Abstract
Background/Objectives.
A rapid diagnosis of ST-segment elevation myocardial infarction (STEMI) is mandatory for optimal treatment. However, a small proportion of patients with suspected STEMI suffer from other conditions. Although case reports have described these conditions, a contemporary systematic analysis is lacking. We report the incidence, clinical characteristics and outcome of patients with suspected STEMI referred for primary percutaneous coronary intervention (PCI) with a final diagnosis other than STEMI.
Methods.
From January 2004 to July 2005, 820 consecutive patients were included with suspected STEMI who were referred for primary PCI to a university medical centre, based on a predefined protocol. Clinical characteristics, final diagnosis and outcome were obtained from patient charts and databases.
Results.
In 19 patients (2.3%), a final diagnosis other than myocardial infarction was established: coronary aneurysm (n=1), (myo)pericarditis (n=5), cardiomyopathy (n=2), Brugada syndrome (n=1), aortic stenosis (n=1), aortic dissection (n=3), subarachnoidal haemorrhage (n=2), pneumonia (n=1), chronic obstructive pulmonary disease (n=1), mediastinal tumour (n=1), and peritonitis after recent abdominal surgery (n=1). These patients less often reported previous symptoms of angina (p<0.001), smoking (p<0.05) and a positive family history of cardiovascular diseases (p<0.05) than STEMI patients. Mortality at 30 days was 16%.
Conclusion.
A 2.3% incidence of conditions mimicking STEMI was found in patients referred for primary PCI. A high clinical suspicion of conditions mimicking STEMI remains necessary. (Neth Heart J 2008;16:325-31.)
Keywords: ST-segment elevation myocardial infarction, differential diagnosis, coronary angiography, percutaneous coronary intervention
A rapid and accurate diagnosis of acute ST-segment elevation myocardial infarction (STEMI) is of crucial importance as early initiation of primary percutaneous coronary intervention (PCI) is beneficial to patients.1-3 In general, plaque rupture or plaque erosion and subsequent platelet aggregation and thrombosis resulting in acute occlusion of a coronary artery is considered the main mechanism of STEMI.4 However, coronary arteries without any stenosis have been reported in 2.6% of patients with suspected STEMI on acute diagnostic coronary angiography (CAG).5 Although some of these patients may actually suffer from myocardial infarction resulting merely from coronary spasm or a thrombus,5-7 others may have alternative diseases and conditions.
Various conditions may present with an identical electrocardiographic pattern as STEMI in clinical practice.8 First, transient ST-segment elevation can be present in acute coronary syndromes, especially in patients with significant coronary artery stenosis but no total occlusion.9 Second, cardiac conditions not affecting the coronary arteries, such as pericarditis and myocarditis, can also present in an infarction-like manner.8,10,11 A third group of conditions which sometimes have a STEMI-like presentation are of vascular origin, such as pulmonary embolism and aortic dissection.12,13 Finally, noncardiac conditions such as acute cholecystitis or pancreatitis may also mimic STEMI.14,15
Although these conditions simulating STEMI have been described in the literature, in particular in case reports, a contemporary systematic analysis is lacking with regard to the incidence and clinical features of conditions mimicking STEMI in patients referred for primary PCI. This study was performed to document the incidence, clinical characteristics as well as the outcome of patients with suspected STEMI and referred for primary PCI who had a final diagnosis other than STEMI.
Patients and Methods
Patients
From 1 January 2004 to 30 July 2005,820 consecutive patients with suspected STEMI referred for primary PCI were included in a registry of patients treated at our catheterisation laboratory. The setting of the study was a university medical centre with 24-hour primary PCI facilities providing emergency care with seven referral hospitals in a region with 750,000 inhabitants. Based on an agreement between cardiologists, all patients with suspected STEMI in this region were transported, referred and treated in our hospital in accordance with an ambulance paramedics driven and computer assisted STEMI protocol. Patients with (1) symptoms suggestive of myocardial infarction for at least 30 minutes, with (2) a time from onset of symptoms of less than 12 hours before presentation and with (3) an electrocardiogram (ECG) with ST-segment elevation of more than 0.1 mV in two or more leads or suspected new onset bundle branch block were transported to our catheterisation laboratory for acute CAG and subsequent primary PCI. The results of the initial assessment were sent by fax to the coronary care unit as well as the interventional cardiologist at the catheterisation laboratory. This strategy has been described previously.16 Upon this action, the catheterisation laboratory was activated. Whenever a diagnosis of STEMI was doubted, communication was established between the ambulance and the coronary care unit. Based on clinical judgement in combination with the information from the ECG, a decision to proceed to the catheterisation laboratory was taken. In the ambulance, aspirin (500 mg), clopidogrel (300 mg), and heparin (5000 IU) were administered. For patients presenting in referral hospitals, a similar medication regimen was followed.
Data collection
Clinical features and outcome of patients were obtained by patient chart and database review and enquiry at the referral hospitals. Patient data were reviewed individually by two authors and consensus was reached in all cases. The following data were collected: age, gender, heart rate and blood pressure on admission, cardiovascular risk factors, biochemical markers on admission, troponin and creatine kinase myocardial isoform (CK-MB) peak values, coronary angiographic data and 30-day mortality. Cardiovascular risk factors included hypertension, diabetes, smoking, a family history of cardiovascular disease, hypercholesterolaemia and a history of angina, myocardial infarction, stroke, coronary artery bypass grafting (CABG) and/or PCI. Major adverse cardiac as well as noncardiac events during hospital admission were documented. A normal CAG was defined as the absence of any coronary sclerosis. Nonsignificant coronary artery disease was defined as a stenosis less than 50%.
Final diagnosis
In those patients in whom no culprit lesion was found on CAG, the final diagnosis was based on agreement of at least three cardiologists. A coronary aneurysm was diagnosed by coronary angiography and confirmed at autopsy if the patient died during coronary angiography. (Myo)pericarditis was diagnosed when fever and/or elevated C-reactive protein (CRP) were present, a friction rub was audible and/or pericardial effusion was seen on echocardiography or computed tomography (CT) of the chest. When positive cardiac biomarkers were also present, a diagnosis of myo-pericarditis was made. Cardiomyopathy was diagnosed using ventriculography and echocardiography or single photon emission computed tomography (SPECT). A diagnosis of Brugada syndrome was made when ST-segment elevation in leads V1 to V3 was present on the ECG with or without right bundle branch block, and when structural heart disease was excluded by coronary angiography, ventriculography, echocardiography and exercise testing.17 Aortic stenosis was diagnosed using auscultation, echocardiography, cardiac catheterisation and CT of the chest. Aortic dissection and a mediastinal tumour were diagnosed by echocardiography or a CT of the chest. Subarachnoidal haemorrhage was diagnosed using CT of the head. The diagnosis of chronic obstructive pulmonary disease and peritonitis were based on clinical findings. Pneumonia was diagnosed by CT of the chest.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and were analysed using the two-sided Student's t test. Categorical variables are expressed as percentage and the Χ2 test was performed for analysis. Fisher's exact test was used when cell size was smaller than 5. An α of 0.05 was considered statistically significant.
Results
Incidence of conditions mimicking STEMI
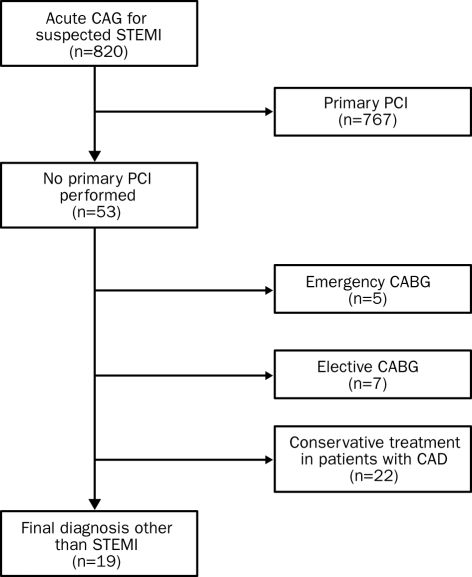
Among the 820 patients with suspected STEMI referred for primary PCI, 767 had coronary angiographic confirmation of the clinical and electrocardiographic diagnosis and underwent primary PCI. From the 53 other patients, five patients underwent emergency coronary artery bypass grafting (CABG), seven patients were scheduled for elective CABG and 22 patients with coronary artery disease were treated conservatively (figure 1). In the remaining 19 patients (2.3%), a final diagnosis other than STEMI was made.
Figure 1.
Selection of study population. In 53 out of 820 patients, CAG was not followed by PCI. Of these patients, 34 were treated with emergency or elective CABG or with conservative therapy, leaving 19 patients with a final diagnosis other than STEMI. CAG=coronary angiography, STEMI=ST-segment elevation myocardial infarction, PCI=percutaneous coronary intervention, CABG=coronary artery bypass grafting, CAD=coronary artery disease.
Clinical characteristics and outcome
Clinical characteristics of these 19 patients were compared with those of the other 801 (97.7%) patients in table 1. Patients with a final diagnosis other than STEMI less often reported angina (p<0.001) and had a lower prevalence of the conventional cardiovascular risk factors smoking (p<0.05) and a positive family history (p<0.05). Other risk factors such as diabetes and dyslipidaemia were rarely present in patients with a different diagnosis. On admission, pathological Q waves were present in two out of 13 (15%) patients with available ECGs. Of those patients in whom an alternative diagnosis was established, one patient (5%) had coronary pathology, nine patients (47%) had cardiac diseases, three patients (16%) had vascular conditions, two patients (11%) had neurological disorders, three patients (16%) had pulmonary conditions and in one patient (5%) abdominal pathology was found (table 2). Aspirin, heparin and clopidogrel were administered before arrival in 16/19 (84%) of these patients. In-hospital and 30-day mortality was 3/19 (16%).
Table 1.
Clinical characteristics of patients with a final diagnosis other than STEMI compared with STEMI patients.
| Characteristics | Other diagnosis (n=19) | STEMI (n=801) | P |
|---|---|---|---|
| Male sex (%) | 74 | 69 | NS |
| Age (years), mean ± SD | 59±14 | 63±13 | NS |
| Systolic blood pressure (mmHg) | 117±37 | 128±27 | NS |
| Diastolic blood pressure (mmHg) | 66±23 | 73±16 | NS |
| Heart rate (beats/min) | 81±13 | 78±19 | NS |
| Systemic hypertension (%) | 26 | 28 | NS |
| Previous MI (%) | 0 | 10 | NS |
| Previous stroke (%) | 0 | 4 | NS |
| Previous angina (%) | 11 | 57 | <0.001 |
| Previous CABG (%) | 0 | 4 | NS |
| Previous PCI (%) | 0 | 8 | NS |
| Diabetes (%) | 0 | 11 | NS |
| Smoking (%) | 21 | 48 | <0.05 |
| Family history (%) | 16 | 45 | <0.05 |
| Hypercholesterolaemia (%) | 16 | 35 | NS |
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as percentage. CABG=coronary artery bypass grafting, CVD=cardiovascular diseases, MI=myocardial infarction, NS=not significant, PCI=percutaneous coronary intervention, STEMI=ST-segment elevation myocardial infarction.
Table 2.
Final diagnoses of 19 patients with suspected STEMI referred for PCI.
| Diagnosis (%) | N | % |
|---|---|---|
| Coronary (5) | ||
| - Coronary aneurysm | 1 | 5 |
| Cardiac (48) | ||
| - Pericarditis | 3 | 16 |
| - Myopericarditis | 2 | 11 |
| - Cardiomyopathy | 2 | 11 |
| - Brugada syndrome | 1 | 5 |
| - Aortic stenosis | 1 | 5 |
| -Vascular (16) | ||
| - Aortic dissection | 3 | 16 |
| Neurological (11) | ||
| - Subarachnoidal haemorrhage | 2 | 11 |
| Noncardiovascular thoracic (15) | ||
| - Pneumonia | 1 | 5 |
| - COPD | 1 | 5 |
| - Mediastinal tumour | 1 | 5 |
| Abdominal (5) | ||
| - Peritonitis after recent abdominal surgery | 1 | 5 |
| Total | 19 | 100 |
COPD=chronic obstructive pulmonary disease, PCI=percutaneous coronary intervention, STEMI=ST-segment elevation myocardial infarction.
Coronary angiography
In the two patients with subarachnoidal haemorrhage, a diagnosis other than STEMI was suspected before or shortly after arrival in the hospital and CAG was not performed. In the other 17 patients, CAG was performed and showed normal coronary arteries in seven patients, nonsignificant coronary artery disease in five, single-vessel disease in one, three-vessel disease in two and a coronary aneurysm in one. Coronary artery lesions were not documented in one patient.
Final diagnoses
Coronary pathology (5%)
A 54-year-old male with suspected STEMI was admitted in profound cardiogenic shock. CAG showed a large aneurysm of the left main coronary artery. Autopsy showed a haemopericard and cardiac tamponade.
Cardiac pathology (48%)
Cardiac diagnoses included pericarditis (n=3), (myo)-pericarditis (n=2), cardiomyopathy (n=2), Brugada syndrome (n=1) and aortic stenosis (n=1) (table 3A). In all five patients with (myo)pericarditis, the CRP was elevated, and fever was present in four patients. CAG in one pericarditis patient showed stable coronary artery disease with stenoses of the left main and circumflex coronary arteries for which elective CABG was performed after five weeks. In one cardiomyopathy patient, systolic cavity obliteration was seen on ventriculography performed during the acute cardiac catheterisation. On echocardiography, marked concentric left ventricular hypertrophy was present. The other cardiomyopathy patient had a dilating cardiomyopathy with depressed left ventricular function. CAG showed normal coronary arteries. In both patients pharmacological therapy was initiated. Brugada syndrome was diagnosed in a 43-year-old male who received a cardiac defibrillator after complete clinical and electrophysiological evaluation. In a 60-year-old male without coronary risk factors and nonsignificant coronary artery disease, aortic stenosis was diagnosed with a peak pressure gradient of 88 mmHg. Troponin and cardiac enzymes were positive (troponin 498 μg/l, CK-MB 118 U/l). He underwent uncomplicated elective aortic valve replacement.
Table 3A.
Clinical characteristics of patients diagnosed with (myo)pericarditis.
| Patient | Gender | Age (years) | BP (mmHg) | HR (/min) | RF | Troponin (μg/l) | CK-MB (U/l) | Leuco (109/l) | CRP (mg/l) | Hb (mmol/l) | Creat (μmol/l) | Glucose (mmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 57 | 130/80 | 80 | 0 | NA | 2 | 9.8 | 60 | 8.7 | 93 | 7.6 |
| 2 | Male | 67 | 121/83 | 89 | 2 | NA | 1 | 12.4 | 47 | 7.7 | 74 | 7.7 |
| 3 | Male | 63 | 140/85 | 80 | 1 | <0.01 | NA | 15.1 | 83.4 | 8.3 | 61 | 6.8 |
| 4 | Male | 31 | 92/54 | 86 | 1 | 8 | 14 | 8 | 59 | 9.3 | 81 | 6.6 |
| 5 | Male | 51 | 130/90 | 100 | 0 | 0.38 | 17 | 7.1 | 110.1 | 8.8 | 105 | 5.6 |
BP=blood pressure, HR=heart rate, CK-MB=creatine kinase myocardial isoform, Creat=creatinine, CRP=C-reactive protein, Hb=haemoglobin, Leuco=leucocyte count, NA=data not available, RF=number of cardiovascular risk factors, for detailed definition see Methods section.
Vascular pathology (16%)
Aortic dissection type A was diagnosed in three patients (table 3B). All three patients underwent emergent supracoronary ascending aorta replacement. Concomitant CABG of the left anterior descending artery (n=1) and left anterior descending artery and right coronary artery (n=1) was performed. Two patients made a full recovery and were in New York Heart Association class I after 17 months of follow-up. Postoperative haemodynamics deteriorated after surgery in the third patient and he died on the first postoperative day.
Table 3B.
Clinical characteristics of patients diagnosed with aortic dissection.
| Patient | Gender | Age (years) | BP (mmHg) | HR (/min) | RF | Troponin (μg/l) | CK-MB (U/l) | Leuco (109/l) | CRP (mg/l) | Hb (mmol/l) | Creat (μmol/l) | Glucose (mmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 59 | 74/40 | 72 | 2 | 186 | 56 | 9.1 | <4 | 7.6 | 115 | 5.4 |
| 2 | Male | 49 | 115/80 | 60 | 0 | 10.9 | 65 | 6.1 | NA | 8.2 | 111 | 7.6 |
| 3 | Male | 61 | 82/30 | 87 | 2 | 3736 | 269 | 14.1 | 18 | 9.4 | 200 | 17.2 |
BP=blood pressure, HR=heart rate, CK-MB=creatine kinase myocardial isoform, Creat=creatinine, CRP=C-reactive protein, Hb=haemoglobin, Leuco=leucocyte count, NA=data not available, RF=number of cardiovascular risk factors, for detailed definition see Methods section.
Neurological pathology (11%)
In two patients with signs of inferior and inferolateral infarction on the 12-lead ECG, a diagnosis ofsub-arachnoidal haemorrhage was made (table 3C). CAG was not performed in these two patients because of a subcomatose state occurring shortly before or after arrival at our hospital. CT in both patients showed bleeding from the anterior communicating artery. One patient developed a hydrocephaly requiring extraventricular drainage and had a rebleed prior to a coiling procedure of the anterior communicating artery. She recovered with moderate cognitive impairment. The other patient received an extraventricular drain but died of rebleeding on the 12th day after admission.
Table 3C .
Clinical characteristics of patients diagnosed with subarachnoid haemorrhage.
| Patient | Gender | Age (years) | BP (mmHg) | HR (/min) | RF | Troponin (μg/l) | CK-MB (U/l) | Leuco (109/l) | CRP (mg/l) | Hb (mmol/l) | Creat (μmol/l) | Glucose (mmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 73 | 88/40 | 70 | 0 | 0.2 | 6 | 5.9 | <4 | 8.5 | 94 | 8.3 |
| 2 | Female | 70 | 220/120 | 105 | 1 | 5.9 | 13 | 29.6 | NA | 7.7 | 132 | 9.2 |
BP=blood pressure, HR=heart rate, CK-MB=creatine kinase myocardial isoform, Creat=creatinine, CRP=C-reactive protein, Hb=haemoglobin, Leuco=leucocyte count, NA=data not available, RF=number of cardiovascular risk factors, for detailed definition see Methods section.
Noncardiovascular thoracic pathology (15%)
A 43-year-old male presenting with shock had normal coronary arteries. A diagnosis of pneumonia was confirmed by CT of the chest showing consolidation in the right lower lobe and a pleural effusion. An exacerbation of chronic obstructive pulmonary disease was diagnosed in an 85-year-old female with a STEMI-like presentation probably due to a combination of hypoxia and an old anterior myocardial infarction in combination with marked left ventricular hypertrophy. A 5 3-year-old male with normal coronary arteries had a mediastinal tumour with pulmonary metastases. He died seven months after presentation.
Abdominal pathology (5%)
A 76-year-old female underwent CAG which showed three-vessel disease but no unstable lesion or occlusion compatible with a culprit vessel. Further analysis established a diagnosis of peritonitis after recent abdominal surgery.
Discussion
This study shows that an alternative diagnosis was made in 2.3% of patients with suspected STEMI referred for primary PCI to a university medical centre receiving STEMI patients based on 12-lead ECG data in combination with clinical assessment performed in the ambulance and supported by communication with coronary care unit staff. Although the clinical characteristics of these patients differ somewhat from those in the STEMI patients, the available data do not allow a solid differentiation between patients who have and those who do not have a myocardial infarction. To our knowledge, this is the first systematic analysis studying the incidence, clinical characteristics and outcome of conditions mimicking STEMI in patients referred for primary PCI in whom STEMI has been excluded and a definitive alternative diagnosis has been established.
Diagnoses other than STEMI
Pericarditis or myopericarditis remains one of the most frequently reported diseases presenting as STEMI.8,10,11,18,19 Costantini and colleagues19 reported that myocarditis patients are generally young males with a low coronary risk profile. In agreement, (myo)-pericarditis was found in five patients in our group who were all male, with an age ranging from 31 to 67, and none of them had more than two cardiovascular risk factors (table 3A). Brugada syndrome, an inherited disease with ST-segment elevation in leads V1 to V3 with or without right bundle branch block on the ECG and no structural heart disease, has been misdiagnosed as STEMI previously.20,21 Coronary aneurysm and aortic stenosis are known causes of myocardial infarction without atherosclerosis.22 Multiple (case) reports have documented cardiomyopathies, aortic dissection and subarachnoidal haemorrhage as the underlying cause in patients with ST-segment elevation on the 12-lead ECG.5,18,23-26 Pneumonia is another condition known to mimic myocardial infarction.27 A mediastinal tumour may lead to compression of coronary, myocardial and/or pericardial structures.
Previous studies
In general, acute myocardial infarction (AMI) is reported to be excluded in 1.4 to 13% of patients with acute chest pain and ST-segment elevation.5,28-30 In suspected AMI patients who were admitted to a coronary care unit and treated with thrombolytic agents, AMI was ruled out in 35/609 (5.7%) of patients as reported by Khoury and colleagues.29 In their report, they did not find demographic or clinical features that distinguished non-AMI patients from AMI patients. Unstable angina accounted for 20 (57%) patients. Other alternative diagnoses included undefined chest pain, pericarditis, pancreatitis, oesophagitis and aortic dissection. Widimsky and colleagues reported normal acute coronary angiograms in 26/1004 (2.6%) of patients with suspected STEMI.5 The diagnosis of AMI was made in a total of seven patients (0.7%) with normal angiograms. Besides (myo)peri-carditis and dilated cardiomyopathy, their alternative diagnoses included hypertension with left ventricular hypertrophy, pulmonary embolism and misinterpretation of the ECG. Remarkably, they found no patients with a noncardiac cause of the STEMI-like presentation except for pulmonary embolism. In a report describing a range of noncoronary diagnoses in 958 STEMI patients by Larson and colleagues, false-positive ST-segment elevation was described in 6.2% of their patients.31 Our findings seem to be in line with the incidence rates as reported in the literature.
Implications
In patients with STEMI, minimising the time to CAG and subsequent reperfusion therapy is essential.1-3 At present, prompt diagnosis of STEMI can be made by ambulance paramedics in approximately 95% of all patients.32 However, our study showed that in 2.3% of patients with symptoms and an ECG compatible with STEMI, a different diagnosis was present. In these patients, CAG is not beneficial and may even be unfavourable. In other words, time pressure may compromise accuracy and may lead to inappropriate activation of the catheterisation laboratory. This may result in delay in establishing the right diagnosis and delivery of appropriate treatment with possibly serious consequences in patients in whom an initial diagnosis of STEMI was made inappropriately. Performing a CAG as the initial diagnostic test may also cause some delay, as echocardiography or CT may be a more appropriate initial test to exclude a diagnosis of STEMI and to differentiate it from other conditions, depending on the clinical situation.
Antithrombin and antiplatelet therapy was also administered in the ambulance to patients who did not have STEMI. Although their use is beneficial in patients with STEMI, these agents are contraindicated in at least some conditions mimicking STEMI, especially in those requiring surgery, and may have influenced the clinical course in these patients by aggravating the underlying condition or inducing complications. Two patients who received antithrombin and antiplatelet therapy died, one with an aortic dissection and one with a subarachnoidal haemorrhage, although the role of aspirin, heparin and clopidogrel on their clinical course is not clear. In patients with an aortic dissection misinterpreted as AMI and treated with thrombolytics, lethal results have been reported.23,24
Limitations
We could not define patient characteristics that identified patients with an alternative diagnosis. Although absence of previous episodes of angina and of pathological Q waves is present in a large majority of patients with an alternative diagnosis, the specificity of these two parameters is far below clinical application.
This is a single-centre study representing only one of the various prehospital diagnostic strategies for STEMI. Considering the known shortcomings of retrospective studies, inaccuracies may have occurred during analysis and some patients may have been misclassified or excluded in the study. Furthermore, with our number of patients included in the analysis, the findings of this study may be influenced by chance. Future larger volume, multicentre studies may thus be warranted.
Conclusion
Our study shows a 2.3% incidence of a final diagnosis other than STEMI in 820 patients with suspected STEMI referred for primary PCI. The diagnoses included cardiac, vascular, neurological, thoracic and abdominal conditions. The clinical characteristics of these patients could not differentiate them from patients with STEMI. Overall, mortality at 30 days was three out of 19 patients, two of whom had received antithrombin and antiplatelet therapy. Depending on the clinical situation, a diagnostic CAG is often necessary but echocardiography or CT may be a more appropriate initial diagnostic test to exclude a diagnosis of STEMI and to differentiate it from other conditions. Thus, maintaining a high clinical suspicion of conditions mimicking STEMI is crucial as the advantages of timely intervention in patients with STEMI should be balanced against the possible risks caused by antithrombin and antiplatelet therapy and by a delay in the treatment of the underlying disease in case of misdiagnosis.
References
- 1.Bradley EH, Herrin J, Wang Y, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med 2006;355:2308-20. [DOI] [PubMed] [Google Scholar]
- 2.Brodie BR, Stuckey TD, Wall TC, et al. Importance of time to reperfusion for 30-day and late survival and recovery of left ventricular function after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 1998;32:1312-9. [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra F, Patel A, Jones M, et al. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2-4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J 2002;23:550-7. [DOI] [PubMed] [Google Scholar]
- 4.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897-902. [DOI] [PubMed] [Google Scholar]
- 5.Widimsky P, Stellova B, Groch L, et al. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Can J Cardiol 2006;22:1147-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3-year follow-up study of 91 patients. Eur Heart J 2001;22:1459-65. [DOI] [PubMed] [Google Scholar]
- 7.Raymond R, Lynch J, Underwood D, Leatherman J, Razavi M. Myocardial infarction and normal coronary arteriography: a 10 year clinical and risk analysis of 74 patients. J Am Coll Cardiol 1988; 11:471-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128-35. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2002;23:1809-40. [DOI] [PubMed] [Google Scholar]
- 10.Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart 2000;84:245-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surawicz B, Lasseter KC. Electrocardiogram in pericarditis. Am J Cardiol 1970;26:471-4. [DOI] [PubMed] [Google Scholar]
- 12.Livaditis IG, Paraschos M, Dimopoulos K. Massive pulmonary embolism with ST elevation in leads V1-V3 and successful thrombo-lysis with tenecteplase. Heart 2004;90:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spittell PC, Spittell JA Jr, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 1993;68:642-51. [DOI] [PubMed] [Google Scholar]
- 14.Ryan ET, Pak PH, DeSanctis RW. Myocardial infarction mimicked by acute cholecystitis. Ann Intern Med 1992;116:218-20. [DOI] [PubMed] [Google Scholar]
- 15.Fulton MC, Marriott HJ. Acute pancreatitis simulating myocardial infarction in the electrocardiogram. Ann Intern Med 1963;59: 730-2. [DOI] [PubMed] [Google Scholar]
- 16.Bouten MJ, Simoons ML. Strategies for pre-hospital thrombo-lysis: an overview. Eur Heart J 1991;12 (Suppl G):39-42. [PubMed] [Google Scholar]
- 17.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992;20:1391-6. [DOI] [PubMed] [Google Scholar]
- 18.Elsman P, Alleman MA, Zijlstra F. [Clinical presentations mimicking acute myocardial infarction; therapeutic pitfalls]. Ned Tijd-schr Geneeskd 1998;142:1057-60. [PubMed] [Google Scholar]
- 19.Costantini M,Tritto C, Licci E, et al. Myocarditis with ST-Elevation Myocardial Infarction presentation in young man. A case series of 11 patients. Int J Cardiol 2005;101:157-8. [DOI] [PubMed] [Google Scholar]
- 20.Ozeke O, Aras D, Deveci B, Yildiz A, Maden O, Selcuk MT. Brugada-like early repolarization pattern misdiagnosed as acute anterior myocardial infarction in a patient with myocardial bridging of the left anterior descending artery. Mt Sinai J Med 2006;73:627-30. [PubMed] [Google Scholar]
- 21.Sajeev CG, Vinayakumar D, Venugopal K. Brugada syndrome simulating acute myocardial infarction. Int J Cardiol 2005;99:155-6. [DOI] [PubMed] [Google Scholar]
- 22.Antman EM, Braunwald E. ST-elevation myocardial infarction: pathology, pathophysiology, and clinical features. In: Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald's heart disease: a textbook of cardiovascular medicine. Philadelphia, PA: Elsevier Saunders; 2005:1141-65. [Google Scholar]
- 23.Blankenship JC, Almquist AK. Cardiovascular complications of thrombolytic therapy in patients with a mistaken diagnosis of acute myocardial infarction. J Am Coll Cardiol 1989;14:1579-82. [DOI] [PubMed] [Google Scholar]
- 24.Kahn JK. Inadvertent thrombolytic therapy for cardiovascular diseases masquerading as acute coronary thrombosis. Clin Cardiol 1993;16:67-71. [DOI] [PubMed] [Google Scholar]
- 25.Gu YL, van den Heuvel AFM, Erasmus ME, Zijlstra F. Aortic dissection presenting as acute myocardial infarction: potential harm of antithrombin and antiplatelet therapy. Neth Heart J 2006;14:147-9. [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura Y, Kaseno K, Kubo T. Transient ST-segment elevation in subarachnoid hemorrhage. J Electrocardiol 1989;22:133-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Cannon CP. Approach to the patient with chest pain. In: Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald's heart disease: a textbook of cardiovascular medicine. Philadelphia, PA: Elsevier Saunders; 2005:1129-39. [Google Scholar]
- 28.Chapman GD, Ohman EM, Topol EJ, et al. Minimizing the risk of inappropriately administering thrombolytic therapy (Thrombolysis and Angioplasty in Myocardial Infarction [TAMI] study group). Am J Cardiol 1993;71:783-7. [DOI] [PubMed] [Google Scholar]
- 29.Khoury NE, Borzak S, Gokli A, Havstad SL, Smith ST, Jones M. “Inadvertent” thrombolytic administration in patients without myocardial infarction: clinical features and outcome. Ann Emerg Med 1996;28:289-93. [DOI] [PubMed] [Google Scholar]
- 30.Terkelsen CJ, Lassen JF, Norgaard BL, et al. Reduction of treatment delay in patients with ST-elevation myocardial infarction: impact of pre-hospital diagnosis and direct referral to primary percutaneous coronary intervention. Eur Heart J 2005;26:770-7. [DOI] [PubMed] [Google Scholar]
- 31.Larson DM, Menssen KM, Johnson RK, et al. False positive ST elevation in patients undergoing direct percutaneous coronary intervention. Circulation 2006;114:II-346 [Google Scholar]
- 32.van 't Hof AW, Rasoul S, van de Wetering H, et al. Feasibility and benefit of prehospital diagnosis, triage, and therapy by paramedics only in patients who are candidates for primary angioplasty for acute myocardial infarction. Am Heart J 2006;151:1255-5.e5. [DOI] [PubMed] [Google Scholar]