Summary
During mitosis, the motor molecule cytoplasmic dynein plays key direct and indirect roles in organizing microtubules (MTs) into a functional spindle. At this time, dynein is also recruited to kinetochores, but its role or roles at these organelles remain vague, partly because inhibiting dynein globally disrupts spindle assembly [1-4]. However, dynein can be selectively depleted from kinetochores by disruption of ZW10 [5], and recent studies with this approach conclude that kinetochore-associated dynein (KD) functions to silence the spindle-assembly checkpoint (SAC) [6]. Here we use dynein-antibody microinjection and the RNAi of ZW10 to explore the role of KD in chromosome behavior during mitosis in mammals. We find that depleting or inhibiting KD prevents the rapid poleward motion of attaching kinetochores but not kinetochore fiber (K fiber) formation. However, after kinetochores attach to the spindle, KD is required for stabilizing kinetochore MTs, which it probably does by generating tension on the kinetochore, and in its absence, chromosome congression is defective. Finally, depleting KD reduces the velocity of anaphase chromosome motion by ∼40%, without affecting the rate of poleward MT flux. Thus, in addition to its role in silencing the SAC, KD is important for forming and stabilizing K fibers and in powering chromosome motion.
Results and Discussion
To prevent the formation of aneuploid cells, the sister kinetochores on each replicated chromosome must become attached to the forming bipolar mitotic spindle such that each is attached to a different pole. In animal cells, the kinetochore fibers (K fibers) that mediate this attachment begin to form at nuclear-envelope breakdown (NEB) while astral microtubules (MTs) growing randomly from the separating centrosomes contact kinetochores [7]. During this “search-and-capture” process, the kinetochore that is closest to and/or facing a pole (centrosome) often attaches before its sister does. When this occurs, the now “mono-oriented” chromosome moves toward the pole with velocities that can exceed 50 μm/min [8]. As spindle assembly continues, the MTs for K fiber maturation are both seeded by the kinetochore itself and captured from the centrosomes [9, 10]. In mammals, the MT-binding capacity of kinetochores varies depending on their surface area [11], but it usually exceeds 20 MTs, and the rate at which kinetochores move becomes progressively attenuated as their K fibers mature (to the 1-2 μm/min velocity seen during anaphase [12]).
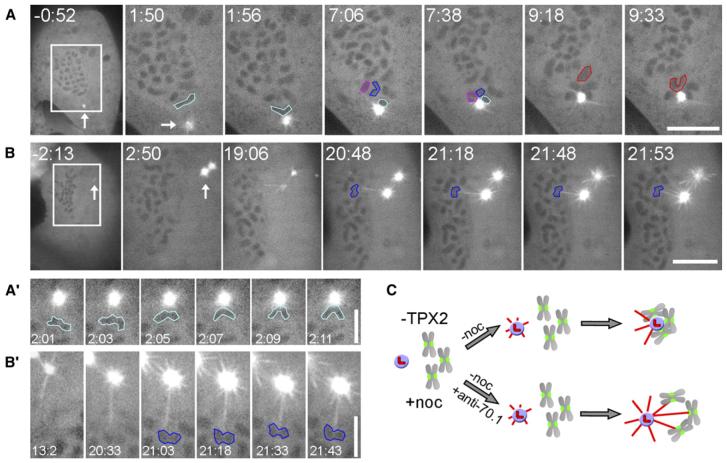
The velocity exhibited by mono-orienting chromosomes, toward the minus ends of their associated K fiber MTs, is comparable to the rate at which cytoplasmic dynein moves vesicles along MTs during interphase [8]. However, although dynein is concentrated at unattached kinetochores [13, 14], its involvement in the rapid motion of attaching chromosomes remains to be demonstrated. As a first step toward this goal, we used the RNAi method of Tulu et al. [10] to deplete TPX2, a spindle-assembly factor, from LLC-PK1 cells expressing GFP-α-tubulin. As detailed previously [10], this treatment blocks kinetochore-associated MT formation but not the capture of kinetochores by astral MTs. Indeed, when TPX2-depleted mitotic cells are allowed to gradually recover from a nocodazole (MT poison) pretreatment, MT arrays form from centrosomes but not kinetochores (Figures 1A and 1B). As during a normal division, when MTs from these arrays contact one kinetochore on an unattached chromosome, the now-mono-oriented chromosome moves toward the centrosome with an average velocity of 29 ± 19 μm/min (n = 10; Figures 1A and 1A′). Importantly, in this experiment, search and capture occurs under conditions in which MTs cannot form at kinetochores, eliminating the possibility that the rapid poleward chromosome motion observed in normal cells is produced by astral MTs interacting with kinetochore-nucleated MTs. When TPX2-depleted mitotic cells are allowed to recover from nocodazole, after being microinjected with a function-blocking antibody against cytoplasmic dynein (ab70.1) [15], astral MTs still grow from the centrosomes as the intracellular nocodazole concentration drops (Figure 1B). However, under this condition, when centromere and/or kinetochore regions are contacted by astral MTs, no motion toward the centrosomes is seen, even after prolonged periods (n = 6 cells; Figure 1B). In these preparations, kinetochores appear to form attachments with astral MTs because, after astral MTs grow into a centromere region, stable connections between them and centromeres are observed (Figure 1B′). From this study we conclude that inhibiting kinetochore-associated dynein (KD) by antibody injection prevents the rapid poleward motion of attaching chromosomes.
Figure 1. Cytoplasmic Dynein Is Responsible for the Rapid Centrosome-Direct Motion of Attaching Kinetochores in TPX2-Depleted Cells.
(A) A LLC-PKa cell depleted of TPX2, during recovery from a treatment with nocodazole (starting at 0:00). As the cell recovers, astral microtubules form around the centrosome, whereas chromosome-mediated microtubule formation is inhibited. Within the first few minutes of recovery, those chromosomes (outlined in different colors) closest to the centrosome exhibited a sudden rapid motion toward the centrosome (arrow), presumably because an astral microtubule contacted one of their kinetochores. Time is given as min:s. The scale bar represents 10 μm.
(A′) Temporal details of chromosome motion toward a centrosome in a TPX2-depleted cell recovering from nocodazole. Time is given as min:s. The scale bar represents 5 μm.
(B) A TPX2-depleted cell that was injected with 70.1 dynein antibody shortly before being released from the nocodazole (at 0:00). As in controls (A), during drug recovery, the centrosomes nucleate numerous astral MTs, some of which grow toward the chromosomes. However, movement of chromosomes (outlined in blue) toward the centrosomes does not occur, even after a prolonged period and even though astral MTs clearly contact the centromere region of at least one chromosome ([B′] shows the chromosome outlined in blue in [B]). The scale bar in (B) represents 10μm; the scale bar in (B′) represents 5μm.
(C) A schematic of this experiment. TPX2-depleted cells are treated with nocodazole to depolymerize MTs. When the drug is washed out, the chromosomes and centrosomes come together in control cells to form a rosette-like structure. By contrast, in dynein-antibody-injected cells, the chromosomes and centrosomes interact but do not move toward each other.
Dynein is targeted to kinetochores by a complex of proteins that includes ZW10, and, as a consequence, mutating or depleting ZW10 selectively prevents dynein from binding to kinetochores [6]. Our observations on antibody-injected TPX2-depleted cells predict that depleting ZW10 will also inhibit the rapid poleward motion of kinetochores during the early stages of spindle assembly. To test this, we used the RNAi protocol of Kops et al. [16] to knock down ZW10 in GFP-CENP-B-expressing U2OS cells. We found that 96 hr after transfection with ZW10 siRNA duplexes, the level of ZW10 in western blots of growing U2OS cultures was reduced by ∼85% (Figure S1A in the Supplemental Data available online). When these cultures were first treated with nocodazole (1.0 μM) for 1 hr before they were processed for the indirect immunofluorescent (IMF) localization of ZW10 and then analyzed by wide-field 3D deconvolution light microscopy, ZW10 was not detected in maximalintensity projections in 25% of the cells, and it was significantly attenuated in the remaining 75% (Figure S1B). Similar analyses also revealed that dynein was either absent or largely depleted from kinetochores (Figure S1C).
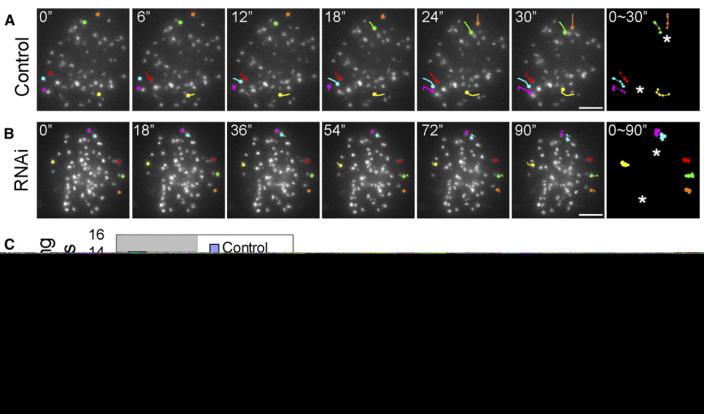
We then followed the behavior of GFP-CENP-B-labeled kinetochores for 5 min after NEB in untreated and ZW10-reduced cells with a framing interval of 6 s. In ten mock-transfected control cells, 89 of the 637 kinetochore regions (13.9%) that could be continuously followed for 5 min after NEB exhibited linear translocations in a fixed direction that lasted up to 18 s (3 frames) and had a maximum velocity between frames of 10-20 μm/min, and another 15 (2.3%) moved at rates exceeding 20 μm/min (Figures 2A and 2C; Movie S1). By contrast, of the 735 kinetochores followed during spindle assembly in 12 ZW10-depleted cells, only 16 (2.1%) exhibited rapid translocations of 10-20 μm/min, and just one moved faster than 20 μm/min (Figures 2B and 2C; Movie S2). These observations confirm our conclusion, drawn from antibody-injected TPX2-depleted cells, that KD powers the rapid poleward motion of kinetochores that results from a successful search-and-capture event.
Figure 2. Depleting Kinetochore-Associated Dynein by ZW10 RNAi Inhibits the Rapid Motion of Kinetochores toward a Centrosome after Nuclear-Envelope Breakdown.
(A) In control GFP-CENP-B U2OS cells, a variable number of kinetochores exhibit rapid poleward motions toward a centrosome shortly after nuclear-envelope breakdown. In the example shown here, six kinetochores (CENP-B-positive regions that are represented by different colors at the 0 time point) underwent sudden rapid linear motions toward a centrosome during a 30 s period. The changing position of these kinetochores during this motion is tracked in the last panel. Asterisks mark the spindle poles. The scale bar represents 5 μm.
(B) Rapid kinetochore motions are rarely seen after nuclear-envelope breakdown in cells depleted of kinetochore-associated dynein by ZW10 RNAi. In the cell depicted here, no kinetochores exhibited a rapid motion. The scale bar represents 5 μm.
(C) Plot depicting the percentage of kinetochores exhibiting rapid motions within 5 min of nuclear envelope breakdown, and their range of velocities, before and after ZW10 RNAi (see text for details).
Why do chromosomes in TPX2-depleted cells show no poleward motion after dynein-antibody injection, whereas chromosomes in ZW10-depleted cells can still become clustered around the centrosomes after NEB? Because the incidence of clustering during prometaphase in ZW10-reduced cells is more than 2.1% of the chromosomes examined, it is too high to be ascribed solely to the presence of residual dynein at some kinetochores. Alternatively, after depletion of ZW10 by RNAi, kinetochores can still nucleate MTs [9]. As shown for K fiber formation in Drosophila S2 cells [9], it is likely that in the absence of ZW10 (and KD), fragments of kinetochore-nucleated MTs move themselves and their attached kinetochore poleward along astral MTs with, for example, the slow minus-end spindle-associated MT motor, HSET. By contrast, when MT nucleation at kinetochores is prevented by the knockdown of TPX2, K fiber formation can only occur via the recruitment of astral MTs. Because there is no dynein at the kinetochore to power poleward motion, the chromosome will not move poleward until the K fiber matures to the point where it can initiate MT poleward flux (see below). The formation of a mature K fiber via recruitment alone, especially under conditions where the chromosome cannot translocate into the centrosome (Figure 1B), can be expected to require many hours [10, 17].
The initial rapid pole-directed motion of a monoorienting chromosome is a highly conserved behavior, having been described in many types of organisms, including diatoms [18], insects [19], amphibians [8], mammals [20], and humans (this study). (As they reattach to metaphase spindles, kinetochores in S. cerevisiae can also move poleward along the surface of a MT derived from a spindle pole [21]. However, under this experimental condition, the motion is relatively slow, is often interrupted by pauses or antipoleward movements, and is mediated by Kar3. HSET, the human homolog of Kar3, is not reported to be a kinetochore component in mammals [22].) In mammals, the presence of dynein in the kinetochore corona, which radiates from the kinetochore plate, greatly increases the surface area exposed to probing astral MTs. The fact that dynein can attach to and move along the wall of a MT means that even glancing contacts with astral MTs result in chromosome attachment and centrosome-directed motion [8]. As a consequence, chromosomes, scattered within the cell at NEB, are rapidly and efficiently tethered and transported into a common region where they promote the formation of a common spindle via their chromatin-associated MT-nucleation activity [23, 24]. The significance of this “collection” is likely of considerable importance in minimizing chromosome loss in large cells, including the early centrosome-mediated cleavage divisions in mammalian zygotes. Furthermore, the movement of a mono-orienting chromosome into a spindle pole actually facilitates its subsequent biorientation and congression by positioning its unattached kinetochore adjacent to the K fibers on bioriented chromosomes [25]. This allows the unattached kinetochore to laterally associate with and glide toward the spindle equator along other K fibers by using its associated CENP-E (kinesin 7) motors.
Early observations on Drosophila ZW10 mutants noted “lagging” chromatids in anaphase; this implied that ZW10 functions in anaphase chromosome motion [26, 27]. However, in addition to binding dynein, the ZW10 complex is also required for localizing spindle-assembly checkpoint (SAC) proteins Mad1 and Mad2 to unattached kinetochores [16, 28]. As a result, in the absence of functional ZW10, the SAC is nonoperable [29], which suggests that the lagging-chromatid phenotype in anaphase is due to premature anaphase onset [16, 30, 31]. However, although premature anaphase clearly contributes to this phenotype, it is also possible that depleting KD, by mutating or knocking down ZW10, also disrupts normal chromosome congression—a condition that would be masked by premature anaphase onset.
To explore this possibility, we defined the duration of mitosis (from NEB to anaphase onset) in untreated GFP-CENP-B U2OS cells, as well as in cells from ZW10-reduced cultures. We found that at 37°C, mitosis in mock-transfected controls took 31 ± 6 min (n = 20, range 22-48), whereas after knockdown of ZW-10, the duration was 42 ± 11 min (n = 20, range 26-65), and more than 60% of these cells entered anaphase with noncongressed chromosomes compared to more than 1% in mock-transfected control cultures (data not shown, see below). When the SAC is rendered nonfunctional by disruption of proteins involved in the monitoring or signal-transduction pathways, vertebrate cells exit mitosis 10-15 min after NEB regardless of the state of spindle assembly [32, 33]. Thus, when ZW10 is reduced within a cell, but not eliminated, the SAC can still delay anaphase onset, but not for prolonged periods. In this regard, Kops et al. [16] depleted 85% of the ZW10 from HeLa cultures by RNAi and found that, in response to nocodazole, the mitotic index was still two times that predicted if the checkpoint was lacking in all cells. They concluded that this unexpected modest increase in mitotic index “almost certainly reflected continued mitotic checkpoint signaling in the ZW10-containing proportion of cells and a complete absence of sustained checkpoint signaling in ZW10-depleted cells.” However, because of their methods, these authors could not determine whether the increased mitotic index was due to a minor proportion of cells that contained a normal complement of ZW10, or to a larger proportion that contained some ZW10 but not enough to sustain a prolonged SAC arrest. At any one time, both situations would give the same modest increased mitotic index.
Our methods directly addressed this issue. In our RNAi preparations, ∼75% of the mitotic cells contained some residual ZW10 (see above), and, although anaphase occurred (prematurely) in the presence of noncongressed chromosomes, it was still delayed 2-3× relative to cells that completely lacked an SAC. The only reasonable interpretation of these data is that the presence of residual ZW10 allows one or more kinetochores to recruit some Mad1/Mad2 and that this in turn leads to the production of enough “wait anaphase” signal to delay anaphase, but not for a prolonged period. The same situation, which is referred to by Weaver and Cleveland [32] as a “weakened checkpoint,” is seen when cells are engineered to contain reduced amounts of other checkpoint proteins, such as BubR1 [34] or Mad2 [35].
Our live-cell data reveal that when ZW10 is substantially reduced, many chromosomes fail to complete congression over a temporal period in excess of that required for all chromosomes to congress in untreated cells. This implies that the lagging-chromatid phenotype seen in anaphase cells depleted of ZW10 is not due solely to a weakened SAC, but also to problems in establishing and maintaining normal kinetochore-to-pole connections during prometaphase. To test this idea, we treated ZW10-depleted U2OS cultures for 2 hr, prior to fixation, with 5.0 μM MG132, a drug that leads to an accumulation of metaphase cells in control cultures by inhibiting proteolysis and anaphase onset. We then compared the ratio of metaphase (all chromosomes positioned on the spindle equator) to prometaphase (i.e., one or more noncongressed chromosomes) cells under both control (MG132 only) and experimental (ZW10 knockdown + MG132) conditions. We counted 200 cells in each of two experiments and found that 92% ± 2% of the cells in control cultures were in metaphase, compared to only 47% ± 4% in ZW10-depleted cultures. This result confirms our finding from live cells that congression is defective, at least for some chromosomes, after depletion of kinetochores of the ZW10/KD complex.
Live-cell studies reveal that KD constantly streams from the kinetochore toward the spindle poles along K fibers. This behavior is thought to remove SAC proteins from the kinetochore [15], as well as to deliver proteins involved in spindle-pole maintenance and function to the centrosomes [3]. To determine whether the selective depletion of KD in ZW10 knockdown cells disrupts spindle-pole formation and structure, we fixed control and ZW10-depleted cultures and stained them for the IMF localization of γ-tubulin, NuMA, Kif2a, and cytoplasmic dynein. Although selectively reducing dynein at kinetochores via ZW10 siRNA leads, as expected, to a diminished level of dynein in the spindle-pole regions, the amount and distribution of γ-tubulin, NuMA, and Kif2a were not noticeably affected (Figure S2).
Dynein is a kinetochore-associated motor that is positioned to generate tension on the kinetochore as it works along K fiber MTs. As R.B. Nicklas and colleagues have elegantly demonstrated by live-cell micromanipulation, “tension, or some consequence of tension, stabilizes the proper spindle attachment” [36], probably by slowing the turnover rate of K fiber MTs [37, 38]. Does depleting ZW10 and KD from kinetochores lead to a reduction in tension at the kinetochore-centromere interface? To answer this question, we compared the distance between the peripheral edges of CENP-B spots (i.e., sister kinetochores) in U2OS cells under control and experimental conditions. We found that in nocodazoletreated (5 μM, 3 hr) cells lacking MTs, this distance was 0.67 ± 0.07 μm (n = 74 kinetochore pairs from six cells), compared to 1.05 ± 0.07 μm (n = 98 kinetochore pairs from ten cells) in untreated metaphase controls. By contrast, on fully congressed chromosomes in ZW10 knockdown cells containing mature spindles, this distance was 0.87 ± 0.09 μm (n = 96 kinetochore pairs from 12 cells); this represents a 47% reduction in tension relative to untreated controls. This finding is consistent with the previous report that globally disrupting the dynein-dynactin interaction in metaphase PtK1 cells with p50 reduces the tension on kinetochores by 40% [15]. It also supports our contention that the phenotype observed in the absence of functional ZW10 arises from premature entry into anaphase under conditions in which chromosome congression is already delayed, because kinetochores lack the dynein necessary for promoting timely tension-mediated K fiber stability.
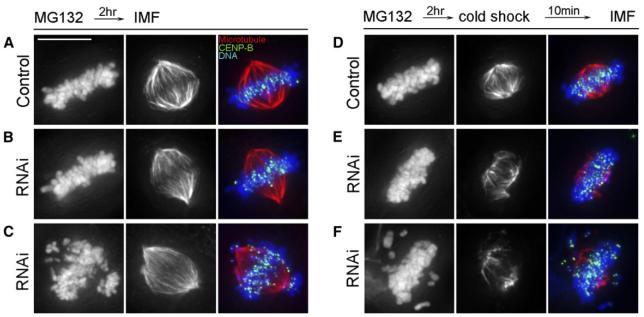
To further evaluate this idea, we incubated mock-transfected control and ZW10-depleted U2OS cultures in MG132 for 2 hr, and then placed them in cold (4°C) medium on ice for 10 min prior to fixation for tubulin IMF. In untreated cells, the enhanced stability of K fiber MTs, relative to other spindle MTs, makes them more resistant to depolymerization by cold [39]. As expected from previous reports, we found that, compared to untreated cells (Figure 3A), metaphase cells in cold-treated U2OS cultures (Figure 3D) contained shorter spindles with greatly reduced numbers of nonkinetochore MTs but prominent K fibers. By contrast, compared to cold-shocked controls (Figure 3D), the number of cold-stable K fiber MTs in metaphase cells containing reduced levels of ZW10 (Figure 3E) was noticeably reduced. To validate this subjective impression, we captured low magnification (20×) images of these cells with a NIKON TE-2000-U microscope equipped with a Hamamatsu ORCA camera and then quantified MT fluorescence. We found that the fluorescence intensity of cold-shocked ZW10-depleted and MG132-treated metaphase cells (n = 27 over two experiments) was only 40.7% ± 4% that of mock-transfected, cold-shocked, MG132-treated controls (n = 22 over two experiments). From these data we conclude that the ZW10/KD complex is required for stabilizing MT attachments to kinetochores, probably by promoting tension, and in its absence congression is delayed and/or disrupted because K fiber MTs are not properly stabilized.
Figure 3. Relative to Spindles in Control Cells, Spindle and Kinetochore Microtubules in Metaphase Cells Depleted of ZW10 Are More Labile.
(A and B) Maximum-intensity projections from deconvolved data sets of representative metaphase spindles from U2OS cultures treated with MG132 for 2 hr (A) or with MG132 for 2 hr after depleting ZW10 with RNAi (B).
(C) Prometaphase cell depleted of ZW10 and treated with MG132 for 2 hr.
(D) Metaphase cell treated as in (A) before incubating at 4°C for 10 min. Cold-shocking metaphase cells leads to a significantly shorter spindle consisting predominantly of kinetochore fibers (cf [A] and [D]).
(E and F) Metaphase (E) and prometaphase (F) spindles in ZW10-depleted cultures treated with MG132 for 2 hr (C) and then incubated at 4°C for 10 min. When compared to cold-shocked controls (D), the spindles in cold-shocked cells depleted of ZW10 (E and F) are significantly shorter and contain fewer, as well as thinner, kinetochore fibers.
The left column shows DAPI staining, the center column shows tubulin fluorescence, and the right column is a merged view also showing CENP-B. The scale bar in (A) represents 10 μm.
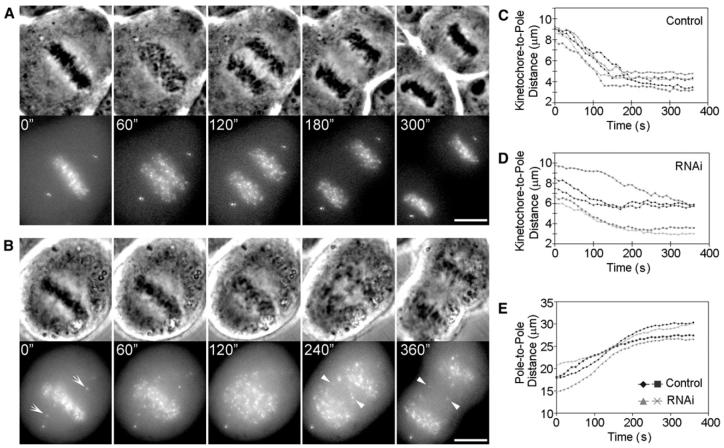
Finally, we asked whether specifically depleting KD in U2OS cells depresses the rate of chromosome poleward motion during anaphase, as previously reported in ZW10 mutant Drosophila spermatocytes [31], and also after dynein-antibody microinjection of Drosophila embryos [40] and PtK1 cells [15]. For this study, GFP-centrin-1-expressing U2OS cells were transfected with GFP-CENPB 48 hr after ZW10 RNAi. Selected prometaphase or metaphase cells were imaged 48 hr later, at 10 s intervals, while they completed mitosis. As noted above for ZW10-depleted U2OS cells expressing GFP-CENP-B, the great majority of these cells initiated anaphase with noncongressed chromosomes, which generated lagging chromatids (Figure 4B). We then determined the rate of anaphase A, i.e., the change in the distance between GFP-CENP-B-labeled kinetochores and GFP-centrin-1-labeled centrosomes, on chromosomes positioned near the spindle's long axis. In controls (Figures 4A and 4C; Movie S3) kinetochores moved poleward with an average rate during the first 2-3 min of 1.4 ± 0.10 μm/min (n = 12; range 1.2-1.7 μm/min). By contrast, in ZW10-depleted cells (Figures 4B and 4D; Movie S4), the rate of kinetochore poleward motion during anaphase A was depressed to 0.8 ± 0.2 μm/min (n = 12; range 0.40 -1.10 μm/min; p < 0.001), whereas the rate of anaphase B (spindle-pole separation) was not affected (Figure 4E). Thus, a major velocity component of anaphase chromosome motion in mammals is due to KD.
Figure 4. The Rate of Anaphase Poleward Chromosome Motion Is Significantly Reduced after Depletion of Kinetochore-Associated Dynein by ZW10 RNAi.
(A) Selected phase-contrast (top) and fluorescence (bottom) micrographs, from a time-lapse series, of a control GFP-CENP-B U2OS cell labeled with GFP-centrin as it undergoes anaphase and cytokinesis. The scale bar represents 10 μm.
(B) Similar to the sequence shown in (A), except in this cell, kinetochore-associated dynein was depleted by ZW10 RNAi. Note that the cell entered anaphase with numerous noncongressed chromosomes (CENP-B dots that are positioned off the spindle equator at anaphase onset are indicated with arrowheads at 0 time). Arrowheads depict some of the lagging chromosomes seen during anaphase. The scale bar represents 10 μm.
(C) Plot depicting the change in kinetochore-to-pole distance versus time for four chromosomes, positioned near the spindle's long axis, during anaphase in untreated control cells.
(D) Similar to (C), except in this case, kinetochore-associated dynein was depleted by ZW10 RNAi. Note that relative to controls, the velocity of anaphase chromosome motion is significantly reduced in the ZW10-depleted cells (See text for details.).
(E) Change in pole-to-pole (centrosome-to-centrosome) distance during anaphase in control and ZW10-depleted cells. Depleting kinetochores of cytoplasmic dynein does not alter the rate or extent of anaphase B (spindle elongation).
In vertebrates, poleward MT-subunit flux contributes 35%-45% of the chromosome velocity during anaphase, whereas 55%-65% has been ascribed to a pacman activity associated with the kinetochore. In our experiments, the reduction of anaphase velocity after depleting ZW10/KD (43%) is closer to that contributed by MT flux than by pac-man. This raises the issue of whether depleting KD depresses flux. To answer this, we measured flux in control and ZW10-depleted U20S metaphase cells expressing photoactivatable GFP-α-tubulin [10, 41]. We found that the flux rate in metaphase and early anaphase U2OS control cells (0.5 ± 0.1 μm/min; n = 8 half spindles) was the same as that published previously [41], and that it did not change (0.5 ± 0.1 μm/min; n = 14 half spindles) after depletion of ZW10 (Figure S3). This is consistent with the report that the flux rate in Drosophila S2 cells does not change after depletion of dynein heavy chain globally by RNAi [4], and it means that dynein, either at the kinetochore or in the spindle, does not contribute to flux.
In summary, recent work on KD has focused on how it mediates silencing of the SAC, and no effort has been made to understand the role of this motor in chromosome behavior during spindle assembly. Here we show that as prometaphase begins, dynein is responsible for rapidly transporting chromosomes toward the forming spindle during the search-and-capture process. We argue that this behavior reduces chromosome loss by facilitating congression and the formation of a common bipolar spindle. Next, we show that by creating tension on the K fiber-kinetochore interface, the ZW10/KD complex stabilizes K fibers during spindle assembly. Indeed, in the absence of this influence, many chromosomes fail to congress even when spindle assembly is prolonged by inhibiting anaphase. This, when combined with premature entry into anaphase, leads to the characteristic lagging-chromatid phenotype seen in ZW10 mutants. Finally, we find, as others have, that dynein is involved in generating the force for chromosome poleward motion, contributing up to 40% of the velocity in mammals after the K fiber matures. Much of the remaining velocity for this motion appears to be generated by poleward MT-subunit flux [41], which, as we and others [4] have shown, is not affected by the absence of KD. Clearly, in addition to its putative role in silencing the SAC, KD also functions to power chromosome movement and to ensure faithful chromosome segregation via its effects on K fiber formation and stability.
Acknowledgments
The authors thank Dr. Duane Compton, Dr. Kevin Sullivan, Dr. Michael Goldberg, and Dr. Alexey Khodjakov for generously supplying GFP-tagged cell lines and/or antibodies; Dr. Chris O'Connell and Dr. Jadranka Loncarek for assistance with microscopy; and the Lab of Cell Regulation for helpful comments. Parts of this work were presented at the 2006 American Society for Cell Biology Meeting (San Diego, California). This research was supported by National Institutes of Health (NIH) GMS grants 40198 to C.L.R and 59057 to P.W.
Footnotes
Supplemental Data Experimental procedures, three figures, and four movies are available at http://www.current-biology.com/cgi/content/full/17/11/973/DC1/.
Supplementary Material
References
- 1.Echeverri CJ, Paschal BM, Vaughan KT, Valee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purohit A, Tynan SH, Vallee RB, Doxsey SJ. Direct interaction of pericentirn with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999;147:481–491. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaetz J, Kapoor TM. Dynein/dynactin regulates metaphase spindle length by targeting depolymerizing activities to spindle poles. J. Cell Biol. 2004;166:465–471. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiato H, Khodjakov A, Rieder CL. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibers. Nat. Cell Biol. 2005;7:42–47. doi: 10.1038/ncb1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr DA, Williams BC, Hays TS, Goldberg ML. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karess RE. Rod-Zw10-Zwilch: A key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Rieder CL. Kinetochore fiber formation in animal cells: Dueling mechanisms come to a draw. Chromosoma. 2005;114:310–318. doi: 10.1007/s00412-005-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiato H, Rieder CL, Khodjakov A. Kinetochoredriven formation of kinetochore fibers contributes to spindle assembly during mitosis in animals. J. Cell Biol. 2004;167:831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieder CL. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- 12.McNeill PA, Berns MW. Chromosome behavior after laser microirradiation of a single kinetochore in mitotic PtK2 cells. J. Cell Biol. 1981;88:543–553. doi: 10.1083/jcb.88.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steuer ER, Wordeman L, Schroer TA, Sheetz MP. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- 14.Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- 15.Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kops G, Kim Y, Weaver BAA, Mao Y, McLeod I, Yates JR, Tagaya M, Cleveland DW. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the “search-and-capture” process during mitotic spindle assembly. Curr. Biol. 2005;15:826–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Tippit DH, Pickett-Heaps J, Leslie R. Cell division in two large pennate diatoms Hantzschia and Nitzschia III. A new proposal for kinetochore function during prometaphase. J. Cell Biol. 1980;86:402–416. doi: 10.1083/jcb.86.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickards GK. Prophase chromosome movements in living house cricket spermatocytes and their relationship to prometaphase, anaphase and granule movements. Chromosoma. 1975;49:407–455. doi: 10.1007/BF00285133. [DOI] [PubMed] [Google Scholar]
- 20.Roos UP. Light and electron microscopy of rat kangaroo cells in mitosis. III. Patterns of chromosome behavior during prometaphase. Chromosoma. 1976;54:363–385. doi: 10.1007/BF00292816. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 22.Mountain V, Simerly C, Howard T, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–365. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadsworth P, Khodjakov A. E pluribus Unum: Towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor TM, Lampson M, Hergert P, Cameron L, Cimini D, Salmon E, McEwen BF, Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams BC, Goldberg ML. Determinants of Drosophila zw10 protein localization and function. J. Cell Sci. 1994;107:785–798. doi: 10.1242/jcs.107.4.785. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Hu X, Ding X, Dou Z, Yang Z, Shaw AW, Teng M, Cleveland DW, Goldberg ML, Niu L, et al. Human Zwint-1 specifies localization of Zeste Wwhite 10 to kinetochores and is essential for mitotic checkpoint signaling. J. Biol. Chem. 2004;279:54590–54598. doi: 10.1074/jbc.M407588200. [DOI] [PubMed] [Google Scholar]
- 28.Buffin E, Lefebvre C, Huang J, Gagou ME, Karess RE. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr. Biol. 2005;15:856–861. doi: 10.1016/j.cub.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Basto R, Gomes R, Karess RE. Rough deal and zw10 are required for the metaphase checkpoint in Drosophila. Nat. Cell Biol. 2000;2:939–943. doi: 10.1038/35046592. [DOI] [PubMed] [Google Scholar]
- 30.Chan GKT, Jablonski SA, Starr DA, Goldberg ML, Yen TJ. Human zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat. Cell Biol. 2000;2:944–947. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- 31.Savoian MS, Goldberg ML, Rieder C. The rate of chromosome poleward motion is attenuated in Drosophila zw10 and rod mutants. Nat. Cell Biol. 2000;2:948–952. doi: 10.1038/35046605. [DOI] [PubMed] [Google Scholar]
- 32.Canman JC, Sharma N, Straight A, Shannon KB, Fang G, Salmon ED. Anaphase onset does not require the microtubule-dependent depletion of kinetochore and centromere-binding proteins. J. Cell Sci. 2002;115:3787–3795. doi: 10.1242/jcs.00057. [DOI] [PubMed] [Google Scholar]
- 33.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Dai W, Wang Q, Liu T, Swamy M, Fang Y, Xie S, Mahmood R, Yang Y-M, Xu M, Rao CV. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 35.Michel LS, Liberal V, Chaterjee A, Kirchwegger R, Pasche B, Gerald V, Dobles M, Sorger PK, Murty VV, Benezra R. Mad2 haploinsufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 36.Nicklas RB, Ward SC. Elements of error correction in mitosis: Microtubule capture, release, and tension. J. Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J. Cell Sci. 2000;113:3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- 38.Nicklas RB, Waters JC, Salmon E, Ward SC. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 2001;114:4173–4183. doi: 10.1242/jcs.114.23.4173. [DOI] [PubMed] [Google Scholar]
- 39.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- 40.Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat. Cell Biol. 2000;2:922–930. doi: 10.1038/35046574. [DOI] [PubMed] [Google Scholar]
- 41.Ganem NJ, Upton K, Compton DA. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 2005;15:1827–1832. doi: 10.1016/j.cub.2005.08.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.