneously. Whole brain analysis rendered a correlation of AMDP- and SAPS-ratings with the differential activation in the left posterior cingulate cortex (PCC) [P = 0.001 uncorr., AMDP (x, y, z = –6, –27, 39, z = 3.82), SAPS (x, y, z = –3, –30, 39, z = 4.05), Table 5]. Volume of interest analysis of beta values from these PCC coordinates revealed no significant correlation with detection latency or number of incongruence detections. Additionally, the correlation of passivity and functional main effect of monitoring on IPL activation was analysed by volume of interest analysis of MC–CC contrast images (extracted beta values from spheres centred on the coordinates of MC correlation maxima given above, Table 3). This analysis revealed a positive correlation of differential MC–CC activation and passivity symptoms in the left IPL (SAPS: x, y, z = –39, –54, 40; Pearson's r = 0.508, P = 0.0266; AMDP: x, y, z = –45, –51, 42, Pearson's r = 0.546, P = 0.0177), while differential activation in the right IPL displayed a trend for SAPS correlation (x, y, z = –42, –54, 42, Pearson's r = 0.417, P = 0.0611) but no significant AMDP correlation (x, y, z = 51, –48, 48, Pearson's r = 0.292, P = 0.1455).
Neither the score for auditory hallucinations nor the global degree of SAPS-ratings exhibited a significant correlation with individual activation levels of the previously described visuomotor action monitoring network.
Discussion
The present study examined if the impairment of causal association between own intentions and external events in patients with psychotic passivity experiences is demonstrably associated with behavioural and neurophysiological monitoring dysfunctions. For that purpose we examined a group of patients comprising individuals with different levels of passivity symptoms as well as individuals without passivity experiences. Action monitoring was examined within a simple visuomotor task, where the patients had to compare their original action with the perceived action outcomes. Test subjects were asked to control if changes in the direction of a moving object (car) were linked to their own action or caused by the computer.
The functional main effect of action monitoring (MC–CC) in patients and in a healthy control group replicated the pattern of the action monitoring network observed in our previous study with healthy participants (Schnell et al., 2007) (Fig. 2). The activated network comprised bilateral IPL and temporoparietal junction, precuneus, caudal anterior cingulate, dorsolateral and right ventrolateral PFC.
Analysis of data from the patients group revealed a simultaneous correlation of passivity symptoms with altered physiological function of this action monitoring network and with impaired behavioural monitoring performance. These observations support the idea that passivity symptoms are caused by defective action monitoring as suggested by Frith and Blakemore (Frith et al., 2000a): Both AMDP- and SAPS- passivity scores were correlated with increased latency in the detection of incongruence between own actions and resulting visual feedback. SAPS scores for passivity symptoms were also correlated with a decreased number of detected incongruence events. These symptom-related changes were obviously not linked to general dysfunctions of the motor system since passivity symptoms were not associated with altered motor performance or action frequency. In the neurofunctional scope both passivity symptom scores were correlated with increased bilateral IPL (BA 40) activation in the MC. A correlation between passivity and the functional main effect of experimental conditions (MC–CC) was observed in the activation of the posterior cingulate gyrus and—by volume of interest analysis—in the left IPL as well.
The IPL had previously been characterized as a key structure of the action monitoring network (Spence et al., 1997; Blakemore and Sirigu, 2003; Farrer et al., 2004; Fogassi and Luppino, 2005; Schnell et al., 2007). The IPL is reactive to both visuo-motor and visuo-proprioceptive incongruence (Shimada et al., 2005; Balslev et al., 2006) and to the observation of external actions (Buccino et al., 2001). Therefore, it is highly suggestive that IPL hyperactivation in passivity experiences indicates increased (false) detection of asynchrony between own actions and observed consequences. Accordingly activation of the left IPL was correlated with increased latency of incongruence detection. The simultaneously observed correlation of passivity symptoms with increased activation of primary motor and sensory areas in the MC underlines the connection between passivity experiences and the motor system. Noteworthy, there was no correlation of activation of those regions or passivity experiences with the number of actions in the monitoring task. Thus increased activation of the motor cortex cannot simply be explained as a secondary result of an increased number of actions due to longer detection latencies in patients with passivity symptoms. It is more likely that increased activation of the IPL causally contributes to the increased latency of visuomotor incongruence detection.
In accordance with the assumptions about passivity related uncertainty in self-other decisions in the monitoring condition, there was a significant correlation between passivity symptoms and the differential activation of the PCC (BA31) during action monitoring compared to unattended actions (MC–CC). Activation in this area is frequently found in response to errors (Menon et al., 2001), but most notably the observed correlation between functional monitoring effort and passivity symptoms can be conceived as a dysfunction of self monitoring since the PCC and the adjacent medial parietal cortex is conceived as a nodal structure in self-representation (Lou et al., 2004). Lou et al. reported functional connections of this area during self-monitoring to both the lateral parietal and the medial prefrontal cortices which are also part of the action monitoring network (Fig. 2). Thus symptom related differential hyperactivation of the posterior cingulate might represent a compensatory effort during conscious self-monitoring in patients experiencing passivity. The correlation between passivity and the differential PCC activation during action monitoring assumedly indicates that the experience of passivity is selectively linked to the self-monitoring component of action monitoring. Hence, we solely found a passivity related disturbance of conscious self monitoring, while the measures of automatic motor performance—like the error rate in movement-target coordination—remained unaffected in accordance with observations reported by Knoblich et al. (2004). Thereby the passivity related increase of differential PCC activation might reflect increased recruitment of evaluative functions for the monitoring of sensory input during self-other judgements (Vogt et al., 1992; Lou et al., 2004; Seger et al., 2004; Schnell et al., 2007). In contrast the passivity related increase of IPL activation in the MC is presumably related to actual signalling of visuomotor incongruence. With respect to the observation of a similar passivity related IPL-hyperactivation in movements without explicit self-monitoring demand (Spence et al., 1997), we assume that dysfunctional IPL activation and respective false signalling of incongruence might occur in both conditions to some degree and is further aggravated by the explicit demand of conscious self monitoring in the MC. Accordingly volume of interest analysis revealed that there is in fact a significant correlation between passivity and the main experimental effect of action monitoring (MC–CC) in the left IPL (Fig. 3) and a trend for correlation with SAPS scores in the right IPL. However, those differential effects in the IPL are considerably smaller than the correlation with differential activation in the PCC.
In summary, these observations correspond to the idea of an exaggerated sense of agency (Frith, 2005) in patients with passivity experiences. An increased number of false asynchrony detections could also explain the emergence of behavioural disturbances when reliable determination of congruence and incongruence is required like in our experiment: In order to sustain normal motor performance, i.e. to keep the car on the track, patients with less reliable signalling of incongruence have to perform more checks before they can definitely decide that the computer is controlling the observed actions. To rely on the false signalling of external control would often result in errors in the underlying task, i.e. the car would leave the track when these patients stop their actions. Hence, the observed passivity related hyperactivation of IPL can be interpreted as an equivalent of psychopathology. The inferior part of the human posterior parietal lobe holds analogous functions like the monkeys area PF (von Economo, 1929), where specialized neurons code for targets of observed external actions (Fogassi and Luppino, 2005). According to Wolperts model of predictive action monitoring (Wolpert, 1997), the prediction of action consequences reduces activation of sensory cortices (Blakemore et al., 1998b). It can be hypothesized that insufficient attenuation of IPL activity by predictive models results in increased IPL reactivity during the observation of own actions. This functional mechanism could explain the correlation of passivity and with the failure of visuomotor action monitoring observed in our experiment. But is this failure of visuomotor action monitoring actually linked to passivity symptoms or more likely associated with schizophrenia in general?
Within the patients group behavioural measures and the activation of the action monitoring network (Fig. 2) were correlated with the individual scores for passivity experiences, but not with the scores for acoustic hallucinations or global SAPS-scores for positive symptoms. Moreover, the low correlation between the SAPS scores for passivity and auditory hallucinations in schizophrenic patients suggests a pathophysiological independence of both symptom categories.
Between-group comparison of healthy controls and schizophrenic patients revealed no group × condition interaction within the action monitoring network. Though we expected passivity symptoms to be correlated with a larger monitoring-dependent (MC–CC) increase of IPL and PCC activation in the patients group (Spence et al., 1997; Farrer et al., 2004), this interaction was not significant.
After all, the absence of significant group × condition interaction effects in the IPL and PCC supports the assumption that our experiment actually induced rather passivity related functional and behavioural differences within the patients group than schizophrenia-related global differences between groups. Thus our findings support the idea that circumscribed pathophysiological changes of the visuomotor action monitoring system are rather linked to passivity symptoms than globally existent in all schizophrenic patients. This concept of a symptom-specific dysfunction of the visuomotor action monitoring network is in fact supported by correlations of passivity symptoms with increased regional blood flow in the right IPL and left premotor cortex (Spence et al., 1997) and decreased right IPL and left prefrontal volumes (Maruff et al., 2005). Correspondingly, a correlation of auditory hallucinations with volume reduction in frontotemporal areas (Gaser et al., 2004) and changes of temporoparietal pathways (Hubl et al., 2004) indicate similar system–symptom associations in auditory monitoring systems.
However, the conclusion that the perceptual modality which provides data for the defective action monitoring process—i.e visual or proprioceptive for passivity experiences, auditory for auditory hallucinations—determines the modality of perceptual symptoms is not accepted unequivocally. Some authors have reported rather general associations of action monitoring deficits with positive psychotic symptoms in schizophrenia. Blakemore et al. (2000) demonstrated a correlation between positive psychotic symptoms and behavioural deficits in the monitoring of congruence between own actions and tactile perceptions. Farrer et al. (2004) found a correlation of first rank symptoms of schizophrenia defined by Kurt Schneider—including passivity and auditory hallucinations—and the modulation of activity in the right angular gyrus by visuomotor incongruence. Franck et al. (2002) had previously found an increase of parietal activity at rest—albeit in superior not in inferior parietal cortex—to be generally associated with schneiderian first rank symptoms. However, in behavioural measures of a task with explicit monitoring demand the same author observed a symptom-specific association of visuospatial monitoring deficits and delusions of influence (Franck et al., 2001). In line with the latter observation the seminal PET study of Spence et al. (1997) provided a basic evidence of symptom-specific contribution of IPL dysfunction to the emergence of passivity symptoms by examining the generation of simple movements in psychotic patients.
The major difficulties to prove the hypotheses of symptom specifity—i.e. that the sensory modality of the defective monitoring mechanism determines the sensory modality of resulting psychotic symptoms—depends on certain assumptions included in experimental designs: The first premise is to address both the function of the action monitoring system and passivity symptoms directly. The PET experiment of Farrer et al. examined the cerebral activation within an action monitoring task. However, the statistical analysis tested for a correlation of activation modulation with a combined score for hallucinations and passivity but not with passivity alone. On this background our experiment is the first study to directly address the connection between passivity symptoms and the capacity to differentiate own and external actions in an action monitoring task, demonstrating both functional and behavioural correlates of passivity.
The second implication in the examination of passivity symptoms is the determination of the task difficulty. Symptom-specific effects of defective action monitoring may only be detectable on moderate levels of perceptual load since higher levels may involve confounding attentional disturbances in schizophrenic patients. To avoid a possible floor effect in schizophrenic patients we reduced the speed of the motor task (Schnell et al., 2007) to increase specificity for a passivity-related dysfunction rather than sensitivity for load-dependent group differences between schizophrenic patients and controls. Accordingly, we found no significant differences in visuomotor performance or fMRI signals between groups, but a significant correlation of passivity symptoms with functional and behavioural data within the patient group. The absence of the group difference which was found in other studies may also be derived from the fact that 11 of 15 patients had never received antipsychotic treatment and that extrapyramidal effects which may generally interfere with motor tasks had been ruled out with standard ratings (EPS, AIMS).
After all, we suggest that dysfunctions of action monitoring are effective on two levels: unspecific global dysfunctions of monitoring might become effective on higher levels of perceptual load. In contrast, symptoms occurring on lower levels of perceptual load are assumedly linked to a localized failure in the monitoring systems for the respective sensory modality. The idea of such a pathophysiological system–symptom association is strengthened by the low correlation between symptom categories like e.g. hallucinations and passivity symptoms (Stuart et al., 1995). Moreover, this concept provides a conceptual link between psychopathological observations and the dysconnection concept of schizophrenia (Weinberger and Lipska, 1995). As the general affection of long pathways implied in reafferent processing might come into effect on higher perceptual loads in all patients, more severe regional dysconnections may result in specific symptoms like acoustic hallucinations (Hubl et al., 2004) already on lower levels of perceptual load.
While the observed correlations indicate a possibility to assign psychopathologic phenomena to the dysregulation of a pertinent neural network, the adaptation of a dimensional approach of symptom assessment is a limitation of this study since the traditional approach of psychopathology is categorical. However, dimensional assessment appears to be the only method to represent not only the quality but also the gradual differences of symptoms experienced in differentially affected individuals. The idea that the rating scales for psychopathology actually represent differences between subjects in a linear way is supported by the study of Gaser et al. (2004) who explored the correlation of SAPS scores for hallucinations with anatomical data. First of all, their findings with different categorical and correlational approaches support the concept that the transition between SAPS scores between 0 and 1 does not necessarily comprise the boundary of a clinical symptom category. Likewise, a score of 1 for a single symptom in the AMDP passivity subscale—which corresponds to e.g. ‘slight derealisation’—does not classify the subject under the clinical category of schneiderian ego-disturbances. A score of 1 rather describes a slight, intermittent disturbance of the monitoring system, which could possibly observed in other psychiatric disorders as well, and rather indicates a state where the markedness of a symptom has not reached significance for diagnostic classification.
Since the descriptive system of psychopathology has not undergone factorial organization, there is no clear concept about the orthogonality of the psychopathological categories and the dependence of items within one category. To address this conceptual issue we performed an additional alternative correlation analysis with the maximum instead of the sum of AMDP and SAPS ratings within the passivity category. This alternative approach rendered correlations in the same areas as the original analysis. A second conceptual limitation of the rating scales results from the selection of included psychopathological phenomena. The definition of passivity provided by both systems is not completely congruent with traditional concepts of disturbed sense of the self (Cutting, 1997) or the concept of ego-disturbances defined by Kurt Schneider (Schneider, 1962). With less conceptual premises the term ‘passivity symptoms’ describes difficulties of determining the agency of actions and cognitive processes, which result in misattribution to external causes. However, the experimentally observed correlation of such experiences with behavioural and functional alterations indicates that the psychopathological category of passivity symptoms of AMDP and SAPS ratings represent a meaningful entity within a neurophysiological approach to psychopathology.
Our experiment demonstrated the possibility to study pathophysiological associations between passivity symptoms and the function of the pertinent action monitoring network with both functional and behavioural data. The results support the idea that passivity experiences in schizophrenia result from a dysfunction in central action monitoring mechanisms with hyperactivation of the IPL, possibly an equivalent of false signaling of visuomotor incongruence and differential hyperactivation of the posterior cingulate cortex, presumably related to increased self-monitoring effort. From the neuroanatomical perspective these findings of IPL hyperactivation in passivity symptoms underline the function of this area as a key element of action monitoring. The findings may promote the understanding of dysfunctional monitoring of own actions in schizophrenia and other psychiatric or neurological disorders.
Acknowledgements
This study was supported by a grant of ‘Cologne Fortune’, a research program of the University of Cologne. We would like to thank Jochen Weber for his advice and assistance in programming the paradigm.
Glossary
Abbreviations:
- AIMS
Abnormal Involuntary Movement Scale
- BA
Brodmann area
- CC
control condition
- EPI
echoplanar imaging
- EPS
Extrapyramidal Motor Side Effects Scale
- FDR
false discovery rate
- HRF
hemodynamic response function
- IPL
inferior parietal lobule
- MC
monitoring condition
- mPFC
medial prefrontal cortex
- PCC
posterior cingulate cortex
- SANS
Scale for Assessment of Negative Symptoms
- SAPS
Scale for Assessment of Positive Symptoms
- SCID
Structured Clinical Interview for DSM-IV Axis I Disorders
- SPM
Statistical Parametric Mapping
References
- AMDP. Das AMDP - System. 7th edn. Göttingen: Hogrefe; 2000. Manual zur Dokumentation psychiatrischer Befunde; pp. 109–116. [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M. Symptoms of schizophrenia. Methods, meanings, and mechanisms. Arch Gen Psychiatry. 1995;52:341–51. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Balslev D, Nielsen FA, Lund TE, Law I, Paulson OB. Similar brain networks for detecting visuo-motor and visuo-proprioceptive synchrony. Neuroimage. 2006;31:308–12. doi: 10.1016/j.neuroimage.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–45. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 1999;11:551–9. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998a;18:7511–8. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998b;1:635–40. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–9. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–4. [PubMed] [Google Scholar]
- Chapman CE, Bushnell MC, Miron D, Duncan GH, Lund JP. Sensory perception during movement in man. Exp Brain Res. 1987;68:516–24. doi: 10.1007/BF00249795. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–62. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. Principles of psychopathology. Two worlds-two minds-two hemispheres. Oxford: Oxford University Press; 1997. pp. 293–314. [Google Scholar]
- Farrer C, Franck N, Frith CD, Decety J, Georgieff N, d'Amato T, et al. Neural correlates of action attribution in schizophrenia. Psychiatry Res. 2004;131:31–44. doi: 10.1016/j.pscychresns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–31. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Fourneret P, Jeannerod M. Limited conscious monitoring of motor performance in normal subjects. Neuropsychologia. 1998;36:1133–40. doi: 10.1016/s0028-3932(98)00006-2. [DOI] [PubMed] [Google Scholar]
- Fourneret P, Franck N, Slachevsky A, Jeannerod M. Self-monitoring in schizophrenia revisited. Neuroreport. 2001;12:1203–8. doi: 10.1097/00001756-200105080-00030. [DOI] [PubMed] [Google Scholar]
- Fourneret P, de Vignemont F, Franck N, Slachevsky A, Dubois B, Jeannerod M. Perception of self-generated action in schizophrenia. Cognit Neuropsychiatry. 2002;7:139–56. doi: 10.1080/13546800143000212. [DOI] [PubMed] [Google Scholar]
- Franck N, O’Leary DS, Flaum M, Hichwa RD, Andreasen NC. Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:277–82. doi: 10.1176/jnp.14.3.277. [DOI] [PubMed] [Google Scholar]
- Franck N, Farrer C, Georgieff N, Marie-Cardine M, Dalery J, d’Amato T, et al. Defective recognition of one's own actions in patients with schizophrenia. Am J Psychiatry. 2001;158:454–9. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]
- Frith C. The neural basis of hallucinations and delusions. C R Biol. 2005;328:169–75. doi: 10.1016/j.crvi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19:359–63. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000a;31:357–63. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000b;355:1771–88. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Volz HP, Buchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14:91–6. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–20. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Goldberg G, Mayer NH, Toglia JU. Medial frontal cortex infarction and the alien hand sign. Arch Neurol. 1981;38:683–6. doi: 10.1001/archneur.1981.00510110043004. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–5. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Guy W. Abnormal Involuntary Movement Scale (AIMS), ECDEU Assessment Manual for Psychopharmacology. Washington DC: US Department of Health: Education and Welfare; 1976. pp. 534–537. [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–68. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Age, sex and regional brain volumes predict perceptual-motor skill acquisition. Cortex. 2005;41:560–9. doi: 10.1016/s0010-9452(08)70196-5. [DOI] [PubMed] [Google Scholar]
- Knoblich G, Stottmeister F, Kircher T. Self-monitoring in patients with schizophrenia. Psychol Med. 2004;34:1561–9. doi: 10.1017/s0033291704002454. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39:101–7. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- Maruff P, Wood SJ, Velakoulis D, Smith DJ, Soulsby B, Suckling J, et al. Reduced volume of parietal and frontal association areas in patients with schizophrenia characterized by passivity delusions. Psychol Med. 2005;35:783–9. doi: 10.1017/s0033291704003113. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Frith CD. Functional neuroanatomy of verbal self-monitoring. Brain. 1996;119:907–17. doi: 10.1093/brain/119.3.907. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE, Gandevia SC. Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res. 1988;70:569–76. doi: 10.1007/BF00247604. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Nieuwenhuis S, Bashore TR. Errors are foreshadowed in brain potentials associated with action monitoring in cingulate cortex in humans. Neurosci Lett. 2003;348:1–4. doi: 10.1016/s0304-3940(03)00566-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schneider K. Klinische Psychopathologie. Stuttgart: Georg Thieme Verlag; 1962. pp. 120–125. [Google Scholar]
- Schnell K, Heekeren K, Schnitker R, Daumann J, Weber J, Hesselmann V, et al. An fMRI approach to particularize the frontoparietal network for visuomotor action monitoring: Detection of incongruence between test subjects' actions and resulting perceptions. Neuroimage. 2007;34:332–41. doi: 10.1016/j.neuroimage.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–77. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shimada S, Hiraki K, Oda I. The parietal role in the sense of self-ownership with temporal discrepancy between visual and proprioceptive feedbacks. Neuroimage. 2005;24:1225–32. doi: 10.1016/j.neuroimage.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120(Pt 11):1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Stuart GW, Malone V, Currie J, Klimidis S, Minas IH. Positive and negative symptoms in neuroleptic-free psychotic inpatients. Schizophr Res. 1995;16:175–88. doi: 10.1016/0920-9964(94)00083-k. [DOI] [PubMed] [Google Scholar]
- Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 2003;160:1881–3. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Corcos DM. Intermittent visuomotor processing in the human cerebellum, parietal cortex, and premotor cortex. J Neurophysiol. 2006;95:922–31. doi: 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Wolpert DM. Computational approaches to motor control. Trends Cognit Sci. 1997;1:209–216. doi: 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- World Medical Association. WMA; 2004. Declaration of Helsinki: Ethical principles for medical research involving human subjects. [PubMed] [Google Scholar]