Abstract
Purpose
RTOG 92-08 began as a single arm, phase II trial for patients with anal cancer consisting of Radiation (RT) + 5-FU + Mitomycin-C with a mandatory 2 week break and was amended after completion to evaluate the same treatment regimen without a treatment break. Long-term efficacy and late toxicity reporting are the specific aims of this study.
Methods and Materials
Survivals were estimated with the Kaplan-Meier method. Overall survival (OS) was compared to RTOG 87-04 with the log-rank test. Time to local failure, regional failure, local-regional failure (LRF), distant metastases, second primary and colostomy failure were estimated by the cumulative incidence method. LRF was compared to RTOG 8704 using the Gray’s test.
Results
Forty-seven patients entered in the mandatory treatment break cohort. The study was reopened in 1995 to the no mandatory treatment break cohort completing accrual with 20 patients in 1996. Of 67 total patients, 1 patient in the mandatory treatment break portion of the study did not receive any protocol treatment and is excluded from analyses. After adjusting for tumor size, neither cohort showed a statistically significant difference in OS or LRF compared to the RTOG 87-04 Mitomycin-C arm. No patient in either cohort experienced a grade 3 or higher late toxicity.
Conclusions
No statistically significant differences were seen in OS or LRF when compared to the Mitomycin-C arm of RTOG 87-04, but the sample sizes for the mandatory break cohort and the no mandatory break cohort are small. Late toxicity was low and similar for the treatment cohorts.
Keywords: Anal cancer, mandatory treatment break, chemoradiation
Introduction
Radiation Therapy Oncology Group (RTOG) 92-08 was a phase II pilot study designed to assess tolerance, local-regional control and survival in patients with anal carcinoma treated with 5-flourouracil (5-FU), Mitomycin-C and irradiation to 59.4 Gy. A higher 1 and 2-year colostomy rate was found when compared to the results of a previous RTOG trial, RTOG 87-04 which delivered 50.4 Gy to patients with residual disease after 45 Gy, 23% versus 6% and 30% and 7%, respectively.1 In the initial report of RTOG 92-08, 11 (24%) patients underwent abdominoperineal resection with colostomy. Nine of the 11 had surgery for recurrent or residual disease documented on biopsy. Increasing the radiation dose did not appear to increase local control when given in split-course fashion when compared to radiation schedules given in conventional fractionation.
The objectives of this analysis were to evaluate the long-term efficacy and late toxicity of RTOG 92-08 with over 10 years of follow-up.
Material and Methods
RTOG 92-08 began as a single arm phase II study of 5-FU, Mitomycin-C and irradiation with a mandatory 2-week treatment break for patients with anal carcinoma. Pre-treatment characteristics, eligibility criteria, and treatment received of patients participating in this study have been previously reported.1 The trial was opened June, 1992 and closed in July, 1993. An interim analysis was performed on the initial patients randomized to the trial and a high rate of colostomy was observed. Because of the high rate of colostomy, it was decided the trial would be re-opened in September, 1995 to evaluate the same treatment regimen without a mandatory treatment break, although allowing a treatment break for severe skin toxicity, and finally closed in 1996. Toxicity was the primary end-point for both parts of the study. Efficacy endpoints for each cohort of RTOG 92-08, the mandatory treatment break and continuous radiation schedule, were compared to the radiation (RT), 5-FU, and Mitomycin-C arm of RTOG 87-04.2 The study was not designed to compare the two RTOG 92-08 cohorts to each other and hence no such comparisons will be made.
Local-regional failure was defined as local or regional recurrence or progression. Distant failure was defined as appearance of distant metastases while second primary failure was defined as an appearance of a second primary. Colostomy failure and colostomy-free survival was defined as a colostomy, abdominoperineal resection or exenteration for any reason and colostomy failure or death due to any cause respectively. Absolute and disease-free survival was defined as death due to any cause and local, regional, distant or second primary failure or death due to any cause respectively.
Overall survival, disease-free survival and colostomy-free survival were estimated with the Kaplan-Meier method and RTOG 92-08 overall survival was compared to RTOG 87-04 using the log-rank test.3, 4 Time to local failure, regional failure, local-regional failure, distant metastases, second primary and colostomy failure were estimated by the cumulative incidence method and RTOG 92-08 local-regional failure was compared to RTOG 87-04 using the Gray’s test.5, 6 Multivariate analyses were performed using Cox proportional hazard models to test for treatment differences while adjusting for unbalanced pre-treatment characteristics and tumor size.7 All analyses were performed using the SAS system version 9.1 (SAS Institute Inc., Cary, NC).
Results
Forty-seven patients were accrued to the mandatory treatment break cohort and an additional 20 patients to the no mandatory treatment break cohort. Of the 67 patients, 1 patient in the mandatory treatment break cohort did not receive any protocol treatment and was excluded from the analyses. Tables 1a and 1b show the pretreatment characteristics for both treatment cohorts of RTOG 92-08 compared to RTOG 87-04. There were no statistically significant associations for any of the pretreatment characteristics for either RTOG 92-08 cohort as compared to the RTOG 87-04 arm. When comparing the RTOG 92-08 cohorts to RTOG 87-04, there is a trend towards more node negative patients on the RTOG 92-08 mandatory break cohort (p=0.08) while the RTOG 92-08 no mandatory break cohort has a trend towards more higher tumor stage patients (p=0.07). All cases underwent radiotherapy review with treatment given per protocol in 83% of patients in the mandatory treatment break cohort compared to 60% in the no mandatory treatment break cohort, while 13% had minor acceptable variations in the mandatory break cohort compared to 20% in the no mandatory treatment break cohort. Ninety-eight percent of cases underwent chemotherapy review with all cases either treated as per protocol or having minor or acceptable variation.
Table 1.
| Table 1a Pre-treatment Characteristics RTOG 92-08 Mandatory break cohort vs. 8704 MMC | |||||
|---|---|---|---|---|---|
| Mandatory Break (n=46) |
Mitomycin-C (n=148) |
||||
| Age | |||||
| Median | 62 | 63 | |||
| Range | 31–83 | 29–85 | |||
| n | % | n | % | p-values† | |
| Gender | 0.71 | ||||
| Male | 15 | 32 | 44 | 30 | |
| Female | 31 | 67 | 104 | 70 | |
| KPS | −/−* | ||||
| 60 | 0 | 0 | 3 | 0 | |
| 70–80 | 9 | 20 | 26 | 20 | |
| 90–100 | 37 | 80 | 119 | 80 | |
| Size | 0.39 | ||||
| 2–5 cm | 25 | 54 | 91 | 45 | |
| ≥ 5 cm | 21 | 46 | 57 | 55 | |
| Tumor Stage | 0.72 | ||||
| T1/T2 | 23 | 50 | 84 | 35 | |
| T3 | 18 | 39 | 49 | 50 | |
| T4 | 5 | 11 | 15 | 15 | |
| T- Stage Categorical | 0.42 | ||||
| T1/T2 | 23 | 50 | 84 | 57 | |
| T3/T4 | 23 | 50 | 64 | 43 | |
| Nodal Stage | −/−* | ||||
| N0 | 41 | 89 | 123 | 83 | |
| N1 | 3 | 7 | 24 | 16 | |
| N3 | 0 | 0 | 1 | 0 | |
| NX | 2 | 4 | 0 | 1 | |
| N-Stage Categorical | 0.08 | ||||
| N0,NX | 43 | 94 | 123 | 83 | |
| N1,N3 | 3 | 7 | 25 | 17 | |
| Table 1b Pre-treatment Characteristics RTOG 92-08 No mandatory break cohort vs. 8704 MMC | |||||
|---|---|---|---|---|---|
| No Mandatory Break (n=20) |
Mitomycin-C (n=148) |
||||
| Age | |||||
| Median | 61 | 63 | |||
| Range | 38–83 | 29–85 | |||
| n | % | n | % | p-values† | |
| Gender | 0.63 | ||||
| Male | 7 | 35 | 44 | 30 | |
| Female | 13 | 65 | 104 | 70 | |
| KPS | −/−* | ||||
| 60 | 0 | 0 | 3 | 2 | |
| 70–80 | 4 | 20 | 26 | 18 | |
| 90–100 | 16 | 80 | 119 | 80 | |
| Size | 0.16 | ||||
| 2–5 cm | 9 | 45 | 91 | 61 | |
| ≥ 5 cm | 11 | 55 | 57 | 39 | |
| Tumor Stage | 0.20 | ||||
| T1/T2 | 7 | 35 | 84 | 57 | |
| T3 | 10 | 50 | 49 | 33 | |
| T4 | 3 | 15 | 15 | 10 | |
| T- Stage Categorical | 0.07 | ||||
| T1/T2 | 7 | 35 | 84 | 57 | |
| T3/T4 | 13 | 65 | 64 | 43 | |
| Nodal Stage | −/−* | ||||
| N0 | 14 | 70 | 123 | 83 | |
| N1 | 4 | 20 | 24 | 16 | |
| N3 | 0 | 0 | 1 | 1 | |
| NX | 2 | 10 | 0 | 0 | |
| N-Stage Categorical | 0.72 | ||||
| N0,NX | 16 | 80 | 123 | 83 | |
| N1,N3 | 4 | 20 | 25 | 17 | |
p-values from Chi-square test
Chi-square test not valid due to expected counts of less than 5
Table 2 shows late RT toxicity graded according to the RTOG/EORTC late morbidity scoring scheme. Late toxicity was mild with no grade 3 or higher late RT toxicity. There was no grade 2 late RT toxicity in the mandatory break cohort and only 1 late grade 2 GI toxicity in the no mandatory treatment break cohort.
Table 2.
Late RT Toxicity
| Mandatory Break (n=44) Grade |
No Mandatory Break (n=19) Grade |
|||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| Skin | 1 | 0 | 0 | 0 |
| GI | 1 | 0 | 0 | 1 |
| GU | 1 | 0 | 0 | 0 |
| Other(non-hema) | 0 | 0 | 1 | 0 |
| Worst overall | 1 | 0 | 1 | 1 |
| (2%) | (5%) | (5%) | ||
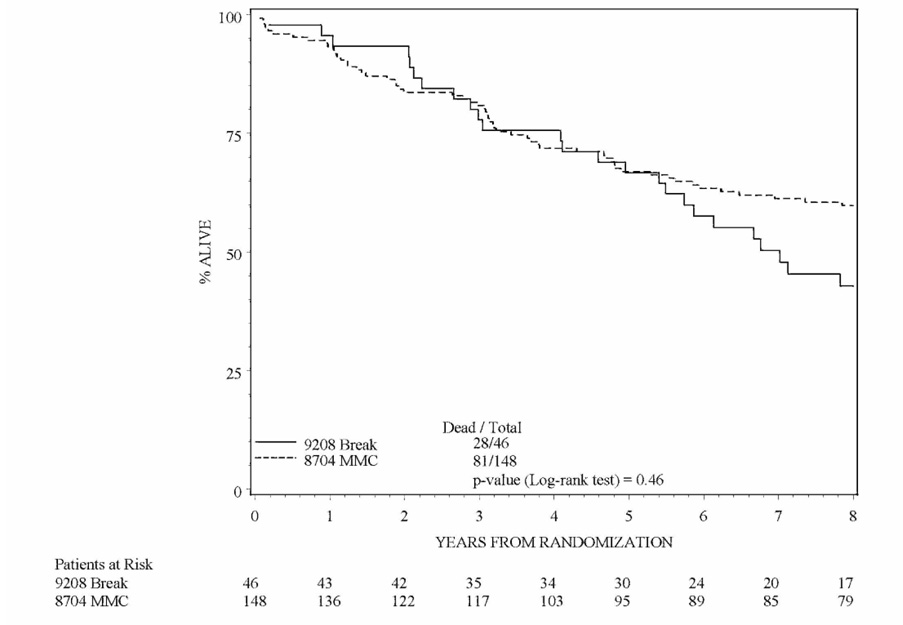
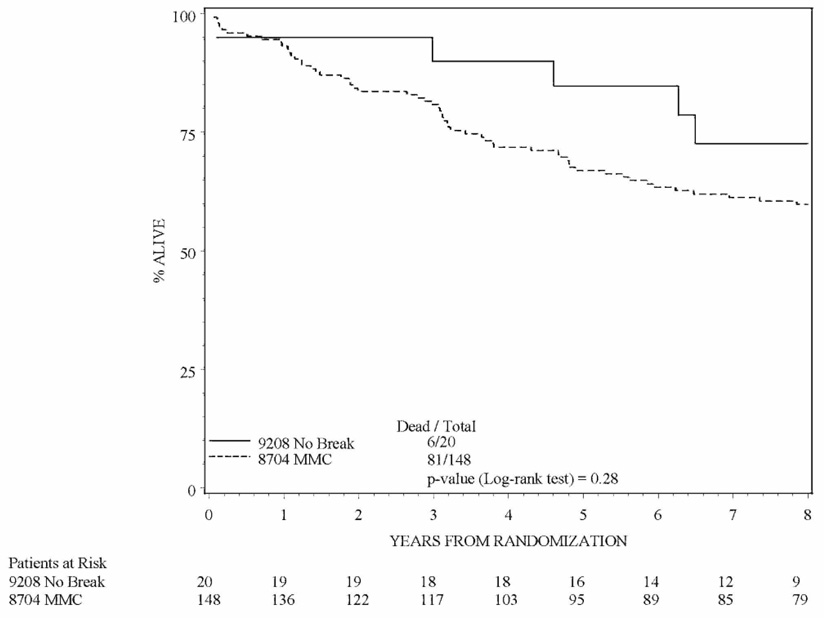
Table 3 and Table 4 show the outcome estimates and follow-up for patients treated on RTOG 92-08. Figures 1a and 1b are Kaplan-Meier estimates comparing patients treated on the mandatory and no mandatory break cohorts of RTOG 92-08 to patients treated on RTOG 87-04. Although the trial was not designed to compare overall survival and local-regional control of each cohort of RTOG 92-08 to each other, it is interesting to note that local failure was lower and absolute, disease-free and colostomy-free survival were higher in patients treated without a mandatory treatment break.
Table 3.
Outcome Estimates
| Mandatory Break (n=46) |
No Mandatory Break (n=20) |
|||||
|---|---|---|---|---|---|---|
| No. of Failures | 5 Year Estimate | 8 Year Estimate | No. of Failures | 5 Year Estimate | 8 Year Estimate | |
| Local failure | 12 | 27% | 27% | 2 | 10% | 10% |
| Regional failure | 4 | 9% | 9% | 2 | 10% | 10% |
| Local regional failure | 13 | 29% | 29% | 3 | 15% | 15% |
| Distant failure | 11 | 18% | 25% | 1 | 5% | 5% |
| Second primary failure | 7 | 2% | 9% | 4 | 5% | 17% |
| Colostomy failure | 11 | 25% | 25% | 2 | 11% | 11% |
| Absolute survival | 28 | 67% | 43% | 6 | 85% | 73% |
| Disease-free survival | 35 | 53% | 34% | 8 | 80% | 63% |
| Colostomy-free | 32 | |||||
| survival | 58% | 34% | 8 | 75% | 63% | |
Table 4.
Median Survival Times and Follow-up for Alive patients on RTOG 92-08
| Mandatory Break (n=46) |
No Mandatory Break (n=20) |
|||
|---|---|---|---|---|
| Absolute Survival | Median survival (all pts) | 7.2 yrs | Median survival (all pts) | 10.6 yrs |
| 95% CI (all pts) | (5.5, 11.9) | 95% CI (all pts) | (8.6, 11.9) | |
| Disease-free Survival | Median DFS (all pts) | 11.9 mon | Median DFS (all pts) | 11.3 mon |
| 95% CI (all pts) | (10.1, 14.2) | 95% CI (all pts) | (10.2, 12.6) | |
| Colostomy-free Survival | Median CFS (all pts) | 11.9 mon | Median CFS (all pts) | 11.3 mon |
| 95% CI (all pts) | (10.1, 14.2) | 95% CI (all pts) | (10.2, 12.6) | |
| Median follow-up (alive pts) | 12 yrs | Median follow-up (alive pts) | 8.8 yrs | |
| Range (alive pts) | 0.2 – 13.4 yrs | Range (alive pts) | 4.2 – 10.2 yrs | |
Figure 1.
Figure 1a: Kaplan-Meier overall survival estimate comparing the mandatory treatment break cohort of RTOG 9208 to the mitomycin-c arm of RTOG 8704. No significant difference in overall survival was found between the two cohorts.
Figure 1b: Kaplan-Meier overall survival estimate comparing the no mandatory treatment break cohort of RTOG 9208 to the mitomycin-c arm of RTOG 8704. No significant difference was found in overall survival between the two cohorts.
Multivariate analysis results for overall survival comparing each cohort of RTOG 92-08 to RTOG 87-04 are shown in Table 5 and Table 6. After adjusting for tumor size, neither cohort showed a statistically significant difference in overall survival or local-regional control when compared to the RTOG 87-04 Mitomycin-C arm. However, it is important to keep in mind that the sample sizes for the mandatory break cohort (n=46) and the no mandatory break cohort (n=20) are very small and RTOG 92-08 was designed for toxicity, not efficacy compared to RTOG 87-04. While there may really be no difference between the RTOG 92-08 cohorts and the Mitomycin-C arm of RTOG 87-04 with respect to overall survival and local-regional control, the power to detect such differences, if they do exist here, is very, very small. The adjusted hazard ratios (HR) also need to be interpreted very carefully. For example, the adjusted HR point estimates of treatment for the no mandatory break cohort look rather strong in favor of the RTOG 92-08 cohort, but the confidence intervals are wide and do contain 1, meaning that we can not make any statistical inferences about a protective effect for the no mandatory break cohort.
Table 5.
Multivariate Analysis for Overall Survival and Local regional failure 87-04 Mitomycin-C and 92-08 Mandatory Break Patients (n=194)
| Endpoints | Adjustment Variables | Comparison | Adjusted HR** | p-value† |
|---|---|---|---|---|
| Overall Survival | Treatment | 8704 Mitomycin-C vs. 9208 Mandatory Break | 1.13 (0.73,1.74) |
0.59 |
| Tumor Size | 2–5 vs. ≥ 5cm | 1.40 (0.96,2.06) |
0.09 | |
| Local Regional Failure | Treatment | 8704 Mitomycin-C vs. 9208 Mandatory Break | 1.03 (0.55,1.93) |
0.93 |
| Tumor Size | 2–5 vs. ≥ 5cm | 1.68 (0.98,2.89) |
0.06 | |
from the Cox regression model
Table 6.
Multivariate Analysis for Overall Survival and Local-regional failure 87-04 Mitomycin-C and 92-08 No Mandatory Break Patients (n=168)
| Endpoints | Adjustment Variables | Comparison | Adjusted HR | p-value† |
|---|---|---|---|---|
| Overall Survival | Treatment | 8704 Mitomycin-C vs. 9208 No Mandatory Break | 0.57 (0.24,1.32) |
0.19 |
| Tumor Size | 2–5 vs. ≥ 5cm | 1.60 (1.04,2.46) |
0.03 | |
| Local Regional Failure | Treatment | 8704 Mitomycin-C vs. 9208 No Mandatory Break | 0.48 (0.15,1.55) |
0.22 |
| Tumor Size | 2–5 vs. ≥ 5cm | 2.68 (1.46,4.94) |
0.002 | |
from the Cox regression model
Discussion
This study evaluated the late toxicity and outcome of patients treated on RTOG 92-08. After 10 years of follow-up, there have been no reported incidences of > grade 3 late toxicity in either treatment cohort. Although patient numbers do not allow adequate statistical evaluation, patients treated with the mandatory break had worse overall, disease-free, and colostomy-free survival compared to patients with similar pretreatment characteristics treated on RTOG 87-04. Patients treated with no mandatory break had outcomes similar to other reported series. Chemoradiation with a total radiation dose of 59.4 Gy was found to provide outcome consistent with previous reports without an increase in late toxicity.
Combined modality treatment of patients with anal cancer can be morbid with the potential of treatment interruptions due to gastrointestinal and dermatologic toxicity. Twelve percent of patients treated on RTOG 87-04 required a 2-week or greater interruption in treatment compared to all patients in the mandatory treatment break cohort of RTOG 92-08. Given the present study and previous reports treatment interruptions should be kept to a minimum for optimal local control.8
In the initial publication of the preliminary results of RTOG 92-08, 26% of patients had greater than grade 3 complications with the majority being hematologic.1 Patients treated on the mandatory treatment break cohort had lower incidence of ≥ grade 3 dermal toxicity compared to patients treated on RTOG 87-04and there was a difference in late toxicity when compared to RTOG 87-04. Five percent of patients treated with 5-FU and mitomycin-C on RTOG 87-04 experienced late grade 4 and 5 toxicities while there were no grade 4 or 5 late toxicity reported in either cohorts of RTOG 92-08. The lack of grade 3 or higher late toxicity in patients treated on either cohorts of RTOG 92-08 was also better than that reported by Bartelink et al.9 They reported differences in late toxicity comparing patients receiving chemoradiation to patients receiving radiation alone, with 28/51 patients receiving chemoradiation listed as having severe late effects. Cummings et al reported an interrupted course of chemoradiation produced less severe normal tissue damage compared to an uninterrupted course of radiation.10 Dermatologic and gastrointestinal late toxicity was also reported in 122/292 (42%) patients receiving chemoradiation in the UKCCCR trial.11 Allal et al reported morbidity correlated significantly with anatomic location of tumor and prescribed external beam dose.12 In addition, Hung et al reported only a 2% (3 patients) chronic toxicity in patients treated with cisplatin-based chemoradiation.13 It is unknown if the lack of late toxicity experienced by patients treated on RTOG 92-08 was secondary to the mandatory treatment break or other treatment related factors.
Local-regional control and survival were the other end-points of interest in RTOG 92-08. 5-year estimates of disease-free survival and colostomy-free survival, 53% and 58% respectively, in patients treated on the mandatory treatment break arm are lower than reported on RTOG 87-04 and by Bartelink, et al.2, 9 The UKCCR trial reported a 3-year local failure rate of 39% in patients treated with chemoradiation.11 Disease-free survival and colostomy-free survival in the no mandatory treatment break cohort of RTOG 9208 were comparable to the above cited results. Patients treated without a split with 5-FU and Mitomycin-C had less failures compared to patients treated with radiation alone or chemoradiation with a treatment interruption.10 The higher failure rate seen in patients with a mandatory break could have been secondary to repair of sublethal damage or tumor repopulation.14
The EORTC reported an overall 5-year survival of 56% for all patients with patients treated with combined modality therapy having a 5-year survival slightly more than 60%. Overall survival was also not significantly different between the two treatment arms in RTOG 87-04 and there was no difference in overall survival between either cohorts of RTOG 92-08 compared to RTOG 87-04. Survival in the no mandatory treatment cohort of RTOG 92-08 was higher compared to the mandatory treatment break cohort of RTOG 92-08 and comparable to the other series with uninterrupted treatment presented above. Once again, the trial was not designed to compare each cohort of 92-08 with each other so statistical comparison of each cohort cannot be performed.
In conclusion, late toxicity was low in the mandatory treatment break and no mandatory treatment break cohorts. However, 5-year estimates of disease-free survival and colostomy-free survival in patients treated on the mandatory treatment break arm are lower than reported on RTOG 87-04 while disease-free and colostomy-free survival in the no mandatory treatment break cohort of RTOG 9208 were comparable to other reported series. Treatment breaks in anal canal treatment should be kept to a minimum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 2007 Gastrointestinal Oncology Symposium, January 19–21, 2007, Orlando Fla.
Conflict of Interest Notification
There is no actual or potential conflict of interest among all of the authors included in this manuscript as it pertains to the publication of this work.
References
- 1.John M, Pajak T, Flam M, et al. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205–211. [PubMed] [Google Scholar]
- 2.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stats Assoc. 1958;53:447–457. [Google Scholar]
- 4.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 5.Kalbfleisch J, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & sons; 1980. [Google Scholar]
- 6.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stats. 1988;16:1141–1143. [Google Scholar]
- 7.Cox DR. Regression models and life tables. J Roy Stats Soc. 1972;34:187–220. [Google Scholar]
- 8.Weber DC, Kurtz JM, Allal AS. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2001;50:675–680. doi: 10.1016/s0360-3016(01)01510-3. [DOI] [PubMed] [Google Scholar]
- 9.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 10.Cummings BJ, Keane TJ, O'Sullivan B, et al. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991;21:1115–1125. doi: 10.1016/0360-3016(91)90265-6. [DOI] [PubMed] [Google Scholar]
- 11.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-flourouracil, and mitomycin. The Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 12.Allal AS, Mermillod B, Roth AD, et al. Impact of clinical and therapeutic factors on major late complications after radiotherapy with or without concomitant chemotherapy for anal carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:1099–1105. doi: 10.1016/s0360-3016(97)00390-8. [DOI] [PubMed] [Google Scholar]
- 13.Hung A, Crane C, Delclos M, et al. Cisplatin-based combined modality therapy for anal carcinoma: a wider therapeutic index. Cancer. 2003;97:1195–1202. doi: 10.1002/cncr.11161. [DOI] [PubMed] [Google Scholar]
- 14.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]