Abstract
Mutations that are supposed to affect right (RV) and left ventricular (LV) electrophysiology equally, often reveal dominant conduction slowing and arrhythmia vulnerability in RV. In this study we investigated the mechanism of dominant arrhythmia vulnerability of RV in senescent mice. We performed epicardial ventricular activation mapping on adult and senescent Langendorff perfused hearts. Longitudinal and transversal conduction velocity, as well as arrhythmia inducibility were determined. Subsequently, hearts were processed for immunohisto-chemistry and Picro Sirius Red staining. Senescent mice revealed decreased conduction velocity, increased aniso-tropic ratio and reduced excitation wavelength in RV, but not in LV. Arrhythmias were mainly induced in RV of senescent hearts. No arrhythmias were induced in adult hearts. Immunohistochemistry revealed that the amount of Connexin 43 and cardiac sodium channel Nav1 .5 were equally decreased, and that collagen content was equally increased in senescent RV and LV. However, patches of replacement fibrosis were found throughout the RV wall, but only in the sub-endocardium and mid-myocardium of LV. The study shows that the dominant arrhythmia vulnerability in RV of senescent mice is caused by the distribution of replacement fibrosis which involves the entire RV but only part of the LV. (Neth Heart J 2008;16:356-8.)
Keywords: tachyarrhythmia, conduction, collagen, gap junction, mapping
For good cardiac function, it is necessary that cardiomyo-cytes are adequately coupled, easily excitable, and that tissue architecture ensures normal impulse propagation. If one or more of these factors are impaired, an arrhythmogenic substrate may arise. Ventricular arrhythmias are considered a major cause of sudden cardiac death. In cardiac diseases such as Brugada syndrome and arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C), mutations are thought to be present that involve the entire heart. Patients with Brugada syndrome may carry mutations in the cardiac sodium channel Nav1.5, which can affect excitability of cardiomyo-cytes.1 ARVD/C may be related to mutations in desmosomal proteins, which can affect the cell-to-cell coupling.2 Despite the fact that these mutations affect the entire heart, the right ventricle (RV) is usually found to be more vulnerable to arrhythmias than the left ventricle (LV) in Brugada syndrome and ARVD/C. The phenotypes mentioned before often become more pronounced as patients age.
Previous studies performed in our department have shown that a decline in cell-cell coupling, caused by reduced (90%) Connexin43 (Cx43) expression, results in conduction slowing and arrhythmia vulnerability predominantly in RV.3 Impaired excitability by reduced (50%) Nav1.5 expression also resulted in conduction slowing, mainly in RV.4 Furthermore, it was found that these conduction abnormalities become more severe with increasing age. To determine why arrhythmia vulnerability is greater in RV than LV we used senescent mice as a model of generally reduced myocardial integrity. Wild-type mice were aged up to about 22 months and compared electrophysiologically and histologically with adult animals (3 months).
Echocardiographic measurements showed that cardiac function was preserved in senescent mice. Fractional shortening, cardiac output and ejection fraction, parameters for LV systolic function, were stable over time. ECG analysis showed that aged animals had increased PQ intervals and QRS durations, which suggests delayed atrioventricular and ventricular conduction.
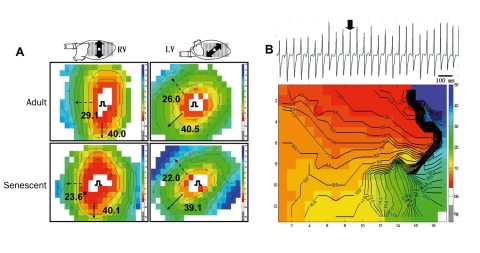
Epicardial mapping showed that transversal conduction velocity (perpendicular to the fibre orientation, CVT) was significantly reduced in senescent RV compared with adult RV, as illustrated in figure 1A. The reduced transversal conduction velocity evokes an increase in anisotropic ratio, a factor significantly contributing to arrhythmogenesis. Longitudinal and transversal conduction velocity in LV and longitudinal conduction velocity in RV were not significantly changed. The ventricular effective refractory period (the longest premature stimulus that is unable to activate the entire heart, ERP) was significantly reduced in senescent RV compared with senescent LV. As a result, the excitation wavelength (product of conduction velocity and refractory period) was significantly reduced in senescent RV only.
Figure 1.
A. Examples of RV and LV epicardial activation maps of adult and senescent mouse hearts. Hearts were paced from the centre of the grid. Conduction velocity (in cm/s) is estimated in longitudinal (bold arrows) and transversal (dashed arrows) direction. Red denotes earliest and blue latest activation. Isochronal lines are set at 1 ms interval. B. Monomorphic sustained ventricular tachycardia in senescent RV. Bold arrow indicates complex used to make the activation map. Anisotropic reentry is evident: the activation front spreads anticlockwise around an area of conduction block (crowded isochronal lines).
We were unable to induce arrhythmias by programmed stimulation in RV and LV of adult mice. In senescent hearts, however, monomorphic ventricular arrhythmias were induced in 16 out of 29 hearts. These arrhythmias were mainly induced in RV: in 13 out of 16 inducible hearts arrhythmias could only be induced in RV, whereas arrhythmias were induced in LV only in one heart. In the two remaining hearts, arrhythmias could be induced from both ventricles. Of the ventricular tachycardias, 24% were non-sustained (5 to 15 complexes), whereas 21% were sustained (>15 complexes). Figure 1B shows a monomorphic sustained ventricular tachycardia, which was induced in RV of a senescent mouse by burst pacing. Figure 1B shows that the impulse revolves counterclockwise around a zone of conduction block, rendered by crowding of the isochronal lines.
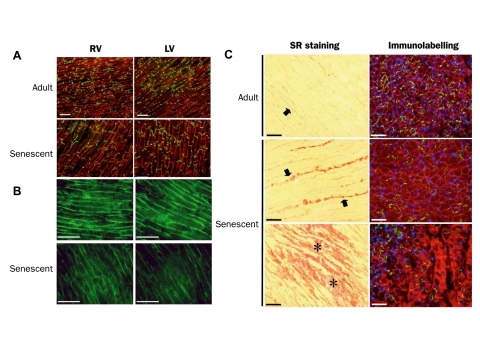
After the electrophysiological measurements, hearts were processed for histological examination. Immunohistochemistry showed that LV and RV had a similar expression pattern for the ventricular gap junction protein Cx43 in adult hearts. Cx43 was expressed homogeneously throughout the ventricles, and could be found both in the intercalated discs and at the lateral borders of the cardiomyocytes. In senescent RV and LV a redistribution of Cx43 from the lateral borders to the intercalated disc occurred (figure 2A, lower panels). There was no difference between the amount of Cx43 and its distribution in RV and LV of senescent hearts.
Figure 2.
A. Expression pattern of cardiac gap junction protein Cx43 in adult and senescent RV and LV. B. Expression pattern of the cardiac voltage gated sodium channel Nav1.5 in adult and senescent RV and LV. C. Left panels show collagen distribution in LV of an adult and senescent mouse heart. Bold arrows indicate interstitial fibrosis, asterisks replacement fibrosis. Right panels show dystrophin (red), Cx43 (green) and nuclei distribution (blue) in areas of interstitial and replacement fibrosis in adult and senescent LV.
Figure 2B shows immunolabelling of the cardiac sodium channel Nav1 .5 in RV and LV of adult and senescent hearts. As can be appreciated from this figure, the Nav1.5 labelling in adult hearts seems uniform throughout the ventricle, with no differences between RV and LV. In senescent hearts, however, signal intensity was reduced and expression became heterogeneous. Also here, no differences were found between RV and LV.
In normal cardiac tissue collagen deposition is present between the fibres of cardiomyocytes (interstitial fibrosis). Disease or ageing may increase interstitial fibrosis. Also patches of fibrosis can arise due to disease or ageing replacing normal healthy cardiac tissue (replacement fibrosis). Sections of adult and senescent hearts were stained with Picro Sirius Red, which marks collagen. As can be seen in figure 2C, the adult heart shows thin strands of interstitial fibrosis (bold arrows). Immunolabelling with dystrophin (cell borders), Cx43, and Dapi (nucleus labelling) in areas of slight interstitial fibrosis showed a homogeneous expression pattern. Senescent hearts, however, revealed increased amounts of interstitial fibrosis, indicated by thick strands between the fibres. Also patches of replacement fibrosis were found in both left and right ventricle of senescent hearts (lower panel). At sites with severe fibrosis, immunolabelling showed that expression of Cx43 is reduced, dystrophin aspecifically reacts with the deposed collagen, and nuclei are virtually absent, indicating that this is true replacement fibrosis.
Most striking was the difference in distribution of these patches of replacement fibrosis in RV and LV. In RV patches were found throughout the entire free wall, whereas in LV patches were only found in the sub-endocardium and mid-myocardium, and virtually absent in the sub-epicardium. Patches of replacement fibrosis were not found in adult hearts.
In summary, this study shows that senescent mice have a decreased conduction velocity, increased anisotropic ratio and reduced excitation wavelength in RV, but not in LV. Arrhythmias could not be induced in adult hearts. RV of senescent mice was more vulnerable to arrhythmias than LV. Cx43 and Nav1.5 expression was decreased, and collagen content increased compared with adults in both RV and LV. Collagen distribution, however, is different in senescent RV and LV: patches of replacement fibrosis could be found throughout the entire RV free wall, in contrast to LV where fibrosis was found only in endocardium and mid-myocardium.
Reduced excitation wavelength is required for maintenance of reentrant arrhythmia alongside an area of conduction block. The patches of replacement fibrosis present throughout the entire right ventricular wall probably form the arrhythmogenic substrate for conduction block and reentry. In LV these patches are not present in the sub-epicardial region of the heart, the area from which the ventricles were stimulated, which could explain the lower arrhythmia vulnerability of LV.
As mentioned before, several cardiac disorders caused by mutations affect RVmore than LV. These disorders are often associated with differences in collagen distribution in RV and LV. This suggests that prevention of collagen development in these hearts could reduce the arrhythmogenic vulnerability of RV. It has been shown that eplerenone, an aldosterone inhibitor, given to cardiomyopathic hamsters, reduced the level of fibrosis by 50% after three months of treatment compared with untreated animals. This drug has a beneficial effect on both cardiac remodelling and the electrical properties of the failing heart.5 Possibly patients suffering from genetic diseases such as Brugada syndrome and ARVD/C could also benefit from treatment with a drug that prevents development of fibrosis. Obviously this assumption requires a further experimental follow-up.
References
- 1.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol 2006;29:1130-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kies P, Bootsma M, Bax J, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm 2006;3:225-34. [DOI] [PubMed] [Google Scholar]
- 3.van Rijen HV, Eckardt D, Degen J, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004;109:1048-55. [DOI] [PubMed] [Google Scholar]
- 4.van Veen TA, Stein M, Royer A, et al. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation 2005; 112:1927-35. [DOI] [PubMed] [Google Scholar]
- 5.De Mello WC. Beneficial effect of eplerenone on cardiac remodelling and electrical properties of the failing heart. J Renin Angiotensin Aldosterone Syst 2006;7:40-6. [DOI] [PubMed] [Google Scholar]