Abstract
Artemin, a member of the glial cell line-derived neurotrophic factor (GDNF) family, supports a subpopulation of trigeminal sensory neurons through activation of the Ret/GFRα3 receptor tyrosine kinase complex. In a previous study we showed that artemin is increased in inflamed skin of wildtype mice and that transgenic overexpression of artemin in skin increases TRPV1 and TRPA1 expression in dorsal root ganglia neurons. In this study we examined how transgenic overexpression of artemin in tongue epithelium affects the anatomy, gene expression and calcium handling properties of trigeminal sensory afferents. At the RNA level, trigeminal ganglia of artemin overexpresser mice (ART-OEs) had an 81% increase in GFRα3, a 190% increase in TRPV1 and a 403% increase in TRPA1 compared to wildtype (WT) controls. Myelinated and unmyelinated fibers of the lingual nerve were increased in diameter, as was the density of GFRα3 and TRPV1-positive innervation to the dorsal anterior tongue and fungiform papilla. Retrograde labeling of trigeminal afferents by WGA injection into the tip of the tongue showed an increased percentage of GFRα3, TRPV1 and isolectin B4 afferents in ART-OE mice. ART-OE afferents had larger calcium transients in response to ligands of TRPV1 (capsaicin) and TRPA1 (mustard oil). Behavioral sensitivity was also exhibited by ART-OE mice to capsaicin and mustard oil, measured using a two-choice drinking test. These results suggest a potential role for artemin-responsive GFRα3/TRPV1/TRPA1 sensory afferents in mediating sensitivity associated with tissue injury, chemical sensitivity or disease states such as burning mouth syndrome.
Keywords: sensory neuron, growth factor, pain, transgenic mice, oral cavity, GFRalpha3
INTRODUCTION
Primary sensory neurons that are responsive to noxious mechanical, thermal or chemical stimuli are dependent on neurotrophic growth factors for their embryonic survival and postnatal maturation (Hjerling-Leffler et al., 2007; Luo et al., 2007). In adult animals, the survival role of growth factors is diminished and they are instead thought to contribute to the maintenance of sensory neuron phenotype and functional properties (Reichardt, 2006). Tissue injury in the adult can lead to enhanced expression of growth factors such as the neurotrophin nerve growth factor (NGF) (McMahon et al., 2006) and the GDNF family member artemin (Malin et al., 2006). NGF and artemin signaling are mediated through binding of receptor tyrosine kinases expressed by nociceptive sensory afferents. A subpopulation of these afferents coexpress the NGF receptor trkA and the artemin receptor components, GDNF-family receptor alpha 3 (GFRα3) and Ret receptor tyrosine kinase. These GFRα3/Ret/trkA neurons are therefore responsive to both artemin and NGF (Malin et al., 2006; Orozco et al., 2001). GFRα3/Ret/trkA neurons are small to medium in diameter and comprise 20–25% of the trigeminal and dorsal root ganglion (DRG) populations (Orozco et al., 2001). Many express calcitonin gene related peptide (CGRP) and the thermal and chemically sensitive transient receptor potential channels, TRPV1 and TRPA1 (Elitt et al., 2006; Story et al., 2003). TRPV1 is a polymodal calcium-permeable cation channel that is activated by heat (> 43°C), protons, anandamide and vanilloid compounds (e.g., capsaicin, resinferatoxin) and is required for the development of hypersensitivity associated with inflammatory stimuli (Davis et al., 2000; Jordt and Ehrlich, 2007; Levine and Alessandri-Haber, 2007; Tominaga and Caterina, 2004). TRPA1 is an ionotropic channel stimulated by chemicals (mustard oil, cinnamonaldehyde), cold and cannabinoids (Bandell et al., 2004; Bautista et al., 2005; Jordt et al., 2004; Patwardhan et al., 2006; Story et al., 2003). Cultured adult DRG neurons can be sensitized to TRPV1 ligands following exposure to artemin or NGF as shown by enhanced calcium transients in response to capsaicin and reduced capsaicin-induced tachyphylaxis (Malin et al., 2006; Shu and Mendell, 2001).
Using transgenic mice that express a K14 promoter-artemin transgene (ART-OE mice), we previously showed that increased levels of artemin in the skin increase TRPV1 and TRPA1 expression in DRG neurons and behavioral sensitivity to noxious heat and cold (Elitt et al., 2006). Ex vivo analysis of cutaneous C-fibers from ART-OE mice also showed reduced heat thresholds and increased firing to an applied heat ramp. These changes in behavior and afferent physiology led us to consider whether artemin expressed in other keratinized, K14-keratin enriched structures such as the tongue would have similar changes in sensitivity. The tongue is highly innervated and routinely comes in contact with noxious thermal, chemical and mechanical stimuli. The anterior two thirds of the tongue is rich in afferents that convey sensory stimuli via the lingual nerve to cell bodies in the trigeminal ganglion. Dysfunction of these afferents is thought to underlie a variety of chronic oral pain disorders such as the oral pain disorder known as burning mouth syndrome (BMS, stomatodynia) (Grushka et al., 1987; Maltsman-Tseikhin et al., 2007). BMS typically affects middle-aged women and is characterized by burning pain localized to the tip and anterior two-thirds of the tongue. Patients with BMS may have a small fiber neuropathy that is associated with sensitization of lingual afferents (Lauria et al., 2005). Although few treatments are available for BMS, topical (Epstein and Marcoe, 1994) and systemic capsaicin (Petruzzi et al., 2004) are reported to provide symptomatic relief suggesting involvement of TRPV1 trigeminal afferents. The increased sensitivity of neurons from ART-OE mice to heat and capsaicin and overlap of TRPV1 and GFRα3 led us to test whether increased artemin alters lingual afferent phenotype and sensitization to chemical stimuli. Results show changes in lingual afferent phenotype and sensitization and support a linkage between artemin level and oral sensitivity to chemical stimuli.
RESULTS
Transcriptional changes in trigeminal ganglia of ART-OE mice
Previous studies of DRG neurons of WT and ART-OE mice have shown that artemin overexpression in skin leads to hypertrophy of GFRα3-positive afferents and a 20.5% increase in total neuron number (Elitt et al., 2006). Nearly all (~95%) GFRα3-positive afferents express the TRPV1 ion channel and many of these neurons exhibit TRPA1 immunoreactivity (Elitt et al., 2006; Orozco et al., 2001). TRPV1 and TRPA1 mRNA levels were also found to be elevated (61% and 210%, respectively) in DRG of ART-OE mice, although the percentage of TRPV1 neurons was not increase (Elitt et al., 2006). To expand our analysis of the role of artemin responsive neurons to trigeminal neurons, SYBR green RT-PCR assays were performed using RNA from trigeminal ganglia of WT and ART-OE mice (Table 1). Analysis shows ART-OE ganglia have a significant increase in TRPV1 (190%), TRPA1 (403%) and GFRα3 (81%), a decrease in TRPM8 (−70%) and no change in ret receptor, TRPV2, TRPV3 and TRPV4. Interestingly, a modest increase in TrkA mRNA (56%) occurred in ART-OE ganglia. Because trkA expression can be increased in response to elevated NGF, this raised the possibility that enhanced expression of artemin indirectly increased NGF expression in the target tissue. However, real time PCR analysis of mRNA from footpad skin and tongues of WT (n=3) and ART-OE (n=3) mice showed no difference in NGF transcript level (tongue, 0.89-fold change, p>0.05; skin, 1.08-fold change, p>0.05).
Table 1.
Change in expression of channel and receptor genes in trigeminal ganglia from WT and ART-OE mice measured using real-time PCR analysis.
| Gene assayed |
Percentage change |
|---|---|
| GFRα3 | +81%* |
| Ret | +1% |
| TrkA | +56%* |
| TRPV1 | +190%** |
| TRPV2 | +7% |
| TRPV3 | +23% |
| TRPV4 | +5% |
| TRPA1 | +403%** |
| TRPM8 | −70%* |
N = 4–6 animals per group.
P<0.005.
P≤0.0005
Phenotypic changes in trigeminal neurons resulting from artemin overexpression
A significant portion of nerve innervation to the tongue arises from neurons in the trigeminal ganglia. To examine the impact of increased artemin on trigeminal neurons, immunolabeling of the ganglia was carried out using antibodies against artemin, GFRα3 and TRPV1. Labeling for TRPA1 was not done due to the lack of a suitable antibody. We first confirmed that tongues from ART-OE mice had increased expression of artemin in the K14-expressing epithelial cells (Fig. 1) as seen in the epidermis of ART-OE mice (Elitt et al., 2006). Increased artemin reactivity was detected in transgenic tongues (Fig. 1A) compared to relatively low staining in WT tongues (Fig. 1B). As in the skin, the density of GFRα3-positive nerve fibers was also increased in ART-OE tongues (Fig. 2A,C) and many of these were TRPV1-immunopositive (Fig. 2B,D). Few TRPV1-positive fibers were visible in WT tongues in comparison to ART-OE mice where staining and overlap with GFRα3 labeling was easily visible in the subepithelial compartment. In contrast to transgenic mice that overexpress BDNF or NT4 (Krimm et al., 2001), aberrant targeting of sensory fibers did not occur in ART-OE transgenics.
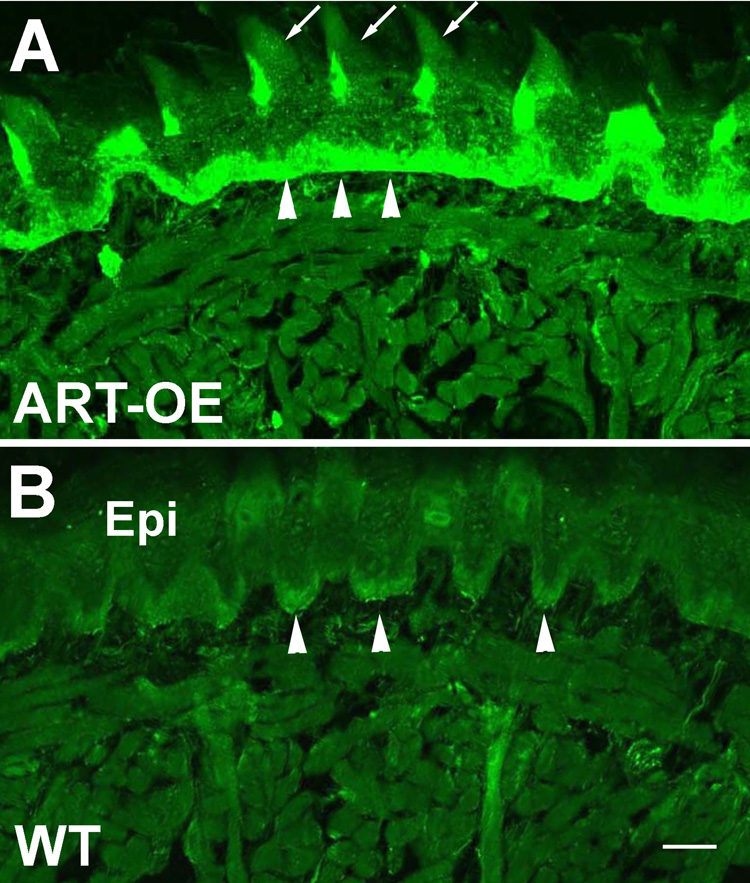
Figure 1. K14-keratin promoter drives increased expression of artemin in the tongue epithelium.
Tongue sections from ART-OE (A) and wildtype (B) mice immunolabeled using anti-artemin antibody. Fluorescent signal is localized to basal epithelial cells (arrowheads in A and B) and in the heavily keratinized filiform papillae (arrows). Epi, epithelial layer. Bar in B = 100 µm.
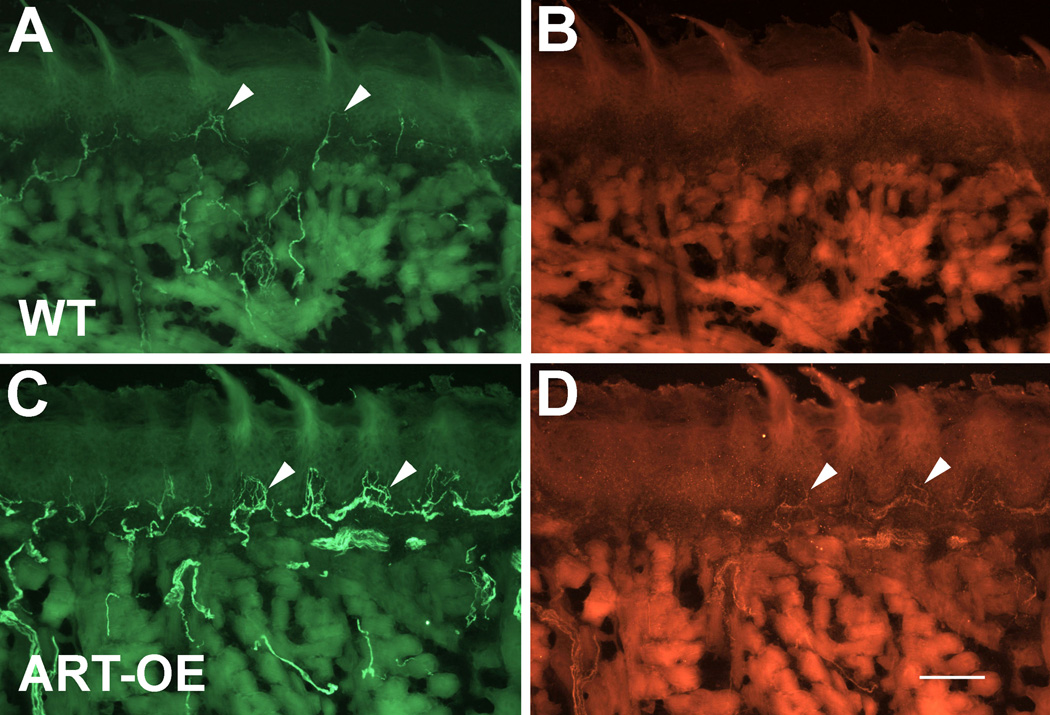
Figure 2. Tongues of ART-OE mice have increased innervation.
Wildtype (A, B) and ART-OE (C, D) sections of tongue immunolabeld with antibodies against GFRα3 (A, C) and TRPV1 (B, D). ART-OE tongues are hyperinnervated in epithelial and subepithelial compartments by GFRα3-positive fibers, some of which contain TRPV1. TRPV1-positive fibers in WT tongues were rare (none apparent in B, but see Fig. 3). Arrowheads in A, C and D indicate positive-labeling of afferents in mucosa. Bar in D = 100 µm.
Although some GFRα3-positive innervation was evident on the ventral surface of the tongue (not shown), the majority of GFRα3-positive fibers were on the dorsal side in both WT and transgenic samples. GFRα3 and TRPV1 labeling was also associated with fungiform papilla structures (Fig. 3) and an increase in labeling intensity was generally seen in ART-OE papilla. Fungiform papillae are innervated by trigeminal afferents of the lingual nerve and geniculate ganglia taste afferents of the chorda tympani nerve (Whitehead et al., 1999). This raises the possibility that some of the fungiform hyperinnervation arose from geniculate ganglia neurons. However, immunolabeling of geniculate ganglia from ART-OE and WT animals showed no significant labeling for GFRα3 and few TRPV1-positive neurons suggesting artemin expression in the tongue epithelium did not affect geniculate neurons (Fig. 4).
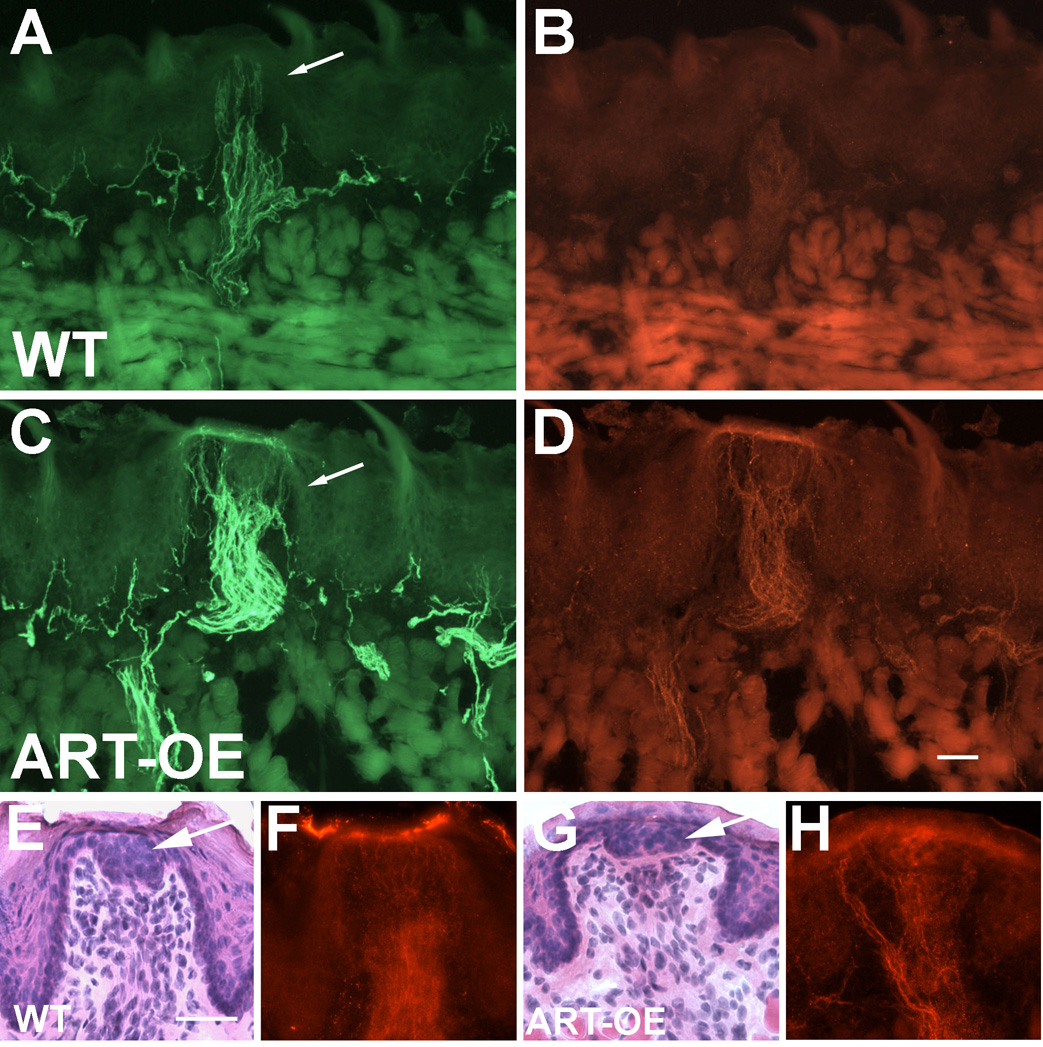
Figure 3. Fungiform papillae are innervated by GFRα3 afferents in WT and ART-OE mice.
Sections of tongue from wildtype (A, B) and ART-OE (C, D) mice show representative fungiform papilla (arrows in A and C) immunolabeled with antibodies against GFRα3 (A, C) and TRPV1 (B, D). GFRα3 (not shown) and TRPV1-fiber labeling to fungiform papillae of ART-OEs (D, H) was generally greater compared to wildtype (B, F) structures. Some fibers appeared associated with the single taste bud structure of the papilla made visible using hemotoxylin and eosin staining (E, G). H&E staining of WT (E) and ART-OE (G) sections was done on sections shown in F and H, respectively. Arrows in E and G show taste bud. Bar in D = 50 µm; bar in E = 40µm.
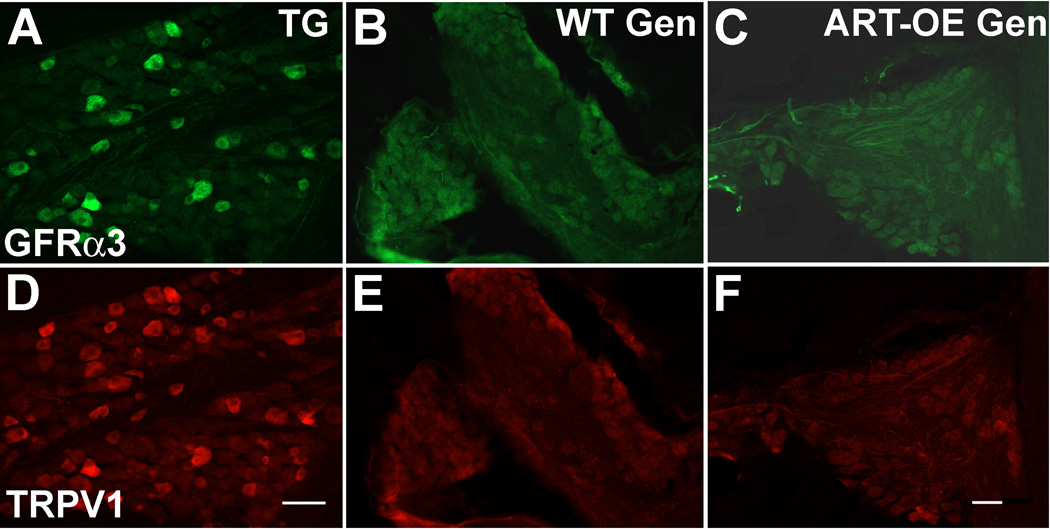
Figure 4. Few geniculate ganglia neurons are immunopositive for GFRα3 or TRPV1.
Immunolabeling for GFRα3 (A-C) and TRPV1 (D-F) on sections of trigeminal (TG, A, D) and geniculate (Gen, B, C, E and F) ganglia. Trigeminal ganglia (from ART-OE mice) were labeled in parallel with geniculate ganglia and serve as a positive control for staining. Few if any geniculate neurons were GFRα3-positive in WT (B) and ART-OE (C) mice. TRPV1 reactivity was evident in many neurons of the trigeminal ganglia (D) but few neurons labeled in geniculate ganglia of WT (E) and transgenic (F) mice. Scale bar in D for A, D = 100 µm; bar in F is for B, C, E and F and = 60µm.
Characterization of backlabeled lingual neurons
To further characterize properties of tongue afferents, neurons that innervate the tip of the anterior tongue were retrogradely-labeled by injection of wheat germ agglutinin (WGA). Immunolabeling of backlabeled neurons in the trigeminal ganglia was then performed for GFRα3, TRPV1, IB4, and CGRP (Fig. 5). WGA injections produced robust labeling primarily on the ventral surface near the exit of the mandibular nerve, similar to areas described in ferrets (Biggs et al., 2007). In WT ganglia approximately 25% of the labeled lingual somata were TRPV1-positive (Fig. 5B), which is similar to the percentage (27.8%) of TRPV1-positive cutaneous neurons (Christianson et al., 2006). However, in contrast to cutaneous afferents, where many (70%) are IB4-positive (Christianson et al., 2006; Lu et al., 2001), very few (10%) lingual neurons bound IB4 lectin and many (50%) expressed CGRP (Fig. 5B). Nearly 25% of labeled lingual somata were GFRα3-positive (Fig. 5B). Thus, lingual neuron properties differ from cutaneous neurons, which are primarily IB4 positive and CGRP negative.
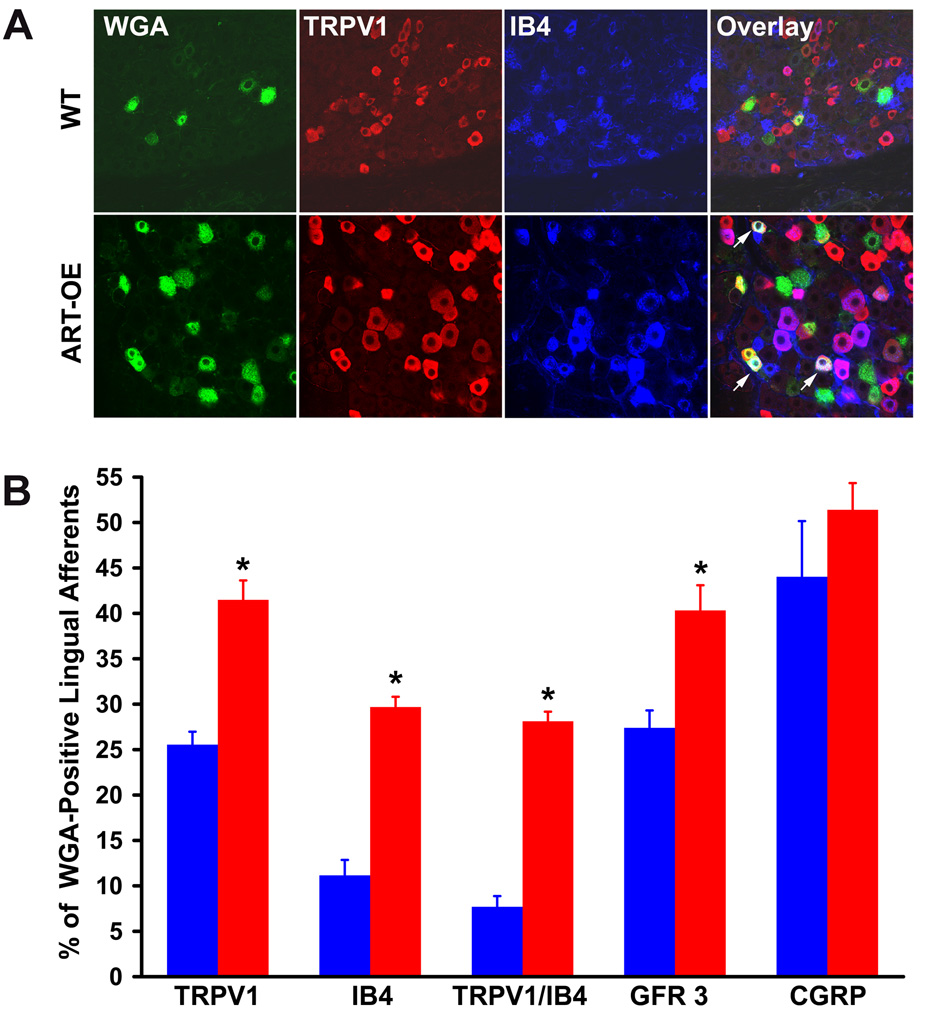
Figure 5. The percentage of lingual neurons that express GFRα3, TRPV1 and IB4 is increased in ART-OE mice.
A. Representative photomicrographs of WGA-labeled lingual-projecting somata from WT (top) and ART-OE (bottom) mice immunolabeled for TRPV1 (red), IB4 (blue) and TRPV1/IB4 overlap. Neurons in ART-OE ganglia exhibited somal hypertrophy and an increased percentage of neurons expressing TRPV1, IB4 and TRPV1/IB4 (arrows, overlap panel). B. Quantification of the percentage of WGA-positive lingual somata that express GFRα3 and TRPV1, IB4 or CGRP. ART-OE ganglia (red bars) had an increased percentage of WGA-positive neurons that co-expressed GFRα3, TRPV1, IB4, or both TRPV1 and IB4 compared to WT mice (blue bars). No significant change in ART-OE WGA-positive neurons that express CGRP occurred. The mean percentage ± SEM is plotted. Analysis was done with n=4 WT and 4 ART-OE mice. Asterisk indicates p<0.05 vs. WT.
In ART-OE mice, an increase occurred in the percent of WGA-positive afferents that were TRPV1-positive (1.62-fold; p≤0.005), IB4-positive (2.66-fold; p≤0.005), both TRPV1 and IB4-positive (3.65-fold; p≤0.005) or GFRα3-positive (1.47-fold; p≤0.005) (Fig. 5B). There was no change in the percentage of WGA-positive neurons that expressed CGRP (Fig. 5B).
Effects of artemin on lingual nerve anatomy
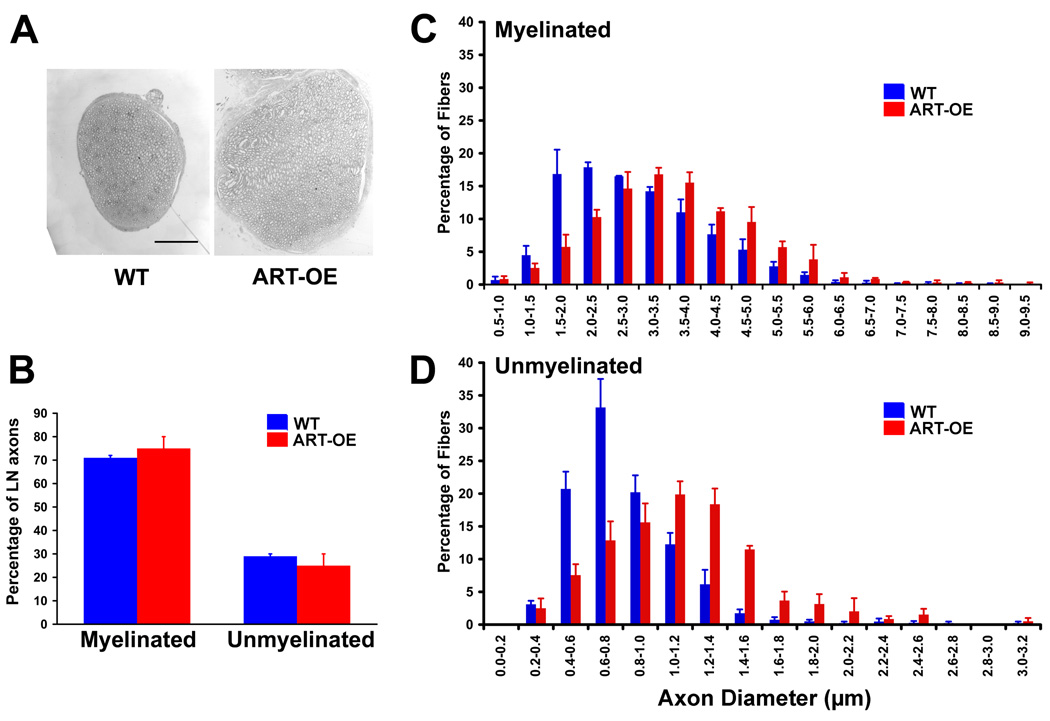
The lingual nerve is comprised of myelinated (Aβ and Aδ) and unmyelinated fibers (Holland and Robinson, 1992). Aβ lingual afferents are mechanoreceptive and unresponsive to thermal or chemical stimuli (Holland and Robinson, 1992; Wang et al., 1993) whereas Aδ fibers are heterogeneous in their response properties and can respond to mechanical, thermal and chemical stimuli (Wang et al., 1993). To determine if increased artemin in the tongue altered lingual nerve anatomy, WT (n=3) and ART-OE (n=3) lingual nerves were examined at the electron microscopic level. A significant hypertrophy of the lingual nerve was evident in ART-OE mice (Fig. 6A). There was no difference in the percentage of myelinated (WT 71 ± 1%, ART-OE 75 ± 5%) or unmyelinated fibers (WT 29 ± 1%, ART-OE 25 ± 5%) (Fig. 6B) although a significant hypertrophy of both myelinated and unmyelinated axon diameters was measured (Fig. 6C, D).
Figure 6. Artemin expression causes hypertrophy of lingual nerve fibers.
A. Low magnification electron microscopy image comparing representative lingual nerve from WT (left) and ART-OE (right) mice demonstrates enlarged nerve diameter in ART-OE mice. B. No difference in the percentage of myelinated and unmyelinated axons in the lingual nerve of WT (blue, n=3) and ART-OE (red, n=3) mice was detected. Rightward shift in distribution of axons in diameter (microns) in ART-OE mice indicates hypertrophy of myelinated (C) and unmyelinated (D) axons in transgenic nerves (p<0.002 and p<0.009, two-way ANOVA). Bar in A = 50µm.
ART-OE mice exhibit oral sensitivity to capsaicin and mustard oil
Because the tongue is an anatomical site that routinely contacts stimuli that activate TRPV1 (capsaicin, heat, low pH) and TRPA1 (mustard oil, cold), we tested whether ART-OE mice had functional consequences related to the anatomical and neurochemical changes. A two bottle drinking aversion test was used in which two bottles, one that contained water with vehicle (0.5% ethanol) and the other containing water plus ligand (capsaicin (CAP) or mustard oil (MO)) in vehicle, were available to separately housed mice. Mice were tested over three days and separate groups of male and female WT and ART-OE mice (10 per group; 40 mice total) were used to assess response to capsaicin or mustard oil (Fig. 7). Although water (containing 0.5% ethanol) consumption was the same across sex and strain (Fig. 7E, F), male and female ART-OE mice drank significantly smaller volumes of water containing capsaicin or mustard oil (Fig. 7A, B). Ligand sensitivity was also evident when volumes were calculated as a percentage of total water consumed (Fig. 7C, D) with the exception of male ART-OE mice. As a percentage, male ART-OE mice did not drink significantly less MO water than male WT mice. This seems to have occurred because these mice reduced their total fluid consumption although females did not. Thus, male ART-OE mice appear to be more sensitive to mustard oil as reflected by the decrease in total drinking behavior.
Figure 7. ART-OE mice display oral sensitivity to capsaicin and mustard oil.
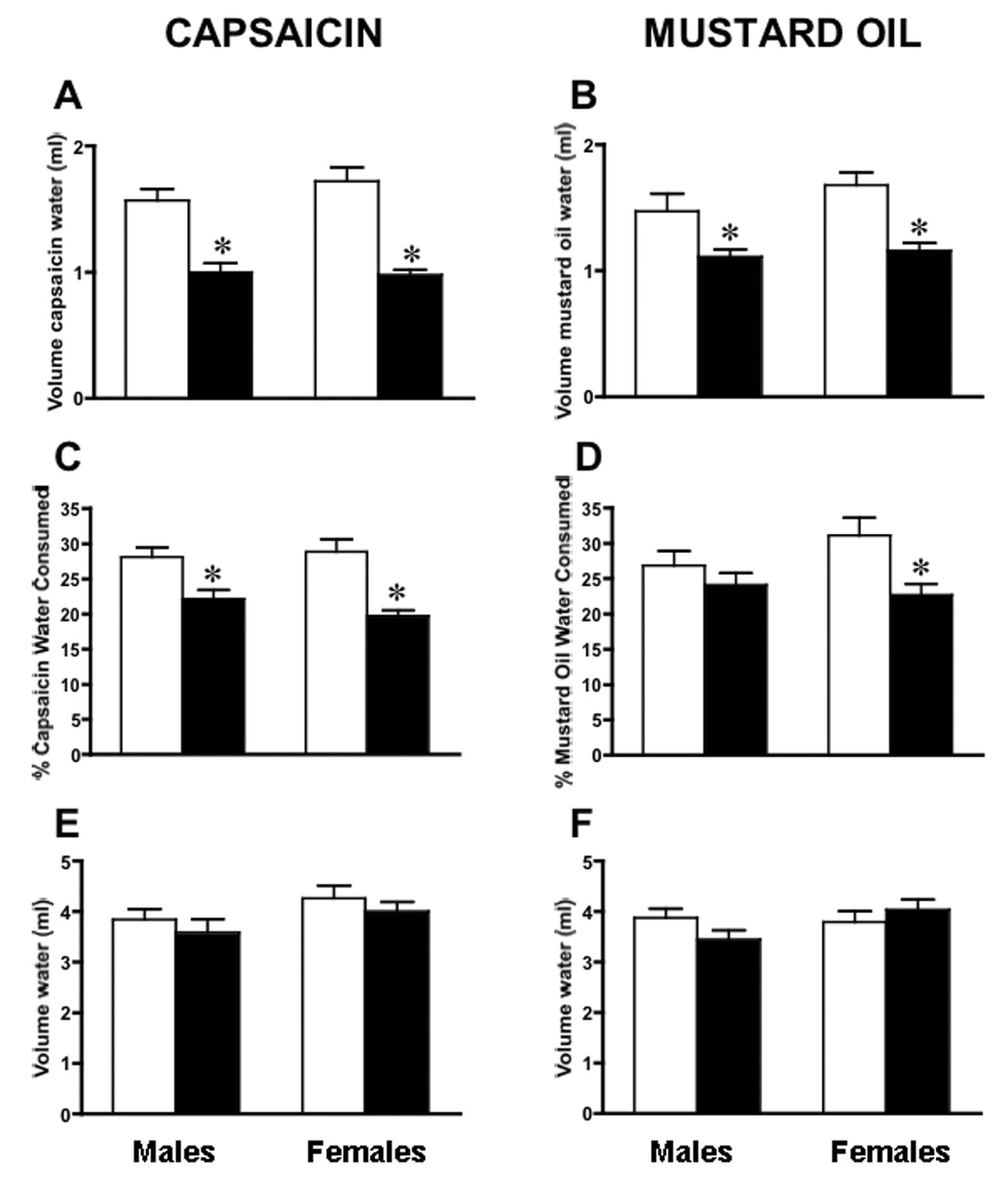
Ten WT (white bars) and 10 ART-OE (black bars) mice of both sex (40 mice total) were tested for oral sensitivity to capsaicin and mustard oil using a two-bottle drinking aversion assay. A. Male and female ART-OE mice drank less capsaicin containing water compared to WT mice (males WT 1.57 ± 0.09 ml; ART-OE 1.00 ± 0.07 ml, p≤0.005: females WT 1.72 ± 0.11 ml; ART-OE 0.98 ± 0.04, p≤0.005). B. Both male and female ART-OE mice drank less mustard oil containing water (males WT 1.47 ± 0.14 ml; ART-OE 1.11 ± 0.06 ml, p≤0.05: females WT 1.68 ± 0.10 ml; ART-OE 1.16 ± 0.06, p≤0.005). C. Same data as panel A but calculated as a percentage of total water consumed (CAP - males WT 29.08 ± 1.37 %; ART-OE 22.16 ± 1.26%, p≤0.005; females WT 28.87 ± 1.90 %; ART-OE 19.74 ± 0.79 %, p≤0.005). D. Calculated as a percentage, female ART-OE mice drank less mustard oil containing water (MO - females: WT 31.15 ± 2.47 %, ART-OE 22.69 ± 1.58 %; p≤0.005). Male ART-OE mice did not drink significantly less mustard oil containing water when calculated as a percentage of total water (see text). E and F. The amount of water (containing 0.5% ethanol) consumed across genotype and sex was unchanged. However, the total amount of water consumed by ART-OE males tested for mustard oil sensitivity was less than WT males (males WT 5.29 ± 0.18 ml, ART-OE 4.52 ± 0.20 ml, p≤0.005). This was not the case for female mice (WT 5.47 ± 0.16 ml; ART-OE 5.19 ± 0.17 ml, p>0.05). Mean ± SEM. Asterisk indicates p<0.05 vs WT.
Calcium handling in response to CAP or MO is enhanced in trigeminal afferents of ART-OE mice
To examine whether increased artemin modulated the functional properties of isolated trigeminal neurons, we measured the response of neurons from WT and ART-OE mice to CAP or MO using fura-2 calcium imaging (Table 2, Table 3). ART-OE mice had a greater number of MO responsive trigeminal neurons compared to WT mice. In addition, calcium transients of ART-OE neurons exposed to MO were of larger magnitude (Farea) compared to WT neurons. These findings indicate a greater number of MO responsive cells that exhibit increased MO-evoked calcium transients are present in ART-OE trigeminal ganglia.
Table 2.
Area and peak measures of calcium transients elicited by brief application of either 100µm mustard oil or 1µM capsaicin to cultured trigeminal neurons from ART-OE and WT mice. Because the percent of lingual (WGA backlabeled) neuron responders is a ratio obtained by dividing the number of responsive cells by the total number of imaged cells, no error is calculated. Trigeminal neuron responders and area and peak data are shown as the mean ± SEM of values.
| % Mustard Oil responders |
F (area) | F (peak) | |
|---|---|---|---|
| Trigeminal | |||
| WT | 28 ± 8 | 12.24±2.06 | 0.31±0.05 |
| ART-OE | 42 ± 2* | 32.67±6.01* | 0.36±0.04 |
| Lingual only | |||
| WT | 35 | 53.25±14.05 | 0.62±0.07 |
| ART-OE | 42 | 76.49±16.16 | 0.63±0.06 |
| % Capsaicin responders | F (area) | F (peak) | |
| Trigeminal | |||
| WT | 41 ± 3 | 53.87± 8.80 | 0.72±0.07 |
| ART-OE | 48 ± 6 | 78.67 ± 10* | 0.70±0.06 |
| Lingual only | |||
| WT | 43 | 99.58±26.82 | 0.52±0.11 |
| ART-OE | 50 | 126.35±14.02 | 0.73±0.05* |
Asterisks indicate p≤0.05.
Table 3.
Summation of cells that were tested and responded to CAP or MO treatment. Not all responder cells were used for area and peak analysis (see Table 2) because they could not be analyzed, i.e., they exhibited an abnormal return to baseline.
| TRIGEMINAL NEURONS | ||||
|---|---|---|---|---|
| CAP | Number of cells tested |
Number of responders |
Number of mice (N) |
# of responders used for magnitude analysis |
| WT | 152 | 65 | 10 | 46 |
| ART-OE | 196 | 85 | 11 | 64 |
| MO | ||||
| WT | 109 | 31 | 5 | 26 |
| ART-OE | 126 | 53 | 6 | 46 |
| LINGUAL NEURONS | ||||
| CAP | ||||
| WT | 69 | 30 | 12 | 13 |
| ART-OE | 116 | 58 | 17 | 29 |
| MO | ||||
| WT | 60 | 21 | 8 | 13 |
| ART-OE | 97 | 41 | 11 | 28 |
In contrast to MO, comparison of the percentage of trigeminal neurons responsive to CAP application was not significantly different between ART-OE (n=11) and WT (n=10) mice) (Table 2). However, CAP responsive trigeminal neurons from ART-OE mice had larger Farea than WT mice. These results suggest that trigeminal ganglia from ART-OE mice do not have more CAP-responsive neurons but that the CAP-responsive neurons that are present exhibit larger calcium transients. This finding is consistent with previous analysis that showed an increase in TRPV1 mRNA in ART-OE dorsal root ganglia but no change in the percentage of TRPV1-positive neurons (Elitt et al., 2006).
To refine the calcium imaging analysis, lingual afferents labeled by injecting WGA conjugated to AlexaFluor 488 into the dorsal tip of the tongue were analyzed (Table 2). Analysis of WGA-labeled lingual afferents from WT (n=12) and ART-OE (n=17) mice showed no difference in the percentage of CAP (WT 43%, ART-OE 50%; WT responsive cells 30; ART-OE responsive cells 58). Similarly, the number of MO responsive cells (WT n=8, ART-OE n=11) was unchanged (WT 35%, ART-OE 42%; WT responsive cells 21; ART-OE responsive cells 41). The magnitude of response to CAP (Area: WT 99.58 ± 26.82; ART-OE 126.35 ±14.02; p>0.05) or MO application (Area: WT 53.25 ± 14.05; ART-OE 76.49 ± 16.16; p>0.05) in WGA labeled afferents was also not significantly changed.
Interestingly, the only significant change in WGA-labeled lingual afferent response properties was indicated by an increase in the peak response (Fpeak) of lingual afferents exposed to CAP suggesting a particular sensitivity to this ligand. In addition, for both genotypes, the total magnitude (Farea) of lingual afferent response to CAP or MO was greater when compared to the whole population of trigeminal afferents (Table 2), suggesting a specialized sensitivity exists for tongue afferents for detection of these chemicals.
DISCUSSION
Increased expression of artemin in keratinized epithelium of the tongue caused a significant alteration in anatomical and physiological properties of trigeminal afferents that innervate the tongue. These changes include hypertrophy of myelinated and unmyelinated fibers of the lingual nerve, increased density of GFRα3 and TRPV1 positive afferents in sub-epithelial and epithelial compartments of the tongue and to fungiform taste structures on the dorsal tongue. The hypertrophy of the lingual nerve and increased innervation density is likely a reflection of the 20% increase in GFRα3-positive neuron survival (Elitt et al., 2006) as well as an increase in branching of GFRα3 afferents in the tongue.
Previous studies of ART-OE mice showed nearly all GFRα3-neurons (97%) to be immunopositive for TRPV1 and TRPA1 channel proteins (Elitt et al., 2006). In the present study, WGA-labeled tongue afferents showed an expected increase in the percentage of GFRα3 and TRPV1-positive neurons in ART-OE mice. Interestingly, an increase also occurred in the percentage of WGA-afferents that coexpressed TRPV1 and IB4. Colocalization of TRPV1 and IB4 is rare in mouse with very few (2–5%) afferents expressing both markers (Zwick et al., 2002). In addition, only 30% of the GFRα3-positive neurons bind IB4 (Orozco et al., 2001). The functional significance of the increase in GFRα3/TRPV1/IB4 overlap is yet to be determined but suggests artemin may regulate expression of the extracellular matrix protein versican, which binds IB4 (Bogen et al., 2005).
An increase in transcripts encoding TRPV1, TRPA1 and GFRα3 was measured in ART-OE trigeminal ganglia. This increase is consistent with coexpression of these markers in the GFRα3-neuron population, the increased density of GFRα3/TRPV1 afferents in the tongue and the aversive behaviors displayed by ART-OE mice to capsaicin and mustard oil. This aversive behavior suggests that GFRα3/TRPV1 afferents in the oral mucosa have a role in the sensitization caused by chemical or thermal stimuli (Szolcsanyi, 1990). The relevance of GFRα3-positive afferents in human oral pathologies is still to be investigated, although both mustard oil and capsaicin elicit a burning sensation when either is applied to the tip of the tongue of human subjects (Green and Schullery, 2003; Simons et al., 2003), suggesting TRPV1/TRPA1 afferents are present.
Although the drinking aversion exhibited by ART-OE mice likely reflects nociceptive input via lingual afferents innervating the tongue surface, sensory input from fibers associated with taste and olfaction may also have influenced consumption. GFRα3-positive fibers were present at high density in fungiform taste structures of WT animals and at a greater density in ART-OE mice. Taste bud cells of zebrafish have also been shown to express artemin (Lucini et al., 2004) and a low-level of artemin expression in tongue epithelium of WT mice is present (this report). Because it is possible that artemin release by non-neuronal cells acts in a paracrine manner to modulate taste-specific afferents, the contribution of geniculate ganglia neurons that also innervate taste buds in fungiform papilla to the increased CAP and MO aversion in ART-OE mice must be considered. A recent study showed TRPV1 mRNA and TRPA1 mRNA expression in geniculate ganglion neurons in rats (Katsura et al., 2006), in contrast to an earlier study in mouse that showed TRPV1 mRNA was not expressed in geniculate neurons (Matsumoto et al., 2001). Our immunolabeling results indicate mouse geniculate neurons do not express GFRα3 and only few of these neurons are TRPV1-positive. Thus, the increased taste aversion displayed by ART-OE transgenics is likely to be primarily generated by trigeminal input.
What is less clear is whether olfactory perception of CAP or MO was sensitized in ART-OE mice. The K14 transgene is expressed in cells of the ventral meatus and vomeronasal organ cells (Takami et al., 1995), making it possible that transgenic artemin expression modified trigeminal sensory innervation to these regions. Both CAP and MO have chemesthetic properties and afferents in the ethmoid branch of the trigeminal nerve in rats respond to TRPV1 ligands (Silver et al., 2006).
RT-PCR analysis showed an increase in the relative amount of trkA mRNA in ART-OE ganglia. To assess if this increase in receptor resulted from an artemin-induced increase in NGF in target tissues, we measured the relative level of NGF mRNA in the skin and tongue of ART-OE mice (see Results). No change in NGF expression occurred suggesting that artemin signaling led to the increase in trkA expression. In vitro assays using cultured primary DRG neurons also showed that artemin (100ng/ml) in the culture medium increased trkA mRNA to a level nearly equivalent to the level seen following NGF addition (data not shown), suggesting artemin indirectly modulates transcriptional control of trkA gene expression. This may occur through artemin activation of Ret, in a manner similar to that reported in a study that showed a GDNF evoked increase in trkA in neuroblastoma cells (Peterson and Bogenmann, 2004).
The increase in TRP channel expression in ART-OE mice may provide insight into the role of artemin and TRPs in injury and disease. In skin of WT mice, artemin expression rises significantly (~10-fold) following adjuvant-induced inflammation. Artemin injection into the foot also caused heat hyperalgesia lasting up to 24h (Malin et al., 2006). The duration of hyperalgesia exceeded 7 days if artemin was coinjected with NGF suggesting synergistic ligand action. Concurrent with the rise of artemin in the periphery was an increase in TRPV1 mRNA in lumbar DRG. These coordinated changes suggest that injury or disease in peripheral tissues may increase artemin expression and in so doing, increase GFRα3/Ret kinase activity. Future studies that examine whether injury to oral epithelium affects artemin and TRP expression and sensitization of GFRα3 afferents will address this possible mechanism.
An interesting pain-associated pathology that is related to changes in sensory innervation to the tongue is burning mouth syndrome (BMS). Biopsies from BMS patients show a reduction in the number of unmyelinated fibers associated with taste papilla (Lauria et al., 2005) and another a reduction in the number of intraepithelial fibers (Yilmaz et al., 2007). Interestingly, although the overall epithelial nerve fiber density was decreased in the study by Yilmaz and colleagues, a significant increase in TRPV1 and NGF-positive fibers and NGF reactivity in basal epithelial cells was found. In addition, pain scores reported by patients in response to applied capsaicin were increased (Yilmaz et al., 2007). These findings are consistent with a role for GFRα3/TRPV1, NGF and artemin responsive fibers as possible contributors to the underlying pathology of BMS.
EXPERIMENTAL PROCEDURE
Animals
Isolation and characterization of transgenic mice that overexpress the coding region of the artemin gene in basal keratinocytes of the epidermis and tongue are described in Elitt et al., 2006. Experiments were performed on 12–16 week-old male and female wildtype and ART-OE mice that were housed in group cages, maintained on a 12:12 hour light-dark cycle in a temperature controlled environment (20.5°C) and given food and water ad libitum. These studies were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the NIH Guide for the Care and Use of Laboratory Animals.
Surgical procedure
Animals were lightly anesthetized with inhaled isoflurane and deeply anesthetized with an intraperitoneal injection of 2.5% avertin (2,2,2-tribromoethanol tert-amyl alcohol diluted in 0.9% saline). The tongue was extracted slightly from the mouth and approximately 1µl of 2% wheat germ agglutinin conjugated to AlexaFluor 488 (WGA-488)(Molecular Probes, Eugene, OR) in sterile saline was injected into the superficial dorsal epithelium at the tip of the tongue using a glass micropipette. Injections were performed bilaterally. The injection sites were carefully washed with saline and dried with a cotton swab to prevent leakage to surrounding tissues. Mice were allowed to recover for two days after which ganglia were removed and processed for immunocytochemistry or cell culture.
Tissue preparation and immunocytochemistry
Animals were deeply anesthetized as described above, transcardially perfused with ice-cold 4% paraformaldehyde in 0.1M phosphate buffer (PB), pH 7.4 and trigeminal ganglia and tongues immediately collected (n=4 WT and 4 ART-OE mice). Other sets of animals (n=3 WT and 3 ART-OEs) were used to immunolabel trigeminal and geniculate ganglia. Ganglia were embedded in 10% gelatin, post-fixed for 1hr in 4% paraformaldehyde, cryoprotected in 25% sucrose at 4°C overnight and then sectioned at 35µm using a sliding microtome. Tongues were embedded in Optimal Tissue Compound (Thermo Fisher Inc) and 20µm sections collected using a cryostat. Sections were washed 3X’s in PB and incubated overnight in primary antibodies diluted in PB containing 0.25% triton X-100 (GFRα3 (1:40) R&D Systems, Minneapolis, MN; TRPV1 (1:500) Oncogene Research, San Diego, CA) or the lectin IB4 conjugated to Alexa Fluor 568 (1:200; Molecular Probes; Eugene, OR). Antibody binding was detected using fluorescent conjugated IgGs used at 1:200–1:1000 dilutions (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Retrogradely-labeled afferents were visualized using a Leica confocal microscope (Leica Microsystems; Wetzlar, Germany). On average, 75 WGA-positive neurons per animal were analyzed at 40X in at least 4 non-overlapping fields from 4 sections (spaced at least 175µM apart). Since cell bodies in ART-OE mice are hypertrophied, only neurons containing a visible nucleus were analyzed to minimize bias from this hypertrophy.
Lingual nerve analysis
Lingual nerve segments were removed, post-fixed 2h in 4% paraformaldehyde and 2% glutaraldehyde, washed in 0.1 M phosphate buffer, immersed in osmium tetraoxide for 90 min at 4°C, deh ydrated in graded ethanols, embedded in Spurr’s resin (EM Corporation) and cut at 0.7–0.8 nm using a ultramicrotome (Reichert Ultracut E). Sections were stained with lead citrate and uranyl acetate and photographed on an electron microscope. Between 250–500 axons were analyzed per animal with the number of myelinated and unmyelinated axons determined from 3–4 cross-sectional images of nerves from 3 WT and 3 ART-OE mice. Axon diameters were measured using NIH Image J software.
SYBR green real time PCR
Total RNA was isolated from trigeminal ganglia as previously described in Elitt et al., 2006. Tissue was homogenized in Trizol (Invitrogen) and RNA in the aqueous phase precipitated using isopropanol. RNA was treated with DNase and 1 µg reverse transcribed using Superscript (Invitrogen). PCR amplification was performed using an Applied Biosystems 5700 real-time thermal cycler. Relative fold change in transcript level was calculated using the ddCt method (Livak and Schmittgen, 2001) using GADPH as a reference standard since its expression (i.e., Ct value) was unchanged in ART-OE ganglia or epithelium. Primers were designed using ABI software and are reported in (Elitt et al., 2006).
Drinking behavior
Mice were tested for oral sensitivity to capsaicin or mustard oil using a modified paired-preference drinking aversion paradigm (Furuse et al., 2002; Simons et al., 2001). A total of 40 mice were used for these experiments (10 WT males, 10 WT females, 10 ART-OE males, 10 ART-OE females). Mice were housed in cages individually and given food and water ad libitum. Each cage was fitted with one bottle that contained normal water plus vehicle (0.5% ethanol) and another bottle that contained 1µM capsaicin or 100µM mustard oil (Sigma-Aldrich, St. Louis, MO). Mice were allowed to drink freely from the two bottles for 24h and then the volume consumed was measured. To ensure that there was not a place preference, the bottle positions were reversed at the end of each day. The average volume consumed over three days of testing and the ratio of capsaicin or mustard oil intake versus total liquid intake (percentage) was calculated. To ensure that there was not a difference in the total volume of liquid consumed by ART-OE mice compared to WT mice, mice were also tested using the same paradigm with both bottles filled with normal water. All mice drank the same volume of water and did not display a bottle preference when both bottles contained normal water. Within group differences between water with vehicle and water with capsaicin or mustard oil were determined using unpaired t-tests.
Calcium imaging of identified lingual afferents
Lingual afferents from male and female mice were backlabeled as described above in Surgical Procedures. Two days following injection, mice were perfused with ice cold Ca+2/Mg+2-free Hanks’ Balanced Salt Solution (HBSS) (Invitrogen) and trigeminal ganglia were dissected and prepared for culture as described (Malin et al., 2007). Dissociated cells were resuspended in F12 media (Invitrogen) containing 10% FBS and penicilin/streptomycin (50 units/ml) and plated onto laminin (0.1mg/mL) and poly-d-lysine (5mg) coated glass coverslips, incubated for 2h at 37°C, fed F12/FBS media and incub ated overnight. Calcium imaging was performed 12–24h post plating. Cells were incubated in HBSS containing 10mg/ml bovine serum albumin (Sigma #A6003) and 2µM of the acetoxymethyl ester of fura-2 (Molecular Probes, Oregon) for 30 min at 37°C. Coverslips were mounted on an Olympus upright microscope stage with HBSS buffer flowing at 5 ml/min. Perfusion rate was controlled with a gravity flow system (VC66; Warner Instruments) and perfusate temperature maintained at 30°C using a heated stage and an in-line heating system (PH1, SHM-6, TC344B; Warner Instruments). Chemicals were delivered with a rapid-switching local perfusion system. Firmly attached, WGA-backlabeled neurons were identified using a 480nm filter and chosen as regions of interest using Simple PCI software (Compix Imaging Systems, Sewickley, PA). Unlabeled, adjacent cells were also identified and imaged. All fields were first tested with brief application of 50mM K+ (high K+) and Ca++ transients imaged to standardize pipette placement and to insure that cells were healthy and responsive. Responses were measured as the ratio of absorbance at 340nm to that obtained at 380nm (ΔF340/380; DG4, Sutter Instruments). Peak responses were > 0.08 ΔF340/380 and were easily distinguished from optical noise (< 0.02 ΔF340/380). Absorbance data at 340 and 380 nm were collected at one per second. Calcium transients were examined in response to local application of either 1µM capsaicin (5–6sec) or 100µM mustard oil (7–10sec) using a rapid-switching perfusion system (Warner Instruments). These doses of capsaicin and mustard oil were chosen based on previous studies in our laboratory showing they elicit responses (>0.08 ΔF340/380) from the maximal number of cells and could be applied repeatedly without significant loss in the number of responding cells. These concentrations are also within the range reported in the literature for calcium imaging in mouse trigeminal neurons.
The number of capsaicin or mustard oil responsive cells was determined as a percentage of total healthy cells (cells that responded to high K+). Ca++ response peak and area (Fpeak, Farea) were calculated using Microsoft Excel for suprathreshold responses (peak response > 0.08 ΔF340/380) as a measure of total Ca++ influx. The portion of the calcium response used for this measurement included the entire curve from the initiation of the response until the point at which the calcium signal returned to the prestimulus baseline. Response parameters were compared for significance using Student’s t test. 10mM capsaicin in 1-methyl-2-pyrrolidinone was used as a stock solution; 1.0 µM capsaicin was made fresh daily in HBSS. 100mM mustard oil in 1-methyl-2-pyrrolidinone was made fresh daily and diluted to 100µM using HBSS.
ACKNOWLEDGEMENTS
We gratefully acknowledge helpful advice from Drs. Michael Gold and Savanh Chanthaphavong and technical assistance from Christopher Sullivan and Mohammed Abdelwahab. This work was supported by grants from the National institute of Neurological Disorders and Stroke (R01 NS33730) and the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK063922).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, Robinson PP. Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. Eur J Pain. 2007;11:192–201. doi: 10.1016/j.ejpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Bogen O, Dreger M, Gillen C, Schroder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. Febs J. 2005;272:1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol. 1994;77:135–140. doi: 10.1016/0030-4220(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Furuse T, Blizard DA, Moriwaki K, Miura Y, Yagasaki K, Shiroishi T, Koide T. Genetic diversity underlying capsaicin intake in the Mishima battery of mouse strains. Brain Res Bull. 2002;57:49–55. doi: 10.1016/s0361-9230(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Green BG, Schullery MT. Stimulation of bitterness by capsaicin and menthol: differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem Senses. 2003;28:45–55. doi: 10.1093/chemse/28.1.45. [DOI] [PubMed] [Google Scholar]
- Grushka M, Sessle BJ, Howley TP. Psychophysical assessment of tactile, pain and thermal sensory functions in burning mouth syndrome. Pain. 1987;28:169–184. doi: 10.1016/0304-3959(87)90114-X. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland GR, Robinson PP. Axon populations in cat lingual and chorda tympani nerves. J Dent Res. 1992;71:1468–1472. doi: 10.1177/00220345920710080201. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Ehrlich BE. TRP channels in disease. Subcell Biochem. 2007;45:253–271. doi: 10.1007/978-1-4020-6191-2_9. [DOI] [PubMed] [Google Scholar]
- Katsura H, Tsuzuki K, Noguchi K, Sakagami M. Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem Senses. 2006;31:681–688. doi: 10.1093/chemse/bjl009. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, Sapelli P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115:332–337. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res. 2001;41:355–363. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- Lucini C, Maruccio L, Tafuri S, Staiano N, Castaldo L. Artemin-like immunoreactivity in the zebrafish, Danio rerio. Anat Embryol (Berl) 2004;208:403–410. doi: 10.1007/s00429-004-0409-y. [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Maltsman-Tseikhin A, Moricca P, Niv D. Burning mouth syndrome: will better understanding yield better management? Pain Pract. 2007;7:151–162. doi: 10.1111/j.1533-2500.2007.00124.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Emori Y, Ninomiya Y, Abe K. A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Res Mol Brain Res. 2001;93:105–112. doi: 10.1016/s0169-328x(01)00129-2. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Bennett DLH, Bevan S. Inflammatory mediators and modulators of pain. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Vol. 5th. Elsevier; 2006. pp. 49–72. [Google Scholar]
- Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S, Bogenmann E. The RET and TRKA pathways collaborate to regulate neuroblastoma differentiation. Oncogene. 2004;23:213–225. doi: 10.1038/sj.onc.1206980. [DOI] [PubMed] [Google Scholar]
- Petruzzi M, Lauritano D, De Benedittis M, Baldoni M, Serpico R. Systemic capsaicin for burning mouth syndrome: short-term results of a pilot study. J Oral Pathol Med. 2004;33:111–114. doi: 10.1111/j.1600-0714.2004.0194n.x. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Silver WL, Clapp TR, Stone LM, Kinnamon SC. TRPV1 receptors and nasal trigeminal chemesthesis. Chem Senses. 2006;31:807–812. doi: 10.1093/chemse/bjl022. [DOI] [PubMed] [Google Scholar]
- Simons CT, Dessirier JM, Jinks SL, Carstens E. An animal model to assess aversion to intra-oral capsaicin: increased threshold in mice lacking substance p. Chem Senses. 2001;26:491–497. doi: 10.1093/chemse/26.5.491. [DOI] [PubMed] [Google Scholar]
- Simons CT, Carstens MI, Carstens E. Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28:459–465. doi: 10.1093/chemse/28.6.459. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Capsaiin, irritation, and desensitization: neurophysiological basis and future perspectives. In: Green B, Mason J, Kare M, editors. Chemical Senses. Vol. New York: Dekker; 1990. pp. 141–168. [Google Scholar]
- Takami S, Getchell ML, Yamagishi M, Albers KM, Getchell TV. Enhanced extrinsic innervation of nasal and oral chemosensory mucosae in keratin 14-NGF transgenic mice. Cell Tissue Res. 1995;282:481–491. doi: 10.1007/BF00318880. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J Gen Physiol. 1993;101:843–866. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC, Ganchrow JR, Ganchrow D, Yao B. Organization of geniculate and trigeminal ganglion cells innervating single fungiform taste papillae: a study with tetramethylrhodamine dextran amine labeling. Neuroscience. 1999;93:931–941. doi: 10.1016/s0306-4522(99)00115-3. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, Anand P. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14:864–871. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]