Abstract
The attachment to and movement of a chromosome on the mitotic spindle are mediated by the formation of a bundle of microtubules (MTs) that tethers the kinetochore on the chromosome to a spindle pole. The origin of these “kinetochore fibers” (K fibers) has been investigated for over 125 years. As noted in 1944 by Schrader [Mitosis, Columbia University Press, New York, 110 pp.], there are three possible ways to form a K fiber: (a) it grows from the pole until it contacts the kinetochore, (b) it grows directly from the kinetochore, or (c) it forms as a result of an interaction between the pole and the chromosome. Since Schrader's time, it has been firmly established that K fibers in centrosome-containing animal somatic cells form as kinetochores capture MTs growing from the spindle pole (route a). It is now similarly clear that in cells lacking centrosomes, including higher plants and many animal oocytes, K fibers “self-assemble” from MTs generated by the chromosomes (route b). Can animal somatic cells form K fibers in the absence of centrosomes by the “self-assembly” pathway? In 2000, the answer to this question was shown to be a resounding “yes.” With this result, the next question became whether the presence of a centrosome normally suppresses K fiber self-assembly or if this route works concurrently with centrosome-mediated K-fiber formation. This question, too, has recently been answered: observations on untreated live animal cells expressing green fluorescent protein-tagged tubulin clearly show that kinetochores can nucleate the formation of their associated MTs in a unique manner in the presence of functional centrosomes. The concurrent operation of these two “dueling” routes for forming K fibers in animal cells helps explain why the attachment of kinetochores and the maturation of K fibers occur as quickly as they do on all chromosomes within a cell.
Introduction
In 1879, Walther Flemming wrote in regard to mitosis that “we do not know, in the movement or changes of position of the threads of a nuclear figure (i.e., chromosomes), whether the immediate causes lie within the threads themselves, outside of them, or both” (Flemming 1879). Since Flemming's time, many investigators have actively pursued the question of how the forces are generated to move the chromosomes during nuclear division (karyokinesis).
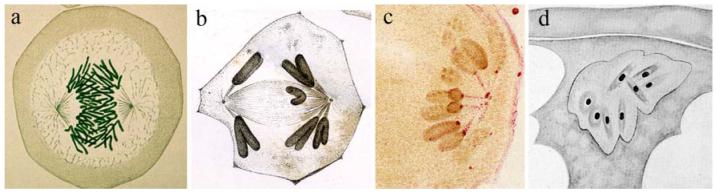
In his fixed preparations of newt cells (Fig. 1a), Flemming could not see that during anaphase each chromatid was connected to a pole of the mitotic apparatus by a prominent fiber. Instead, Van Beneden, Hermann, and Druner independently made this observation (Fig. 1b), and, in 1891, Hermann even predicted that these “mantle” fibers were the principle agents by which the daughter chromosomes were “dragged” apart (see pp. 78-79 in Wilson 1911).
Fig. 1.
Early evidence for kinetochores and K fibers. a Flemming's (1882) drawing of a newt cell in anaphase of mitosis. He did not observe in his preparations that each chromosome was connected to a spindle pole by a discrete fiber. b Druner's (1895) drawing showing K fibers during anaphase in an insect spermatocyte. c Metzner's (1894) drawing depicting kinetochores during anaphase. d Hughes-Schrader's (1924) illustration of prometaphase in A.wheeleri oocytes. Note that each chromosome appears to be organizing its own minispindle within an intact nuclear envelope
As late as 1925, Edmund B. Wilson, in the third edition of his monumental treatise, The Cell in Development and Heredity, characterized the point that a mantle fiber inserts into the chromosome simply as “a small area” (see p 131 in Wilson 1925). However, as early as 1894, Metzner noted that this area contained a small, discrete staining structure or “kinetic region” that led the way during poleward chromosome motion (Fig. 1c; Metzner 1894). Later work revealed that chromosome fragments lacking this region were expelled from the spindle and exhibited no directed motion (Carlson 1938). Thus, by the time Franz Schrader published the first edition of his book on mitosis in 1944 (Schrader 1944), it was widely accepted that Metzner's kinetic region was responsible for attaching a chromosome to, and somehow moving it on, the spindle. Schrader listed 27 terms that had been used previously to describe this region, and he finally settled on Moore's term “kinetochore” (see discussion in Sharp 1934).
Near the time that Schrader (1944) published his book, polarized light microscopy (LM) of living cells by Schmidt (1939), and later by Inoue (1952, 1953), proved that the fibrous nature of the spindle seen in fixed preparations was not an artifact. These studies also revealed that the spindle is highly dynamic in that it grows and shrinks (assembles/disassembles) in response to certain drugs and environmental changes. Thus, by the mid-twentieth century, it was evident that mantle fibers, which were now termed chromosomal or “kinetochore fibers” (K fibers), were real, dynamic, and responsible for generating and/or transmitting the forces for chromosome motion. The prevailing idea at this time was that a K fiber “pulled” on its associated kinetochore, and thus on the chromosome, with a force that was proportional to its length (Ostergren 1945).
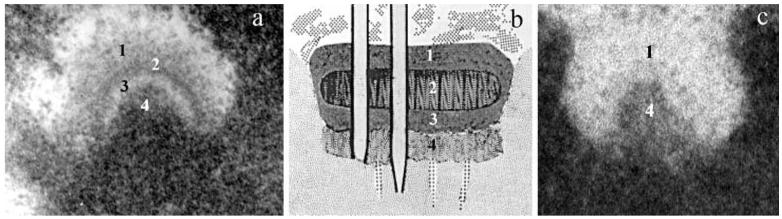
Electron microscopic (EM) studies in the early to mid-1960s, starting with that of Harris (1961), revealed that the spindle and its associated K fibers were composed primarily of microtubules (MTs). Subsequent EM work on the kinetochore in mammals led to the view that this assembly is structured as a “trilaminar” disk in which its associated MTs (K-MTs) are embedded in an “outer” thin, circular electron-dense plate. This plate is separated from an inner chromosome-associated dense layer by an electron-lucent clear zone (Brinkley and Stubblefield 1966; Jokelainen 1967; Roos 1973; Fig. 2a). Jokelainen (1967) emphasized that “the outward surface of the outer kinetochore layer is consistently covered by a corona of low-density material that is practically devoid of cytoplasmic particulate structures.” (Although the kinetochore is often referred to as an organelle, because it lacks a surrounding membrane it is really a macromolecular assembly like the chromosome.)
Fig. 2.
Mammalian kinetochore structure. a An unattached kinetochore from a newt cell viewed in a 0.25-μm section after conventional fixation, embedding, and staining. Note the extensive corona material (1) and the trilaminar structure of the kinetochore proper (2-4). b Jokelainen's (1967) early drawing of a kinetochore in a fetal rat cell. c An unattached kinetochore from a colcemid-treated PtK1 cell, viewed in a 0.25-μm section, after high-pressure freezing and freeze substitution. Numbers 1 and 4 note the corona and kinetochore proper, respectively. See text for details. (Micrograph in c is courtesy of B.F. McEwen and Y. Dong from the Wadsworth Center.)
The multiple functional roles of the kinetochore and the distribution of its associated proteins are still modeled exclusively in the context of this trilaminar structure (Fig. 2b). However, this appearance is likely a fixation artifact caused by shrinkage of the chromosome away from the kinetochore during the dehydration steps used in conventional EM embedding. When viewed after high-pressure freezing and freeze substitution, which minimizes structural changes, the kinetochore in mammals appears as a mat or ball of fibrous material connected directly to a more densely staining surface of the centromeric heterochromatin (McEwen et al. 1998; Fig. 2c). This mat is surrounded on its cytoplasmic surface by a 100- to 150-nm-wide corona that contains a loose network of light-staining, thin (approximately 9-nm diameter) fibers that exclude ribosomes and other particles. As a rule, the number of MTs that bind to a kinetochore as its K fiber matures is set by its surface area, with kinetochores in mammals binding 20-40 MTs (Rieder 1982).
The development of MT subunit tagging and live-cell fluorescence imaging in the 1980s confirmed Inoue's conclusion that K fibers are highly dynamic structures in which their constituent K-MTs exhibit a coordinated behavior (Mitchison et al. 1986; Cassimeris et al. 1988; Gorbsky et al. 1987; Mitchison 1989). In addition, during this decade, the first non-axonemal MT motor proteins were discovered (Vale et al. 1985), as were the first antibodies to the kinetochore-centromere complex (Moroi et al. 1981; Brenner et al. 1981). After these discoveries, research on how chromosomes move focused on a search for spindle- and kinetochore-associated molecular motors and how K-fiber MTs (K-MTs) form and function.
Several excellent reviews have recently been published on the kinetochore and its role in spindle assembly, chromosome motion, and mitotic progression (Biggins and Walczak 2003; Maiato et al. 2004b; Wadsworth and Khodjakov 2004; Kline-Smith et al. 2005; Rogers et al. 2005). None of these, however, focuses on the formation of K fibers, on which important new data have recently become available. What follows are a brief chronological summary and an evaluation of the more seminal discoveries that have led to our current view of how K fibers form in vertebrate somatic cells.
Kinetochore fiber formation: the early years (1911-1980)
An early notion for how K fibers form was outlined by E.B. Wilson in 1911 and was based on the work of Hermann, Van Beneden, and others (Wilson 1911). During the early stages of mitosis in animals, each spindle pole is defined by a centrosome and its associated radial or “astral” arrays of fibers (i.e., MTs). Wilson concluded that mantle fibers (K fibers) “are essentially a part of the asters, i.e., are those astral rays that come into connection with the chromosomes” (p. 315). This simple idea remained relatively unchallenged until 1924 when Hughes-Schrader reported that each meiotic tetrad in Acroschismus wheeleri oocytes appears to organize its own minispindle (Hughes-Schrader 1924) and that all of the minispindles subsequently coalesce to form a single bipolar spindle (Fig. 1d; see Rieder and Nowogrodzki 1983). In the first edition of his book, Schrader (1944) interpreted this and similar data on coccid spermatocytes to mean that K fibers can arise “chiefly or entirely through the activity of the kinetochore alone” (p. 32).
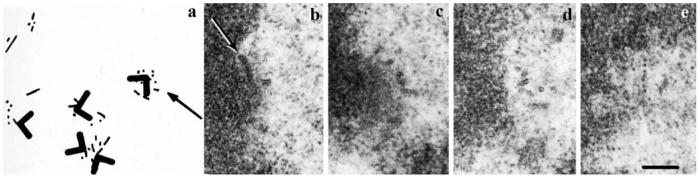
The notion that K fibers grow from the kinetochore garnered strong, subsequent support from Dietz's (1966) phase-contrast observations on live crane fly spermatocytes. He noted that functional K fibers and bipolar spindles form in these cells even when the two astral MT arrays (centrosomes) are physically inhibited from separating. Around this time, Forer (1965), investigating the same material with polarized LM, reported that holes generated in K fibers with a UV microbeam move poleward at rates similar to those of anaphase chromosomes. An Occam's razor interpretation of these findings was that K fibers form by growing from the kinetochore, i.e., each kinetochore nucleates its associated MTs. This interpretation gained experimental support in the mid-1970s when many laboratories reported that kinetochores on isolated mammalian chromosomes nucleated MTs in the presence of tubulin (e.g., McGill and Brinkley 1975; Telzer et al. 1975). Ris and Witt (1981) and Witt et al. (1980) even extended this finding to chromosomes in situ. Using serial section EM, they observed that when Chinese hamster ovary (CHO) or mouse cells were allowed to recover from prolonged treatments with drugs that inhibit MT assembly (colcemid), numerous small MTs appeared first within the kinetochore corona (Fig. 3). Thus, as emphasized by Pickett-Heaps and Tippit (1978) by the end of the 1970s, the idea that the kinetochore generates its associated MTs was “virtually unquestioned in nearly every paper or review on mitosis,” and it formed “a basic building block upon which most models of mitosis (were) erected.”
Fig. 3.
Kinetochores nucleate MTs during a recovery from colcemid treatment. a Reconstruction from 33 serial 0.25-μm-thick sections of several kinetochores (chevron indicates kinetochore outer disk, chevron pointing away from the chromosome) in a CHO cell fixed 15 min into a recovery from a prolonged colcemid block. Note that numerous short MTs appear first near each kinetochore. b-e Four serial sections through the kinetochore depicted by the arrow in a. Note that numerous short MTs appear in the corona material (from Witt et al. 1980)
Kinetochore fiber formation: the middle years (1981-2000)
Despite the data that kinetochores nucleate MTs in cells recovering from drugs, in animals chromosomes exhibit a number of behaviors and features during spindle formation that suggest that this nucleation activity does not occur in untreated cells. For example, the closer a kinetochore is to a centrosome at nuclear envelope breakdown (NEB), the more rapidly it attaches to the spindle (Roos 1976; Rieder and Borisy 1981). During NEB in newt lung cells, one or more chromosomes sometimes become positioned well removed from the two asters (Fig. 4). Under this condition, the attachment of these “lost” chromosomes to the spindle can be delayed for hours (Rieder and Alexander 1990), and throughout this delay there is no evidence of MT formation in the vicinity of the kinetochores. Furthermore, the formation of K fibers on sister kinetochores in mammalian cells usually occurs asynchronously. As a result, cells in early prometaphase contain a variable number of mono-oriented chromosomes (Fig. 4) in which one kinetochore is attached to a spindle pole by a K fiber, while the other kinetochore, which is positioned on the other side of the primary constriction and faces in the opposite direction, remains free of MTs (see, e.g., Roos 1973; Mole-Bajer et al. 1975). As emphasized by Rieder and Borisy (1981), these features are more consistent with the idea that K fibers normally form in animals as kinetochores capture astral MTs growing from the centrosome.
Fig. 4.
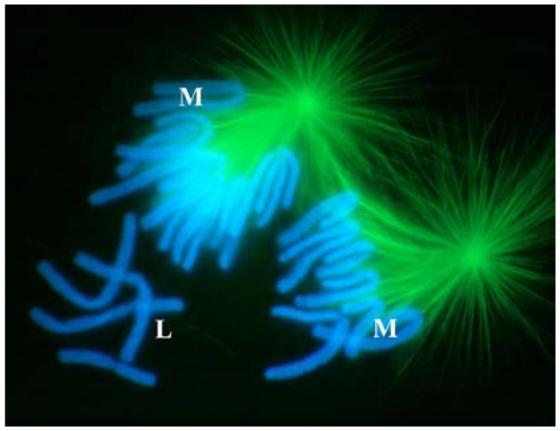
The kinetochores on newt chromosomes (blue) that are not incorporated into a forming spindle show no evidence of nucleating MTs (green). This indirect immunofluorescence micrograph of an early prometaphase newt cell contains several lost (L) and mono-oriented (M) chromosomes. Many of the mono-oriented chromosomes can clearly be seen to possess just a single K fiber. Note the absence of MTs in the vicinity of the lost chromosomes
Within the living cell, as in vitro, MTs are polarized structures that elongate by preferentially adding subunits onto their fast-growing (plus) ends, which are distal to their site of nucleation. Thus, if the MTs used for construction of the K fiber are nucleated by the kinetochore, their plus ends should be located near the spindle poles, away from the kinetochore, and their minus ends should remain associated with the kinetochore. This was indeed reported to be the case for MTs nucleated by kinetochores on isolated chromosomes (Bergen et al. 1980). However, in situ MT polarity studies on whole cells by Euteneuer and McIntosh (1981) demonstrated that all MTs within a half spindle, including K-MTs, possessed the same polarity, which was plus end distal to the spindle pole. This finding was not consistent with the idea that kinetochores act as MT-nucleating centers, and it implied that the nucleation of MTs by kinetochores on isolated chromosomes was an in vitro artifact. The in situ polarity studies were, however, consistent with the idea that K fibers normally form as kinetochores capture the plus end of MTs growing from the spindle pole. Yet, unlike MTs generated from kinetochores on isolated chromosomes, MTs nucleated by kinetochores in cells recovering from drug treatments were subsequently shown to have the correct polarity, i.e., their plus ends are at the kinetochore (Euteneuer et al. 1983). This finding meant that kinetochores either work differently than every other MT-nucleating center identified to date, which was considered highly unlikely, or that the results obtained from drug-treated cells were not physiologically relevant. Thus, by the mid-1980s, the question of how K fibers formed in vertebrates remained unresolved.
Kinetic analyses by Mitchison and Kirschner (1985a) revealed that MTs nucleated in the presence of tubulin by kinetochores on isolated CHO chromosomes grow with complex kinetics. After an initial lag phase, they found that MTs “are continuously nucleated with both plus and minus ends distally localized,” a feature that was “inconsistent with the formation of an ordered, homopolar K fiber in vivo.” In a companion paper, these same authors (Mitchison and Kirschner 1985b) reported that kinetochores on isolated CHO chromosomes could also “capture” preformed MTs and move them in an ATP-dependent manner. This study also noted that MT subunits were added in vitro to growing K-MTs at the kinetochore. Shortly thereafter, tubulin microinjection approaches were used to extend this important in vitro conclusion to live cells (Mitchison et al. 1986).
Mitchison and Kirschner's (1984) discovery that kinetochores on isolated chromosomes can capture preformed MTs, combined with their earlier in vitro finding that centrosome-nucleated MTs constantly grow and shrink at their plus ends (i.e., they exhibit dynamic instability), led to a “search-and-capture” model for K-fiber formation in animals. This scenario (Kirschner and Mitchison 1986), which is similar to that suggested earlier by Wilson and later by others, envisions that K fibers form in animal cells as kinetochores capture and stabilize astral MTs generated from the centrosome. This model was subsequently validated using live-cell, video-enhanced LM by Hayden et al. (1990) and Rieder and Alexander (1990). Their studies in newt cells revealed that when a growing astral MT contacts one of the two unattached kinetochores on an unattached chromosome, the kinetochore immediately associates with the MT lattice and begins moving towards the pole on the surface of the MT at a very high rate of speed. This means that kinetochores begin to experience a poleward force as soon as they become associated with an MT anchored in the spindle pole. Since MT density is highest near the spindle poles, as an attaching kinetochore moves poleward, it captures the sides or plus ends of additional MTs. The plus ends of those MTs that are initially laterally associated with the kinetochore remain dynamically unstable and inevitably shortened until they become associated with the kinetochore corona. As a result, over time the K fiber is formed as more and more MT plus ends become stably associated with the kinetochore. About this time, cytoplasmic dynein was found to be a major component of unattached kinetochores (Pfarr et al. 1990). This motor molecule is the only kinetochore-associated MT minus end motor identified to date; it remains the most likely force-producing candidate for the rapid poleward motion exhibited by attaching kinetochores in many cell types.
The search-and-capture hypothesis rapidly gained widespread acceptance in part not only because it was supported by direct evidence but also because it explained several behaviors (see above) seen during early prometaphase in animal somatic cells. Indeed, by the end of the twentieth century, the textbook consensus for how the kinetochore acquires its MTs during mitosis in animal cells was, and still is, that it captures MT plus ends growing from the spindle pole.
Kinetochore fiber formation: the current view
Although it provides a solid conceptual framework for how K fibers and spindles form, the random nature of the search-and-capture process is not consistent with the kinetics of K-fiber formation in animal cells. While it is predicted that this mechanism would take only minutes to capture a kinetochore (Hill 1985; Holy and Leibler 1994), hours would be required to capture all 96 kinetochores in a human cell (Wollman et al. 2005), which is much longer than the normal duration of mitosis. In addition, it does not explain how all captured kinetochores become saturated with 20-40 MTs in a span of just 15-20 min (McEwen et al. 1997; Wollman et al. 2005). Clearly, other processes must be occurring to facilitate K-fiber formation and maturation. Also, as originally formulated, search and capture does not explain how K fibers form in cells lacking centrosomes (and thus asters), including higher plants and many animal oocytes (e.g., Heald et al. 1997; Karsenti et al. 1984; Szollosi et al. 1972). In these cells, another mechanism must exist for generating K-MTs.
Our knowledge of how K fibers form in cells lacking centrosomes is based on two decades of investigating the assembly of meiotic spindles in Xenopus oocyte extracts. In these extracts, spindles “self-assemble” around chromatin in the absence of centrosomes (reviewed in Heald et al. 1997). The process is initiated by the nucleation of MTs in the vicinity of the chromosomes after NEB. The nucleation of these MTs results from the production of a Ran-guanosine triphosphate (GTP) gradient around the chromosomes by chromatin-bound regulator of chromosome condensation 1 (RCC1), a Ran-GTP exchange factor. The enhanced production of Ran-GTP near chromosomes, which occurs during mitosis in response to the cyclin-dependent kinase 1-mediated phosphorylation of RCC1, frees TPX2 from its carrier (Gruss and Vernos 2004; Li and Zheng 2004). TPX2 is an MT-associated protein intimately involved in chromatin-associated MT nucleation (reviewed in Kufer et al. 2003). After MTs are generated near the chromosomes, they then elongate and are “sorted” into a bipolar array with the proper MT polarity by various MT-associated motor molecules, some of which are bound to the chromosomes (reviewed in Gaglio et al. 1996; Walczak et al. 1998).
Before 1980, the prevailing idea was that the mechanism responsible for K-fiber formation (and chromosome motion) was highly conserved and was the same in all cells. However, the work in Xenopus and in yeast led to the view that multiple redundant mechanisms often exist within a cell to affect many of the various processes that make up mitosis. This appreciation prompted the question of whether the self-assembly pathway for K-fiber formation, used by plants and many animal oocytes, also exists in cells that normally use centrosomes to form their spindles and K fibers (Hyman and Karsenti 1998). The answer to this question required the development of approaches for removing centrosomes from animal somatic cells entering mitosis. The results of these studies revealed the answer to be “yes”: in the absence of centrosomes and their associated astral MT arrays, animal somatic cells form functional bipolar spindles with relatively normal kinetics via a self-assembly process, whether they be derived from flies (Debec et al. 1995) or mammals (Khodjakov et al. 2000; Hinchcliffe et al. 2001).
Given that many (if not all) animal cells are capable of building functional spindles without centrosomes, it became important to determine if the presence of centrosomes inhibits or depresses the self-assembly pathway or if it is intrinsic and always working in the background. This question was difficult to address because in the presence of centrosomes the density of astral MTs in the vicinity of the chromosomes after NEB is extremely high. Yet, by imaging rat kangaroo (PtK1) cells expressing green fluorescent protein (GFP)-tagged tubulin, Khodjakov et al. (2003) were able to show that K fibers often form without a direct connection to a centrosome in cells recovering from monastrol treatment as well as in untreated cells. It was unclear, however, whether the MTs for constructing these K fibers were spontaneously nucleated in the cytoplasm (and captured by the kinetochores), nucleated directly at the kinetochores, or derived from the correction of a syntenic attachment (in which both sister kinetochores initially become attached to a single pole).
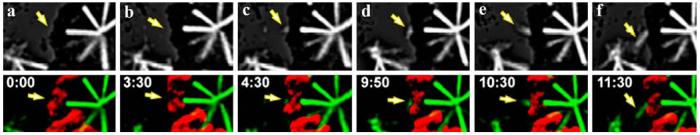
The next year, Maiato et al. (2004a), working with flattened centrosome-containing Drosophila S2 cells expressing GFP-tagged α-tubulin, found that K-MTs routinely form and grow from unattached kinetochores that are not facing a centrosome (Fig. 5). As in PtK1 cells, the growth of K fibers from kinetochores in S2 cells was seen only after a lag period; furthermore, once initiated, each fiber grew as a linear track toward a random point within the cell. During the course of spindle assembly, the ends of these elongating K fibers were then captured by astral MTs and transported toward the centrosome by a process requiring cytoplasmic dynein (Khodjakov et al. 2003; Maiato et al. 2004a). Here it is interesting to note that this mechanism involves cooperation between the kinetochore (MTs) and astral MTs (a spindle pole), which, although not exactly equivalent to Schrader's third possible route for K-fiber formation (see Abstract), orients and anchors K fibers generated by the second route.
Fig. 5.
Kinetochores in animal cells can nucleate MTs in the presence of a functional centrosome. a-f Frames from a fluorescence time-lapse recording of a Drosophila cell stably expressing GF/α-tubulin. The top frames show a deconvolved fluorescence image, while the bottom frames are an overlay of tubulin fluorescence (green) and chromosomes (red). The yellow arrow notes the nucleation and growth of MTs from an unattached kinetochore on a mono-oriented chromosome that is not facing a centrosome (from Maiato et al. 2004a)
The demonstration that kinetochores can seed MT formation in untreated animal cells containing centrosomes has several interesting ramifications. As noted by Wadsworth and Khodjakov (2004) and others (Gruss et al. 2002; Gadde and Heald 2004), it implies that spindle assembly “proceeds by a generally conserved acentrosomal mechanism in all higher eukaryotes, regardless of the presence of a centrosome.” In this view, the real function of the centrosome during mitosis is to generate two astral MT arrays; the presence of these arrays are needed to “collect” preassembled chromosome-generated minispindles into a single common bipolar spindle and also to properly position the spindle within the cell. Thus, since the discovery of K fibers in the late nineteenth century, the two “dueling” notions of how they form have resolved into a draw. Schrader's original conclusion in 1944 is correct: K fibers can form via two pathways, and in animals both work concurrently in the same cell (Schrader 1944).
How do kinetochores seed what will be their own MTs, and, once initiated, how do these MTs elongate? Although the molecular details remain incomplete, the first part of this question is better understood than the second. There is ample experimental evidence (see above) that a Ran-GTP gradient is established around sister kinetochores at NEB by RCC1 [also known as centromere-associated protein D (CENP-D); see Kingwell and Rattner 1997], which is highly concentrated at the centromere. As this gradient forms, TPX2 is released from its importin-α binding partner. This then allows TPX2 to promote the formation of short MT “stubs” on the kinetochore corona (Ris and Witt 1981), where tubulin subunits are highly concentrated (Pepper and Brinkley 1977; Mitchison and Kirschner 1985a). This scenario explains the observation reported by Witt et al. (1980), and later by De Brabander et al. (1981), that K-MTs first form between kinetochores that are in close proximity as cells recover from drugs that inhibit MT assembly. This is where the levels of Ran-GTP would be expected to be the highest.
Once nucleated in the kinetochore corona, short MTs begin to elongate by the addition of MT subunits onto their plus ends. The elevated levels of tubulin in the kinetochore region may facilitate this process. Once the corona-associated MTs begin to elongate, the MT minus ends are pushed away from the kinetochore. This may result from the action of kinetochore-associated stationary MT plus end motors, like CENP-E, that work along the MT lattice near its plus end (see Maiato et al. 2004b). Alternatively, the polymerization process itself may provide the force for pushing the MT minus end away from the kinetochore. Thus, as first implied by Witt et al. (1980) and Ris and Witt (1981), the kinetochore is indeed a unique type of MT-nucleating center: unlike the centriole and centrosome where the minus ends of nucleated MTs remain associated with the organelle, the minus ends of MTs nucleated by the kinetochore are pushed away from the organelle. As also noted by Ris and Witt (1981) (see also Witt et al. 1981), the subsequent binding of these MTs to the outer plate (or kinetochore proper) “is a separate and subsequent step in kinetochore MT bundle formation.” This could occur, e.g., as the plus ends of TPX2-induced MTs become associated with Ndc80, a complex of kinetochore-associated proteins including Hec1 and Nuf2 that is required for the stable attachment of MT plus ends to the kinetochore (reviewed in DeLuca et al. 2005).
In mature (metaphase) K fibers, MT subunits are constantly inserted into K-MT plus ends at the kinetochore, while they are removed at the same rate from the MT minus ends anchored in the polar region. This property of the K fiber is known as poleward MT subunit flux (Mitchison 1989). The kinetochore proteins that are involved in poleward MT subunit flux are just beginning to be identified. Unlike other MT TIP proteins (e.g., EB1, APC), clathrin-associated sorting proteins (CLASPs) target and bind kinetochores in the absence of MTs. Recently, Maiato et al. (2005) reported that knocking CLASPs down in S2 cells by RNAi does not inhibit K-fiber formation. However, it does prevent MT flux by inhibiting the incorporation of tubulin subunits into the MT plus ends at the kinetochore. This finding has an intriguing implication. It suggests that the growth of nascent K-fiber MTs from the kinetochore, and the subsequent maintenance of these MTs once the fiber matures by subunit flux, occurs by different mechanisms: the former appears to be CLASP independent, while the latter is not. One explanation for this result is that as MTs nucleated at the kinetochore elongate to form the K fiber, their MT plus ends are only loosely associated with the kinetochore/corona. Then, once the MT minus ends of the growing K fiber become incorporated into a spindle pole, the fiber begins to generate a force on the kinetochore as it becomes anchored in the pole and MT flux is initiated. In turn, the resulting tension on the kinetochore may induce it to become more tightly associated with its associated MT plus ends. After this more stable association is established, the CLASP protein then becomes essential for the continued maintenance of the now fluxing fiber, which requires the continuous addition of subunits into the K-MT plus ends.
Given that kinetochores can participate in forming their own K-MTs, why do many animal cells often contain unattached kinetochores for extended periods (see above, Fig. 4, and Rieder et al. 1994)? One possibility is that the ability of a kinetochore to generate its own MTs in the presence of a centrosome differs in different parts of the spindle. For example, mono-oriented chromosomes that undergo repeated oscillatory motions, as those associated with the periphery of the spindle often do, may not remain stationary long enough to generate a sufficient Ran-GTP gradient in the kinetochore region to seed MT assembly. The absence of MTs in the vicinity of “lost chromosomes” in newt cells (Fig. 4) may be related to the fact that they are well removed from the forming spindle and its various factors that influence MT assembly. Also, these cells have been reported to contain very little free MT protein (i.e., to be “starved” for tubulin; see Sato et al. 1976). Another question is why all of the growing K-MTs seeded by the kinetochore appear to elongate toward a common point in the cell. Does this occur because the capture sites in the kinetochore are rigid with respect to one another so that the elongating MTs remain parallel as they grow, or is it because neighboring K-MTs rapidly become cross-linked to one another by structural (e.g., nuclear mitotic apparatus) or motor (e.g., Eg5, dynein) proteins as they begin to elongate?
Summary
After 125 years of investigation, it is now clear that there are two routes for forming K fibers. Furthermore, both of these pathways can work simultaneously in the same animal cell during prometaphase, and both involve the capture of MT plus ends by the kinetochore. In the first route, components in the kinetochore corona trap and stabilize astral MTs that grow into their vicinity. This path prevails at NEB, and, with it, the initiation of K-fiber formation coincides with kinetochore orientation toward a pole and attachment to the forming spindle. The second route occurs after a lag period and involves the centromere-mediated assembly of short MT “stubs” in the kinetochore corona. These then begin to elongate by subunit addition onto their kinetochore-associated MT plus ends, which pushes the MT minus ends away from the kinetochore. These MTs become incorporated into the forming K fiber by laterally associating with other K-MTs, and/or the spindle pole, by a search-and-capture mechanism involving astral MTs and cytoplasmic dynein. The extent that this self-assembly route contributes to K-fiber formation in the presence of a centrosome remains to be determined. It is likely to be significant since inhibiting the self-assembly pathway in HeLa cells by knocking down TPX2 leads to the production of spindles that appear depleted of K fibers (Gruss et al. 2002; Moore et al. 2002). In addition, the self-assembly route provides a straightforward explanation for why nascent K fibers, generated from the capture of one or just several astral MTs, mature with a kinetics that exceeds that predicted from search and capture.
Acknowledgements
I thank Alexey Khodjakov and Helder Maiato for lively discussions related to this topic, Roger Sloboda and Susan Nowogrodzki for editorial assistance, and Bruce McEwen and Yimin Dong for the micrograph in Fig. 2c. This paper is dedicated to Dr. Hans Ris, who was my mentor from 1977 to 1980. The work in my laboratory is sponsored by National Institutes of Health General Medical Sciences grant 40198.
Footnotes
Communicated by E.A. Nigg
This article is dedicated to the memory of Hans Ris.
References
- Bergen LG, Kuriyama R, Borisy GG. Polarity of microtubules nucleated by centrosomes and chromosomes of Chinese hamster ovary cells in vitro. J Cell Biol. 1980;84:151–159. doi: 10.1083/jcb.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Walczak CE. Captivating capture: how microtubules attach to kinetochores. Curr Biol. 2003;13:R449–R460. doi: 10.1016/s0960-9822(03)00369-5. [DOI] [PubMed] [Google Scholar]
- Brenner S, Pepper D, Berns MW, Tan E, Brinkley BR. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981;91:95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma. 1966;19:28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- Carlson JG. Mitotic behavior of induced chromosomal fragments lacking spindle attachments in the neuroblasts of the grasshopper. Proc Natl Acad Sci U S A. 1938;24:500–507. doi: 10.1073/pnas.24.11.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L, Inoue S, Salmon ED. Microtubule dynamics in the chromosomal spindle fiber: analysis by fluorescence and high-resolution polarization microscopy. Cell Motil Cytoskelet. 1988;10:185–196. doi: 10.1002/cm.970100123. [DOI] [PubMed] [Google Scholar]
- Debec A, Detraves C, Montmory C, Geraud G, Wright M. Polar organization of gamma-tubulin in acentriolar mitotic spindles of Drosophila melanogaster cells. J Cell Sci. 1995;108:2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- De Brabander M, Geuens G, De Mey J, Joniau M. Nucleated assembly of mitotic microtubules in living PTK2 cells after release from nocodazole treatment. Cell Motil. 1981;1:469–483. doi: 10.1002/cm.970010407. [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED, McEwen BF. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz R. The dispensability of the centrioles in the spermatocyte division of Pales ferruginea (Nematocera) In: Darlington CD, Lewis L, editors. Chromosomes today. Oliver and Boyd; Edinburgh: 1966. pp. 161–166. [Google Scholar]
- Druner L. Studien uber den mechanismus der zellteilung. J Zeits Naturwiss. 1895:271–344. [Google Scholar]
- Euteneuer U, McIntosh JR. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981;89:338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, Ris H, Borisy GG. Polarity of kinetochore microtubules in Chinese hamster ovary cells after recovery from a colcemid block. J Cell Biol. 1983;97:202–208. doi: 10.1083/jcb.97.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming W. Beitrage zur kenntnis der zelle und ihrer lebenserscheinungen. Arch Mikrosk Anat. 1879;18:302–436. [Google Scholar]
- Flemming W. Zellsubstanz, kern und zelltheilung. F.C.W. Vogel; Leipzig: 1882. p. 424. 1882. [Google Scholar]
- Forer A. Local reduction of spindle fiber birefringence in living Nephrotoma suturalis (Loew) spermatocytes induced by ultraviolet microbeam irradiation. J Cell Biol. 1965;25:95–117. doi: 10.1083/jcb.25.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol. 2004;14:R797–R805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Sammak PJ, Borisy GG. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J Cell Biol. 1987;104:9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Vernos I. The mechanism of spindle assembly: functions of Ran and its target TPX2. J Cell Biol. 2004;166:949–955. doi: 10.1083/jcb.200312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Wittmann M, Yokoyama H, Pepperkok R, Kufer TA, Sillje H, Karsenti E, Mattaj IW, Vernos I. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat Cell Biol. 2002;4:41–49. doi: 10.1038/ncb870. [DOI] [PubMed] [Google Scholar]
- Harris P. Electron microscope study of mitosis in sea urchin blastomeres. J Biophys Biochem Cytol. 1961;11:419–431. doi: 10.1083/jcb.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TL. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- Holy TE, Leibler S. Dynamic instability of microtubules as an efficient way to search in space. Proc Natl Acad Sci USA. 1994;91:5682–5685. doi: 10.1073/pnas.91.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Schrader S. Reproduction in Acroschismus. J Morphol. 1924;39:157–207. [Google Scholar]
- Hyman A, Karsenti E. The role of nucleation in patterning microtubule networks. J Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Inoue S. The effect of colchicine on the microscopic and submicroscopic structure of the mitotic spindle. Exp Cell Res. 1952;(Suppl 2):305–311. [Google Scholar]
- Inoue S. Polarization optical studies of the mitotic spindle. I. The demonstration of spindle fibers in living cells. Chromosoma. 1953;5:487–500. doi: 10.1007/BF01271498. [DOI] [PubMed] [Google Scholar]
- Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Karsenti E, Newport J, Kirschner M. Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J Cell Biol. 1984;99:47s–54s. doi: 10.1083/jcb.99.1.47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell B, Rattner JB. Mammalian kinetochore/centromere composition: a 50 kDa antigen is present in the mammalian kinetochore/centromere. Chromosoma. 1997;95:403–417. doi: 10.1007/BF00333991. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Sandall S, Desai A. Kinetochore-spindle microtubule interactions during mitosis. Curr Opin Cell Biol. 2005;17:35–46. doi: 10.1016/j.ceb.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112:159–163. doi: 10.1007/s00412-003-0265-1. [DOI] [PubMed] [Google Scholar]
- Li HY, Zheng Y. Phosphorylation of RCC1 in mitosis is essential for producing a high RanGTP concentration on chromosomes and for spindle assembly in mammalian cells. Genes Dev. 2004;18:512–527. doi: 10.1101/gad.1177304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during mitosis in animals. J Cell Biol. 2004a;167:831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci. 2004b;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- Maiato H, Khodjakov A, Rieder CL. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibers. Nat Cell Biol. 2005;7:42–47. doi: 10.1038/ncb1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BF, Hsieh C-E, Mattheyses AL, Rieder CL. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107:366–375. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M, Brinkley BR. Human chromosomes and centrioles as nucleating sites for the in vitro assembly of microtubules from bovine brain tubulin. J Cell Biol. 1975;67:189–199. doi: 10.1083/jcb.67.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R. Beitrage zur Granulalehre. I. Kern und kerntheilung. Arch Anat Physiol. 1894:309–348. [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol. 1985a;101:755–765. doi: 10.1083/jcb.101.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. II. Microtubule capture and ATP-dependent translocation. J Cell Biol. 1985b;101:766–777. doi: 10.1083/jcb.101.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Evans L, Schultz EE, Kirschner MW. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986;45:515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Mole-Bajer J, Bajer A, Owczarzak A. Chromosome movements in prometaphase and aster transport in the newt. Cytobios. 1975;13:45–65. [Google Scholar]
- Moore WJ, Zhang C, Clarke PR. Targeting of RCC1 to chromosomes is required for proper mitotic spindle assembly in human cells. Curr Biol. 2002;12:1442–1447. doi: 10.1016/s0960-9822(02)01076-x. [DOI] [PubMed] [Google Scholar]
- Moroi Y, Hartman AL, Nakane PK, Tan EM. Distribution of kinetochore (centromere) antigen in mammalian cell nuclei. J Cell Biol. 1981;90:254–259. doi: 10.1083/jcb.90.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergren G. Transverse equilibria on the spindle. Bot Not. 1945;4:467–468. [Google Scholar]
- Pepper DA, Brinkley BR. Localization of tubulin in the mitotic apparatus of mammalian cells by immunofluorescence and immunoelectron microscopy. Chromosoma. 1977;60:223–235. doi: 10.1007/BF00329772. [DOI] [PubMed] [Google Scholar]
- Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J, Tippit DH. The diatom spindle in perspective. Cell. 1978;14:455–467. doi: 10.1016/0092-8674(78)90232-5. [DOI] [PubMed] [Google Scholar]
- Rieder CL. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cyt. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The attachment of kinetochores to the pro-metaphase spindle in PtK1 cells. Recovery from low temperature treatment. Chromosoma. 1981;82:693–716. doi: 10.1007/BF00285776. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Nowogrodzki R. Intranuclear membranes and the formation of the first meiotic spindle in Xenos peckii (Acroschismus wheeleri) oocytes. J Cell Biol. 1983;97:1144–1155. doi: 10.1083/jcb.97.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H, Witt PL. Structure of the mammalian kinetochore. Chromosoma. 1981;82:153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Sharp DJ. Spindle microtubules in flux. J Cell Sci. 2005;118:1105–1116. doi: 10.1242/jcs.02284. [DOI] [PubMed] [Google Scholar]
- Roos UP. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41:195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Roos UP. Light and electron microscopy of rat kangaroo cells in mitosis. III. Patterns of chromosome behavior during prometaphase. Chromosoma. 1976;54:363–385. doi: 10.1007/BF00292816. [DOI] [PubMed] [Google Scholar]
- Sato H, Ohnuki Y, Fujiwara K. Immunofluorescent anti-tubulin staining of spindle microtubules and critique for the technique. In: Goldman AR, Pollard T, Rosenbaum J, editors. Cell motility, book A. Cold Spring Harbor conference on cell proliferation. vol 3. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1976. pp. 419–433. [Google Scholar]
- Schmidt WJ. Doppelbrechung der kernspindel und zugfasertheorie der chromosomenbewegung. Chromosoma. 1939;1:253–264. [Google Scholar]
- Schrader F. Mitosis. Columbia University Press; New York: 1944. p. 110. [Google Scholar]
- Sharp LW. Introduction to cytology. McGraw-Hill; New York: 1934. [Google Scholar]
- Szollosi D, Calarco PD, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Telzer BR, Moses MJ, Rosenbaum JL. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc Natl Acad Sci USA. 1975;72:4023–4027. doi: 10.1073/pnas.72.10.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P, Khodjakov A. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Vernos I, Mitchison TJ, Karsenti E, Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Wilson EB. The cell in development and inheritance. MacMillan; New York: 1911. p. 483. [Google Scholar]
- Wilson EB. The cell in development and heredity. MacMillan; New York: 1925. p. 1232. [Google Scholar]
- Witt PL, Ris H, Borisy GG. Origin of kinetochore microtubules in Chinese hamster ovary cells. Chromosoma. 1980;81:483–505. doi: 10.1007/BF00368158. [DOI] [PubMed] [Google Scholar]
- Witt PL, Ris H, Borisy GG. Structure of kinetochore fibers: microtubule continuity and inter-microtubule bridges. Chromosoma. 1981;83:523–540. doi: 10.1007/BF00328277. [DOI] [PubMed] [Google Scholar]
- Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the “search-and-capture” process during mitotic spindle assembly. Curr Biol. 2005;15:826–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]