Abstract
With increasing knowledge of basic molecular mechanisms governing the development of heart failure (HF), the possibility of specifically targeting key pathological players is evolving. Technology allowing for efficient in vivo transduction of myocardial tissue with long-term expression of a transgene enables translation of basic mechanistic knowledge into potential gene therapy approaches. Gene therapy in HF is in its infancy clinically with the predominant amount of experience being from animal models. Nevertheless, this challenging and promising field is gaining momentum as recent preclinical studies in larger animals have been carried out and, importantly, there are 2 newly initiated phase I clinical trials for HF gene therapy. To put it simply, 2 parameters are needed for achieving success with HF gene therapy: (1) clearly identified detrimental/beneficial molecular targets; and (2) the means to manipulate these targets at a molecular level in a sufficient number of cardiac cells. However, several obstacles do exist on our way to efficient and safe gene transfer to human myocardium. Some of these obstacles are discussed in this review; however, it primarily focuses on the molecular target systems that have been subjected to intense investigation over the last decade in an attempt to make gene therapy for human HF a reality.
Keywords: gene therapy, heart failure, cardiomyocyte, myocardial contractility
Heart failure (HF) (a cardiac condition that impairs the ability of the ventricle to fill with or eject blood) is the ultimate consequence of a vast number of cardiovascular diseases and constitutes 1 of the leading worldwide causes of morbidity and mortality. Pharmacological intervention with β-adrenergic receptor (β-AR) antagonists, inhibitors of angiotensin II and aldosterone, and diuretics are currently standard treatments for HF. Introduction of these pharmacological interventions have substantially increased survival and decreased morbidity in HF; however, these agents are far from ideal. As a consequence, there is an urgency to discover and develop novel and improved therapeutic strategies. Over the last 10 to 15 years, there has been a substantial increase in the understanding of cellular and molecular alterations that take place in HF, and this knowledge has resulted in the testing of hypotheses based on targeting molecular entities that appear to be involved in HF pathogenesis. Importantly, these scientific developments have provided a rationale for correcting human HF by directly intervening within the genetic/molecular foundation of the cardiomyocyte.
With the emergence and growth of translational cardiovascular research, the application of gene therapy for HF is no longer a futuristic “maybe.” However, to date, only 2 human clinical trials (both targeting SR Ca2+-ATPase [SERCA2a]) have been initiated with the aim of treating chronic human HF through gene delivery. These studies will be of critical importance to a few other genes/targets that are on the verge of human trials. Needless to say, this area of research is still in its infancy, and there are large numbers of signaling pathways that have potential pathophysiological significance, and these are currently at different stages of investigation. This review details the present status of HF gene therapy, emphasizing the most promising (and most explored) molecular target systems, as well as discussions regarding some prerequisites needed for successful human intervention.
Practical Considerations
Gene Therapy for HF: Searching for the “Golden Bullet”
In principle, gene therapy in HF must be aimed at correcting key molecular mechanisms in cardiac tissue that reduce/reverse the inevitable cardiac deterioration. This requires introduction of DNA/RNA that targets specific cardiomyocyte processes that alter HF outcomes. As such, prevention of lethal arrhythmias, acute cardiac decompensation, and prevention of end-stage pump failure must be achieved. This is a formidable task, and although it is unlikely that a simple “one target fits all” approach will be adequate, specific targets are being investigated and do appear to prevent or rescue HF in animal models. This single target approach is potentially possible because, although the etiologies off human HF are diverse, there are common biological and molecular signaling pathways within the failing cardiomyocyte that appear to exist. The ideal HF gene therapy strategy may depend on the specific cause of the condition (ie, ischemic, valvular, hypertensive, genetic, etc), perhaps requiring application of a combinatorial approach that targets different cardiac cells and signaling pathways. As an example, gene therapy for HF established as a consequence of ischemic heart disease may ideally involve: (1) targeting vascular cells by stabilizing coronary plaques and induction of neoangiogenesis; (2) providing means to prevent viable cardiomyocytes from dying (via inhibiting apoptosis and/or necrosis) and providing long-term inotropic support without adverse effects; (3) preventing or reducing myocardial remodeling by targeting fibroblasts; and (4) targeting myocardial electrophysiological abnormalities reducing the risk of arrhythmias.
Common to the above type of interventions is the need for a thorough basic understanding of the mechanisms governing specific abnormalities in order for effective therapeutic targeting. As an example of such targeting, the genetic intervention could be: (1) overexpression of a target molecule; (2) alteration of the target’s intracellular shuttling routes though introduction of decoy molecules; (3) loss of function approaches using dominant negative molecules or by introduction of RNA interference; (4) correcting deleterious gene mutations/deletions at the genome or primary mRNA level; or (5) installing genetically modified donor cells (stem cells and/or differentiated cells). In addition, appropriate interventional gene delivery techniques and vehicles/vectors need to be further developed to effectively achieve the above therapeutic approaches. Some of these topics are addressed in separate reviews within this series and are mentioned only briefly below. Our review herein focuses on means and targets to improve the function of the failing cardiomyocyte as a way to reverse myocardial dysfunction and remodeling.
Vehicles for Myocardial Gene Therapy in HF
Gene therapy in HF will certainly require efficient myocardial transduction and long-term transgene expression and only viral vectors appear to meet such a requirement. Even in settings where transient expression of a transgene may be sufficient (ie, induction of neoangiogenesis by secreted growth factors), results from larger controlled preclinical studies have been disappointing. This appears to be a consequence of low efficacy (see elsewhere1 and references therein). A more detailed description of viral vectors and their application within cardiovascular disorders will be the topic of separate review. We discuss briefly below the most commonly used viral vehicles (adenoviruses [AdVs] and adeno-associated viruses [AAVs]) for HF gene therapy.
AdVs are easily manipulated, have a rather large transgene cloning capacity (≈7 to 8 kb), and can also be produced to high titers.2 Importantly, in vivo experimental studies have demonstrated effective myocardial transduction with AdVs to alter global cardiac function.2 The major disadvantage of AdVs is an inflammatory response generally seen on in vivo cardiac delivery, which culminates into a transient transgene expression and a secondary immune response if attempting to intervene again.3 Newer generations of AdVs devoid of more of the immunogenic viral epitopes (so-called “gutted” AdVs) may rectify this problem.
AAVs are generally considered more promising for gene therapy of chronic diseases such as HF because they readily infect cardiac tissue, produce stable and long-term transgene expression and are much less immunogenic.2 Moreover, AAVs have not been shown to cause any known human disease.4 A major disadvantage of AAVs is their somewhat low packaging capability (≈4 to 5 kb). Furthermore, the presence of naturally occurring antibodies against some AAVs in the human population may limit their value.4,5 Reports have also demonstrated unfavorable genomic integration sites and development of hepatocellular carcinoma in mice subjected to AAV gene therapy.6 A promising feature with AAVs is that some serotypes display tropism toward cardiac tissue,7,8 and this may be exploitable to improve HF gene therapy strategies.
Gene Delivery Techniques
The ideal mode of HF gene delivery would be to use a vector that could be administered intravenously with efficient and specific uptake into cardiomyocytes. This could be an unreachable goal in humans because of the large blood volume and subsequent dilution issues; however, the cardiac muscle tropism of some AAV serotypes even in large animals7,8 may prove that this approach is feasible. Overall, there is a concern about “scalability” of doses of vectors used in small animal models when attempting to translate into large animals and humans. Indeed, what has been used in mice and rats have been relatively large doses of viruses and it is not clear whether these will be sufficient to effectively transduce larger hearts or whether high titers potentially needed for human HF gene therapy are safe and even achievable. Importantly, the cardiac muscle tropism of some AAVs may lessen the amount of virus needed for effective human cardiac gene transfer.
Gene delivery strategies to the heart have been targeted interventions to the myocardium using either an intravascular approach through the coronary arteries or direct delivery to cardiac muscle. Intracoronary gene delivery is obviously clinically relevant and appealing; however, this approach is generally inefficient for myocardial gene transfer unless certain adjuvants and specific conditions are used (ie, increasing vascular bed permeability with agents such as histamine, vascular endothelial growth factor, or substance P and increasing perfusion pressure and allowing sufficient cell contact time for vehicle).9,10 Importantly, some of these modifications may be challenging and problematic in larger animals and humans. However, there has been some success in larger animals where blocking venous return on coronary delivery of virus, or retrograde infusion of virus has been used.11,12 In an attempt to further optimize intracoronary delivery, recirculation of virus by closed-loop systems is also being developed.13
Direct intramyocardial injection of viral vectors is also a technique that can support sufficient gene transfer including myocardial gene delivery in larger animals.14 The efficacy of this mode of cardiac gene delivery is naturally limited by accessible myocardial tissue and the areas injected. Moreover, some damage of tissue will occur along the needle track. However, this mode of vehicle delivery is conceptually appealing in human HF, especially during procedures where the myocardium is readily accessible (ie, cardiothoracic surgery).2
Promising HF Gene Therapy Targets
Targeting Proteins Involved in Cardiomyocyte Ca2+ Handling
The handling of Ca2+ during excitation–contraction (EC) coupling is a prominent feature of the cardiomyocyte. This important feature of the cardiomyocyte has long been a focus of molecular HF research because it is deranged in failing cells and importantly; this area will be targeted in the first human HF gene therapy trials. A detailed description of cardiac EC Ca2+ handling in normal as well as failing cardiomyocytes is beyond the scope of this review, but some points are important to address as they lay the groundwork for promising gene therapy targets. In brief, EC coupling begins with the initiation of a cardiomyocyte action potential and Ca2+ enters the cell through voltage-gated L-type Ca2+-channels. This initial influx of Ca2+ triggers the ryanodine receptor (RyR) to extrude Ca2+ from the sarcoplasmic reticulum (SR) into the cytosol.15 This Ca2+-induced Ca2+ release triggers cardiomyocyte contraction through Ca2+ binding to troponin C within the myofilaments of the sarcomere.15 Just as important is the removal of Ca2+ from the cytosol that initiates relaxation of the sarcomere. The cardiac SERCA2a and sarcolemmal Na+−Ca2+ exchanger (NCX) quantitatively are the major players in Ca2+ extrusion in larger animals.15 In addition to these nodal participants of EC coupling, molecules that regulate these proteins are critical for maintaining Ca2+-cycling stability.
Distorted handling of Ca2+ in the cardiomyocyte is a hallmark of HF. There is a decreased SR Ca2+ content and a prolonged Ca2+ transient, which is generally considered to be a consequence of increased NCX, a reduction of SERCA2a and a decreased phospholamban (PLN)/SERCA2a ratio, as well as an augmented open probability of the RyR (ie, “leakiness”).16,17 Some divergences between these specific changes have been found depending the model of HF, as well as some species variations.18 In addition to these changes causing dysfunctional contractile performance, the decreased clearance of cytosolic Ca2+ may increase the risk of arrhythmias and also may precipitate pathological cardiac remodeling. 17,19 Of importance, there is abundant data to suggest that targeting molecules involved in dysfunctional cardiomyocyte Ca2+ are attractive targets for HF gene therapy.
SR Ca2+-ATPase
SERCA2a is the cardiac isoform of this family of Ca2+-ATPases, and a loss of its activity and resultant decrease in SR Ca2+ uptake is a feature of the failing cardiomyocyte including in human HF.20 SERCA2a activity in myocytes is tightly controlled by PLN, a small inhibitory peptide that regulates SERCA2a on a beat-to-beat basis. The inhibitory action of PLN is set by its phosphorylation status.21 When PLN is dephosphorylated it binds and inhibits SERCA2a. In contrast, when PLN is phosphorylated (primarily via protein kinase [PK]A but also calmodulin kinase II), its inhibitory activity on SERCA2a is alleviated. Conceptually, SERCA2a gene therapy in HF (replacing lost activity and tilting the PLN/SERCA2a equilibrium22) is a rational venture as it would remove cytosolic Ca2+ faster in diastole and increase contractile reserves by increasing SR Ca2+ concentration. As an important proof-of-concept for human gene therapy, failing human cardiomyocytes showed restoration of impaired Ca2+ homeostasis and normalization of dysfunctional contractile responses after SERCA2a gene transfer.23
The therapeutic potential of SERCA2a gene transfer to failing myocardial tissue has also been addressed in small animal models of HF. Intracoronary Adv-SERCA2a delivery to rats in HF resulting from transaortic constriction (TAC) led to improved systolic and diastolic function along with dramatically improved survival 28 days after gene delivery.24,25 Importantly, these observations have been corroborated by findings in transgenic SERCA2a mice subjected to TAC.26 The therapeutic potential of SERCA2a gene delivery has also been recently addressed in a large animal model of HF.27 In this study, HF was induced by mitral valve impairment in pigs and AAV1-SERCA2a was administered by so-called antegrade epicardial coronary artery infusion on establishment of volume overload HF. Two months after SERCA2a gene delivery, clear improvements of left ventricular (LV) contractile performance and myocardial remodeling was observed. 27 Consistent with the beneficial effects of increasing SERCA2a content to improve failing cardiomyocyte function is the opposite findings in heterozygote SERCA2a knockout (KO) mice subjected to TAC. In this experimental setting, an augmented hypertrophic response and a faster development of LV dilatation was observed.28
Despite positive results in the above animal models, there are data from other studies that support a more tempered optimism for SERCA2a gene therapy. First, transgenic rats with cardiac SERCA2a overexpression were subjected to myocardial infarction (MI), and even though there were some beneficial effects on LV performance 1 month after MI, there were no differences between the groups when examined at 6 months.29 Moreover, there was increased mortality seen in SERCA2a-treated rats within the first 24 hours after MI, which was apparently attributable to ventricular arrhythmias. 29 In addition, gene transfer of SERCA2a to isolated failing canine cardiomyocytes, although rescuing diastolic dysfunction led to a loss of β-AR responsiveness and inotropic support.30
Despite some of the above inconsistencies/concerns with SERCA2a gene transfer to cardiomyocytes, approval has been gained for the first human HF gene therapy trials. Two phase I clinical studies have been initiated where HF patients are to receive SERCA2a via myocardial gene delivery. In 1 study (CUPID—Calcium-Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease—FDA approved, January 2007), intracoronary delivery of AAV1-SERCA2a (denominated MYDICAR; see http://www.celladon.net) will be given to HF patients. A report detailing the protocol design is now in press.30a In brief, 1 part of the study is a dose-escalation study (4 doses per patient appropriately spaced in time) aimed at identifying safe and efficient dose of AAV1-SERCA2a delivered by antegrade epicardial coronary artery infusion to patients with ischemic or nonischemic dilated cardiomyopathy. Once the safe, efficient dose is identified, a smaller placebo-controlled randomized trial will be undertaken. Importantly, patients receiving AAV1-SERCA2a are required to also receive an implantable intra-cardiac defibrillator. Thus, the potential occurrence of ventricular arrhythmias discussed above will be addressed. The other phase I trial will deliver AAV6-SERCA2a to patients undergoing implantation of a left ventricular assist device with the primary goals of assessing safety and biological effects.
Phospholamban
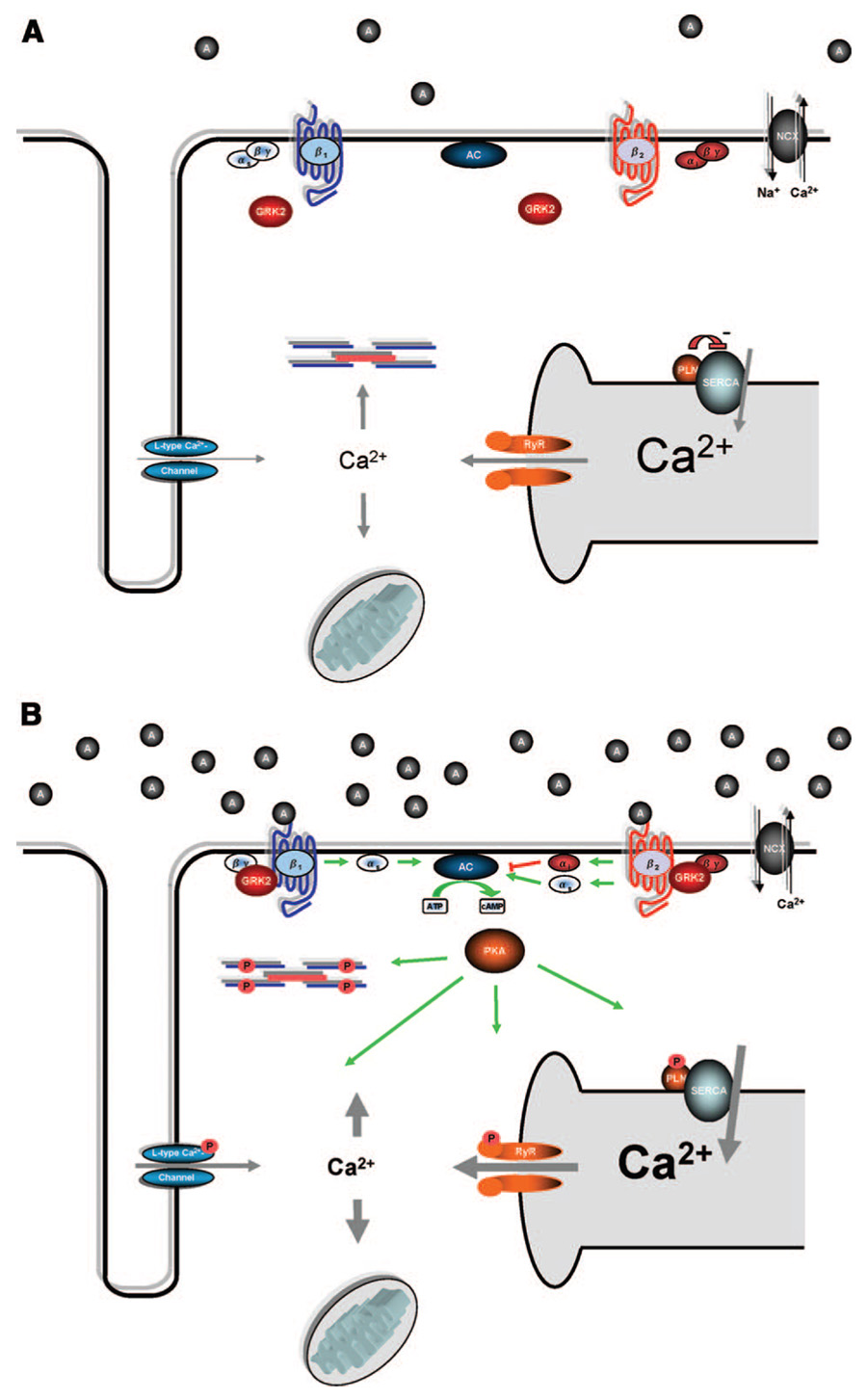
Through its potent action on SERCA2a activity, PLN plays a nodal role in the regulation of SR Ca2+ homeostasis mediating slower cytosolic Ca2+ decay in cardiomyocytes, which translates into prolonged diastolic relaxation both in vitro and in vivo.31,32 Increased levels of PLN in cardiomyocytes has been shown to be detrimental as higher levels of cardiac PLN overexpression in transgenic rabbits resulted in cardiomyopathy.33 Transgenic mice overexpressing a mutant PLN “superinhibitor” of SERCA2a in cardiomyocytes also had deranged Ca2+ handling and depressed contractile performance leading to cardiomyopathy.34 Consistent with these data are the findings that reduction of PLN levels (in KO mice) promote a dose-dependant hyperdynamic condition with increased cardiac performance and blunted contractile responses to β-AR stimulation (Figure 1 and Figure 2), the latter an apparent consequence of β-AR signaling being maximal.35
Figure 1. Simplified β-AR and Ca2+ signaling in the normal, nonfailing heart under resting conditions.
(A) and after adrenergic stimulation (B). A, Sarcolemmal depolarization activates the L-type Ca2+ channel, and Ca2+ enters the cell, triggering the RyR to release Ca2+ from the SR (Ca2+-induced Ca2+ release). Elevated cytosolic Ca2+ binds troponin C and activates myofilaments resulting in contraction of the cardiomyocyte. During diastole, Ca2+ is removed from contractile proteins and the cytosol inducing relaxation of the sarcomere involving SERCA2a, NCX, and the mitochondrial Ca2+ uniporter. B, Adrenergic stimulation of β-ARs results in activation of AC, the generation of cAMP, and activation of PKA. The main targets of PKA include the L-type Ca2+-channel, the RYR, PLN, and troponin I, which, in concert, mediate positive inotropic and lusitropic responses. Adrenergic stimulation also induces GRK2 membrane translocation to initiate receptor desensitization.
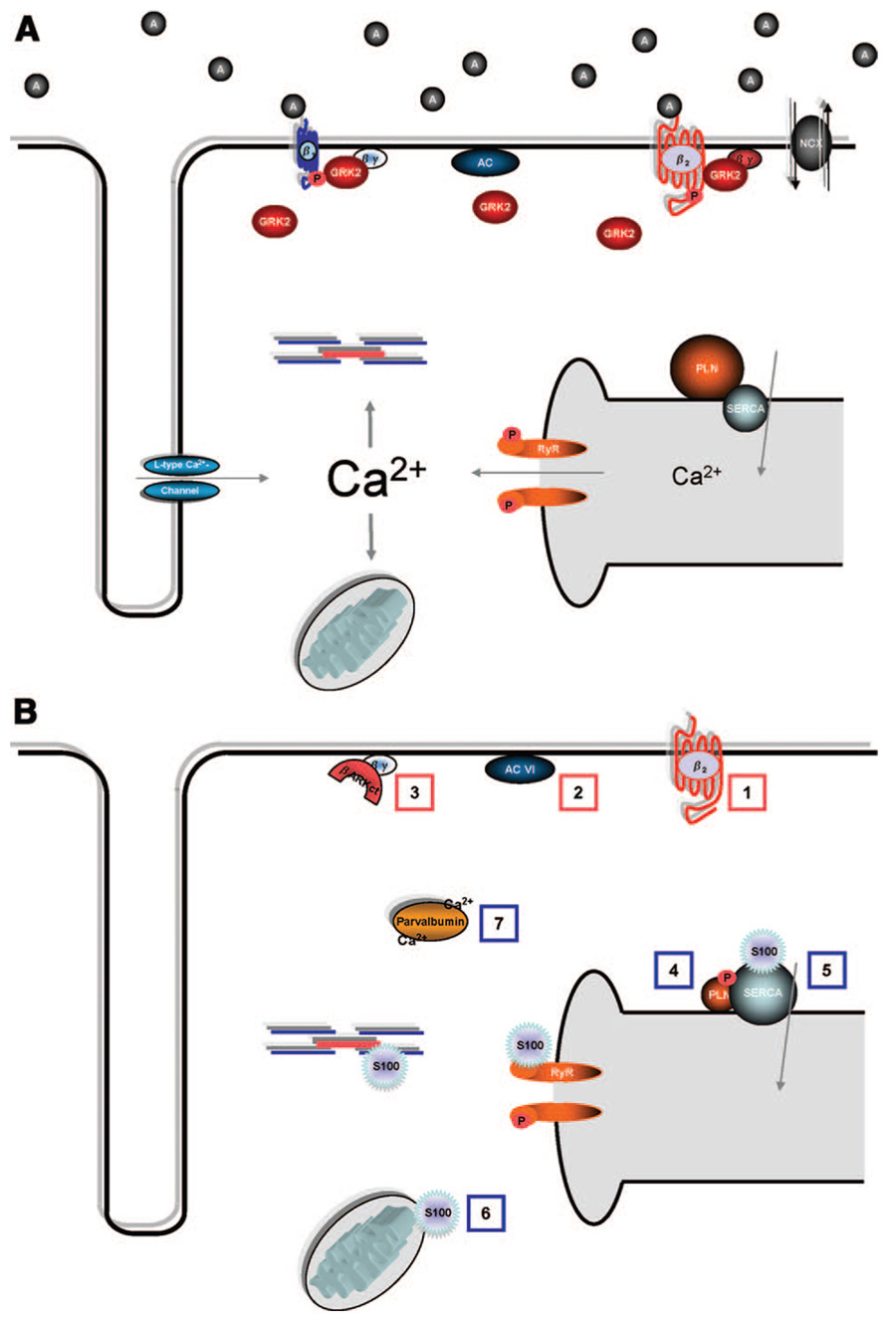
Figure 2. Signaling in the failing heart.
(A), with emphasis on potential targets for gene therapy (B). A, Hallmarks of molecular signaling in the failing heart include defective β-AR signaling and distorted Ca2+ cycling. β1-ARs are selectively downregulated, while β1- and β2-ARs are uncoupled from their effectors; both processes are mediated by GRKs, with GRK2 representing the predominant form in cardiac myocytes. GRK2 is significantly upregulated in the failing heart. Downstream, decreased SR Ca2+ content and a prolonged Ca2+ transient are generally attributed to increased NCX, decreased SERCA2a, decreased PLN/SERCA2a ratio, and leakiness of the RyR. B, Promising targets for HF gene therapy to normalize these changes include: (1) β2-AR overexpression; (2) AC6 overexpression; (3) βARKct expression and GRK2 inhibition; (4) expression of a constitutively phosphorylated from of PLN; (5) overexpression of SERCA2a; (6) S100A1 overexpression; and (7) parvalbumin expression.
Being such a central player in the handling of Ca2+ and contractile function, the loss of PLN in various models of experimental HF has been studied. Cross-breeding PLN KO mice into 2 mouse models of genetic cardiomyopathy (MLP KO and CSQ-overexpressing mice) halted/rescued the detrimental phenotype.36,37 However, in a study using a TAC model of mouse HF, smaller positive effects of PLN ablation was observed.38 To also show that PLN ablation does not always lead to beneficial effects in HF, ischemic injury was exacerbated in isolated PLN KO hearts after ischemia/reperfusion.39 Furthermore, somewhat neutral effects of PLN ablation in the development of cardiomyopathy and LV dysfunction have been found in some HF mice overexpressing tumor necrosis factor-α, Gαq, tropomodulin, or a mutant myosin-binding protein C.40–43 Importantly, however, all of these studies do show the expected alterations in Ca2+ handling and contractility in isolated cardiomyocytes. Overall, these findings are interesting as they point out that genetic interventions promoting improved cardiomyocyte contractility and Ca2+ handling in HF cannot on a general basis predict a positive outcome in global cardiac function and remodeling. Moreover, these results point out that caution is needed in the interpretation of positive data in genetic mouse models of HF.
In attempts to translate the above findings of PLN lowering using gene therapy in experimental HF models, PLN inhibitory or dominant-negative molecules have been used. These studies have generally used mutant forms of PLN, such as a phosphomimetic mutation at serine-16 (S16E), the site of PKA action, which promotes SERCA2a activity.44 Interestingly, AAV-mediated overexpression of PLN-S16E prevented cardiac deterioration in cardiomyopathic hamsters as well as in post-MI HF rats.45,46 Lowering PLN levels by antisense technology has also been shown to correct contractility and Ca2+ handling in failing human cardiomyocytes.47 Prevention of HF development in a larger animal has also been achieved by PLN-S16E gene therapy because sheep with cardiomyopathy demonstrated improved cardiac function compared to controls after intracoronary delivery of AdV-PLN-S16E.13
Even with a substantial amount of evidence showing that abolishment of PLN and/or removal of the inhibitory effects of PLN on SERCA2a can be beneficial in experimental HF, it is still unclear what the outcome would be if used in a human HF population. Of note, there are several documented cases of inherited human cardiomyopathies where the culprit has been found to be mutations and deletions in the PLN gene or its promoter and where the outcome is a loss of PLN inhibition on SERCA2a. Elegant studies have also shown that some of these human cardiomyopathies can be recapitulated in the mouse.48,49 Moreover, a naturally occurring PLN “knockout” mutation in the human population is associated with severe cardiomyopathy.50 This is a perfect example of the difficulties involved in extrapolating findings from murine models to the human population and shows that the enthusiasm for PLN-lowering in human HF should be tempered. However, PLN ablation genetically could be a very different biological event compared to a more acute intervention of lowering PLN levels in HF and probably more studies targeting PLN are warranted.
Protein Phosphatase 1 and Inhibitor Protein-1
The inhibitory action of PLN on SERCA2a is subject to tight secondary control mediated by protein phosphatase (PP)1 dephosphorylation. PP1 activity itself is also subject to rigorous control via the actions of the phosphatase inhibitors, inhibitor protein (I)-1 and I-2. β-AR–mediated PKA activation promotes I-1 to attenuate PP1 activity toward PLN, and this results in sustained PKA-mediated phosphorylation of PLN, which increases SERCA2a activity.51 Similar to murine gene manipulations targeting SERCA2a activity more directly, transgenic cardiac overexpression of PP1 or deletion of I-1 results in reduced cardiac performance, decreased β-AR-mediated Ca2+ signaling and contractility, and development of cardiomyopathy.52 Similar findings have been reported with the overexpression of the PP2 isoform.53 I-1 levels and PP1 activity are reduced in human HF,51 and, thus, increasing I-1 levels in HF would constitute an appealing target. In an article by Pathak et al, this was specifically addressed in both mouse and rat models.54 Transgenic mice overexpressing I-1 showed prevention of LV dysfunction following TAC, whereas beneficial cardiac effects were seen after intracoronary delivery of AdV-I-1 to rats with cardiac dysfunction after TAC.54 These findings were corroborated in a recent study in which cardiac dysfunction in cardiomyopathic hamsters was halted by PP1 inhibition after AdV-mediated delivery of I-2.55 Therefore, targeting PP1 and its inhibitory proteins to ultimately increase SERCA2a pump activity may constitute a promising gene therapy strategy in human HF, although there is clearly a need for further studies using different etiologies of HF and preferably larger experimental animal models.
Parvalbumin
Parvalbumin is an EF-hand Ca2+ sequestering protein that is exclusively expressed in fast-twitch skeletal muscle and neurons. It can bind 2 Ca2+ or Mg2+ ions per molecule, and the concentration and fluctuation rate of these ions determine the specific occupancy.56 In contrast to overexpression of SERCA2a, parvalbumin introduction into cardiomyocytes offers an energy-independent removal of cytosolic available Ca2+ and, thus, in theory, constitutes a potentially appealing mode of correcting the prolonged diastolic Ca2+ decay generally seen in HF without further energy deprivation. As proof-of-principle, AdV-mediated expression of parvalbumin increased that rate of Ca2+ removal and improved the rate of relaxation in cardiomyocytes from hypothyroid rats, as well as in the heart after in vivo delivery.57 Similar findings were seen after parvalbumin gene treatment of dysfunctional rat cardiomyocytes expressing a mutant α-tropomyosin (A63V), as well as in vivo using a cross-breeding strategy between α-tropomyosin-A63V transgenic mice and parvalbumin transgenic mice.58 Selectivity of parvalbumin Ca2+ binding to the relaxation phase of the Ca2+ cycle is determined by the rate of Ca2+ influx and expression levels of parvalbumin.59 Thus, there are some complex kinetic issues that could pose a potential problem in human HF gene therapy because differences in heart rate and/or expression levels of parvalbumin may cause Ca2+ sequestration during systole, which would compromise the force of contraction. This is the conclusion from a study of AdV-mediated parvalbumin to diastolic dysfunctional cardiomyocytes harvested from dogs subjected to TAC.30 In this study, there was clear improvement of relaxation kinetics although at higher parvalbumin concentrations, sarcomere shortening was depressed.30 Overall, although potentially promising, further investigations addressing the impact of long-term parvalbumin expression in relevant models of HF is warranted.
S100A1
S100A1 is a molecule that appears to play multiple and unique roles in cardiomyocyte Ca2+ cycling. S100A1 belongs to the S100 protein family (the largest EF-hand Ca2+-binding protein subfamily) and is predominantly and highly expressed in cardiomyocytes, where it localizes to the SR and the sarcomere and within the mitochondria.60 Importantly, myocardial levels of S100A1 are decreased in HF.60,61 S100A1 delivery to cardiomyocytes results in an increase of isometric contraction followed by an increase in the amount of Ca2+ pumped into the SR.62,63 These actions of S100A1 are independent of the β-AR system because there are no changes in cAMP or PKA activity.62 However, β-AR stimulation in the presence of overexpressed S100A1 results in maximal contractile performance.64,65
Interestingly, the regulatory targets of S100A1 at the level of the SR have been shown to be both RyR and SERCA2a. S100A1 stabilizes RyR in diastole (reducing the frequency of Ca2+ sparks) and augments Ca2+ release during systole.61,66 Moreover, S100A increases SERCA2a activity during the relaxation phase.60,66 Thus, in theory, S100A1 gene therapy for HF may offer a promising and novel mode of action that will not only promote beneficial cardiomyocyte EC Ca2+ handling but also potentially limit arrhythmias through stabilization of RyR and decrease in diastolic Ca2+ leak.
To date, animal models have illustrated the potential of S100A1 gene therapy for HF. First, S100A1 KO mice, although presenting with relatively normal cardiac function basally, display a substantial worsened LV function both after TAC and MI with significantly lower survival.67,68 Importantly, the opposite result was seen in transgenic mice with cardiac-specific overexpression of S100A1 where LV function was stabilized after MI and inotropic reserve maintained. 68 Moreover, AdV-mediated S100A1 gene transfer to failing rat cardiomyocytes resulted in restoration of disturbed Ca2+ handling through increased reuptake of SR Ca2+ during the relaxation phase and a lowering of the RyR-mediated Ca2+ leak, which also resulted in the reversal of fetal gene expression in this model.69
A recent study has also shown chronic benefits of S100A1 gene delivery to the post-MI rat heart using an AAV vector.61 In this study, S100A1 myocardial gene transfer was performed using an AAV6 vector with a novel cardiomyocyte-specific promoter/enhancer (α-cardiac actin enhancer/elongation factor-1 promoter).61 Intracoronary delivery of AAV6-S100A1 was performed in rats 10 weeks after MI where significant LV dysfunction and HF was evident and 2 months after gene delivery, S100A1-treated HF rats presented with significantly enhanced cardiac function and a reversal of LV remodeling compared to control HF rats.61 This chronic rescue of HF was also evident at the single cardiomyocyte level, where restoration of intracellular Ca2+ transients and contractility was also seen.61 This study also had data supporting potential clinical importance because it showed additive beneficial effects combining S100A1 gene therapy with the β-AR blocker metoprolol.60 Studies using large animal models as well as studies using human cardiomyocytes are definitely needed to continue to advance the idea that S100A1 addition could be a useful molecular therapeutic tool to combat human HF.
Targeting the β-AR System in HF
A hallmark of HF is alterations in cardiac β-AR signaling. It is now close to 3 decades since Bristow and colleagues demonstrated lower myocardial β-AR density and decreased responsiveness to β-agonists in failing human myocardium.70 These observations triggered a vast amount of basic and clinical research investigating the molecular mechanisms responsible for these alterations. Several members of the β-adrenergic signaling pathway have been examined in respect to their role in maintenance of normal cardiac function, as well as their influence on HF development. Clinically, 2 pharmacological observations are clear: (1) use of β-AR antagonists in HF is generally beneficial71; and (2) sustained inotropic support in human HF through increased β-AR activation is detrimental.72 However, numerous basic investigations indicate that molecular manipulation of key players in the β-AR pathway that improve signaling and responsiveness can be beneficial in animal models of HF. This includes inhibition of receptor desensitization and increased adenylyl cyclase (AC) activity (see below). Naively viewed, these basic observations appear paradoxical considering the above-described clinical data, although the molecular complexity within the β-AR signaling pathway does not allow for a simplified “on is bad, off is good” interpretation.73 Indeed, a more refined understanding of the balanced interplay of the different components of the β-AR signaling pathway may allow for novel molecular therapies that hold promise above and beyond what is currently being used clinically to improve cardiac function in human HF.
The complexity of the β-AR system in the heart begins at the level of the receptor. Both the β1-AR and the β2-AR are expressed in cardiomyocytes (see elsewhere74 and references therein). In human myocardium, the β1-AR accounts for 75% to 80% of total β-AR density.75 In human HF, the β1-AR is selectively downregulated (≈50%), and the remaining β1- and β2-ARs are partially uncoupled from their downstream signaling components including the heterotrimeric G protein Gs and AC.75 This selective β1-AR downregulation increases the importance and contribution of β2-ARs for inotropy.75 Importantly, recent data have shown that β1- and β2-ARs have distinct signaling and functional consequences, and, hence, they appear to regulate different aspects of catecholamine-dependent cardiac signaling and function.73 Downstream of AC activation by β1-AR stimulation, PKA is considered the key effector, whereas, recently, calmodulin kinase II has also been identified as an important cAMP-independent β1-AR effector.76 Of interest to this discussion, PKA has numerous intracellular targets, including key nodal regulators of EC Ca2+ homeostasis, discussed above such as PLN and RyR. In contrast to β1-AR stimulation, β2-ARs do not elicit the same myocyte contractile response, and the level of cAMP generation (at least globally) is negligible.77
The interaction between activated β-ARs and their G protein is tightly regulated by kinases that dampen the receptor activity by phosphorylation of serine and threonine residues at the carboxyl terminus of the receptor.78 This phenomenon is classically termed desensitization. Agonist-dependant desensitization (homologous desensitization) is mediated by a family of kinases known as the G protein– coupled receptor (GPCR) kinases (GRKs), whereas heterologous desensitization can occur with PKA and other kinases (ie, PKC).78 The potential importance of GRK-mediated β-AR desensitization in HF is discussed below.
β2-AR Overexpression
With the simple hypothesis that β-AR downregulation in the failing heart is maladaptive, studies in the 1990s used transgenic approaches to ask whether overexpression of β-ARs could alter the function and fate of the heart. The results of these investigations illustrate the complexity of cardiac β-AR signaling when pondering specific molecular manipulations to improve the function of the failing heart. Because dampening β-AR activity in theory may be considered an inborn protection mechanism for the failing heart (preventing arrhythmias and conserving energy), it was not surprising that transgenic mice with cardiac overexpression (30-fold) of the human β1-AR caused severe cardiomyopathy.79 In contrast, mice with cardiac overexpression of human β2-ARs, at much higher levels than in the abovementioned β1-AR mice, displayed enhanced systolic and diastolic performance without clear signs of deterioration.80,81 β2-AR–overexpressing mice also showed preserved contractility after MI.82
However, there is cautionary evidence to warrant against β2-ARs as a potential therapeutic molecule as transgenic mice with extremely high levels (>200-fold) of human β2-ARs did develop fibrotic cardiomyopathy and HF after 40 weeks.81 Moreover, cardiac β2-AR–overexpressing mice crossbred with HF mice overexpressing Gαq displayed augmented cardiac dysfunction and worsened hypertrophy.81 To increase the complexity of these findings and the “sorting out” of good versus bad β-ARs, a recent study in mice has shown that despite robust overexpression of the murine β1-AR in the hearts of transgenic mice, no overt cardiomyopathic phenotype was observed.83 The major difference between this mouse and the mouse described above79 was the origin of the receptor (human versus mouse) and a possible significant signaling difference is the presence of constitutive receptor signaling activity (none for the mouse and present for the human).83
The distinct difference between β1- and β2-AR overexpression in the hearts of transgenic mice and the detrimental versus beneficial phenotype has been attributed to the unique characteristic of β2-ARs coupling to both Gs and Gi.78 Importantly, studies have shown that whereas β1-AR–mediated signaling leads to myocyte cell death including via apoptosis, β2-AR stimulation leads to cell survival signaling combating apoptosis.84,85
These results have led to testing whether raising β2-AR levels in the failing heart may be protective or beneficial outside of the mouse and studies have used AdV-mediated β2-AR gene delivery to larger animals. Supporting evidence was first found in rabbits because cardiac performance was improved after myocardial gene transfer of Adv-β2-AR either via a global gene delivery method86 or via intracoronary delivery.87 AdV-β2-AR cardiac gene transfer in a heterotopic rat heart transplant model improved basal, as well as zinterol-stimulated (a β2-AR selective agonist), cardiac performance. 88 Another gene therapy study with AdV-β2-AR was its use in a heterotopic transplantation model in the rabbit, in which failing hearts were transplanted and β2-AR overexpression accelerated reverse remodeling because of mechanical unloading following transplant and this included improved LV function in these failing hearts.89
Although these studies provide intriguing data suggesting that β2-AR enhancement via molecular upregulation may be beneficial, a note of caution is perhaps best served here because of conflicting reports (especially in the mouse). Furthermore, none of the above studies has examined long-term effects of myocardial β2-AR overexpression in HF, including impact on survival.
Targeted Inhibition of GRK2
Studies over the last 2 decades have shown that limiting β-AR desensitization via GRK2 inhibition is a promising molecular strategy in HF.73,78 The ubiquitously expressed GRK2 is the highest expressing GRK in the heart. GRK2 is a cytosolic protein, and upon GPCR stimulation, it is shuttled to the plasma membrane where it binds to dissociated and membrane-embedded βγ-subunits of heterotrimeric G proteins (G βγ), allowing for phosphorylation of agonist-occupied receptors.78 The in vivo effects of GRK2 on cardiac β-ARs was demonstrated in transgenic mice with cardiac-targeted GRK2 overexpression as these mice presented with blunted β-AR responsiveness both for AC activation and LV contractility. 90 This is of potential clinical importance as GRK2 is upregulated in the failing human heart.91 The binding of Gβγ to GRK2 has been exploited experimentally as a peptide containing this region within the carboxyl terminus of GRK2, termed βARKct, has been used to inhibit GRK2-promoted β-AR desensitization, as well as other Gβγ processes.73,78,92 Importantly, transgenic mice with cardiac-targeted βARKct expression display enhanced in vivo cardiac function90 and reversal of the transgenic GRK2 phenotype.93
The upregulation of myocardial GRK2 in human HF demonstrates that the decreased β-AR responsiveness typically found is probably attributable to augmented GRK2-mediated desensitization and receptor–G protein uncoupling. 73,78 Importantly, numerous studies have also demonstrated similar upregulation of myocardial GRK2 in several experimental animal models of cardiac dysfunction. 94–98 A decade ago, the dampening of β-AR signaling in HF caused by increased GRK2 was thought to be protective; however, data from our laboratory have shown that GRK2 activity in the failing heart appears to be pathological,73,78 and its inhibition by the βARKct can lead to both the prevention and rescue of HF.93,95,96,99–104 This includes HF rescue in post-MI rabbits in which AdV-βARKct was administered via percutaneous intracoronary catheterization.102 As an important proof-of-concept for human HF, βARKct gene transfer to failing human cardiomyocytes significantly improved β-AR signaling and contractile dysfunction.105 Of added interest, a study in βARKct transgenic mice with a murine model of HF showed an additive/synergistic effect on survival with concurrent administration of a β-AR antagonist,101 which demonstrates that the 2 strategies are not opposing. It is important to point out that because GRK2 acts to desensitize other GPCRs in the heart, the mechanism of the βARKct probably extends beyond its effects on β-AR signaling.
Importantly, neutral studies have also been reported using βARKct transgenic mice with some murine models of HF.106,107 Moreover, a recent report shows that abolishment of GRK2 (using a newly developed conditional KO mouse for cardiomyocyte GRK2) precipitates cardiomyopathy on chronic catecholamine exposure.108 Interestingly, we now have data using these GRK2 KO mice that show unequivocally that loss of GRK2 in cardiomyocytes preserves LV contractility, improves LV remodeling, and increases survival in post-MI HF (P.W.R., et al, unpublished data, 2008). The contrasting results between these 2 studies are probably a consequence of the model of LV dysfunction but also do show that caution is still warranted with GRK2 targeting. Overall, the data do support the use of the βARKct and GRK2 inhibition as a promising target for HF, and preclinical large animal studies are underway to potentially bring this molecule to clinical trials.
AC Type 6
There are 9 isoforms of AC expressed in mammals (AC1 through AC9109), and AC5 and AC6 are the predominant cardiac isoforms.77 Gene intervention promoting increased cardiac AC levels constitutes in theory an appealing venture because data indicate AC6 as rate-limiting in the β-AR/Gαs/ AC-mediated generation of cAMP. Perhaps more importantly is data demonstrating that overexpression of AC6 does not chronically and constitutively activate cAMP generation, as seen for β1-ARs and Gαs-overexpressing transgenic mice.106,110,111 In fact, cardiac AC6 overexpression leads to increased LV function and increased cAMP levels during β-AR stimulation with no basal changes.112 This may constitute an important difference between using AC6 overexpression to improve β-AR–mediated cAMP production compared with enhancing signaling at the level of the receptor or G protein.113
The consequence of AC6 overexpression in HF has been tested experimentally in different animal models. Initial studies investigated the effect of cardiac AC6 overexpression in cardiomyopathic transgenic mice with myocardial overexpression of Gαq.114,115 AC6 overexpression in the hearts of these mice led to improved LV function, reversal of dysfunctional β-AR signaling, and increased survival.116,116 Importantly, these data have been corroborated in a more relevant gene therapy setting as Gq-overexpressing mice on intracoronary delivery of an AdV-AC6 vector had significantly improved cardiac function when studied 2 weeks after gene delivery.117
The beneficial cardiac effects of increased AC levels appear restricted to the AC6 isoform as overexpression of AC5 does not change outcomes with cardiac Gq overexpression. 118 In fact, the loss of AC5 (using KO mice) leads to improvements in cardiac function after TAC.119 Moreover, a recent report shows that AC5 KO mice have an increased lifespan and are protected against cardiac stress.120 The apparent discrepancy between manipulating AC6 versus AC5 in the heart is, as yet, unresolved although it is interesting that cardiac AC5 overexpression (as opposed to cardiac AC6 overexpression) is associated with increased basal cAMP generation.121
One caveat to the above studies is that the Gq cardiomyopathic mouse model shows little resemblance to human HF. Thus, it is important to note that AC6 overexpression has been tested in other models of cardiovascular stress. For example, mice with cardiac overexpression of AC6 had a significantly lower mortality than control mice 1 week after induction of MI.122 Findings of significant increments in both isoproterenol and forskolin-stimulated cAMP production and LV contractility after intracoronary delivery of AdV-AC6 in normal, as well as pacing-induced, HF pigs has also been reported.123,124 These studies show that the use of AC6 as a gene therapy strategy does hold some promise for use in HF and clinical trials have been planned. In theory, AC6 gene delivery could mediate inotropic support within the β-AR signaling pathway that, like inhibition of GRK2 with βARKct, would be temporally restricted without constitutive cAMP elevation.
Gene Targeting Monogenetic Causes of HF
Even though monogenetic causes of cardiomyopathy constitute a small percentage of the overall HF population, this diverse group of disorders potentially holds promise for more immediate gene therapy application. Being of monogenetic cause, they are (at least in theory) devoid of the complexity seen in most HF conditions. It is also hard to envision these disorders to ever be the focus of pharmaceutical drug development, and, thus, rectification of these disorders by gene therapy may be the only realistic treatment. The genetic basis for these mostly inherited diseases are widely heterogeneous and involve proteins constituting the sarcomere, the cytoskeleton, members of the Ca2+ handling family, and more.125,126 For many of the cardiomyopathies caused by mutations in genes encoding sarcomeric proteins, it may be feasible to correct the altered function, and possibly prevent the development of HF, by simply overexpressing the wild-type protein. The rationale for this is the observation that some overexpressed, tagged exogenous sarcomeric proteins assemble into the sarcomere in an orderly, stoichiometric fashion. 127 This presents the possibility of “removing” endogenously produced protein from the sarcomere by tipping the equilibrium. As of yet, no experimental studies have tested this theory; however, such experiments are feasible because mouse models that mimic known inherited human cardiomyopathies are available.
For cardiomyopathies precipitated by mutations in larger proteins, gene therapy is and will be much more technically challenging, if even possible. For example, Duchenne’s muscular dystrophy represents such a disease in which severe skeletal muscle weakness and cardiomyopathy are the major symptoms.128 The mutant protein responsible is dystrophin and several different mutations or deletions in its large gene have been documented to give rise to a variety of different degrees of clinical manifestations.129 In severe forms, the protein is significantly truncated or even absent. Importantly, it has been shown that introduction of a dystrophin minigene can substantially alleviate the clinical symptoms.130 Genetic correction at the primary mRNA level by exogenously induced “exon skipping” has also been demonstrated to be efficient as this technology leads to the loss of exons harboring deleterious mutations during mRNA splicing resulting in production of a slightly truncated, but functional, protein.131 Using the same principle with antisense oligonucleotides, the feasibility of correcting Duchenne gene defects by exon-skipping was also recently demonstrated in human patients.132 Even though these results are promising, major challenges remain for treating both skeletal muscle as well as cardiomyocyte weaknesses in these patients.
Concluding Remarks
As more mechanisms involved in HF progression are uncovered, there will be an increasing number of molecules to potentially target in human HF. This review has focused on biological systems that have been explored the most over the last several years and those that have an ongoing emphasis. It is likely that with increasing basic understanding of cardiomyocyte processes that other targets will emerge that show equal or even superior promise than those identified to date. Obviously, all of the studies and data referred to in this review are primarily from experimental animal models that have a variable resemblance to actual settings seen in a human HF population. For the majority of human HF cases, etiologies are complex and a “one gene fits all” approach seems overly simplistic. Moreover, it is likely that when entering the human patient population, translation from experimental animal models will be difficult. Thus, it seems imperative that any candidate gene is subjected to a large amount of scrutiny including extensive studies in large animal models before attempting human trials. Further improvement in viral vectors and gene delivery methodology is also needed.
Despite these obstacles, gene therapy holds great promise in the aspect of improving survival and quality of life for HF patients. By specifically targeting molecular pathways known to be detrimental in HF, the stagnation currently seen for pharmacological therapies can be addressed. Importantly, a few of the targets discussed above are in preclinical stages with the goal of clinical trials in the near future. The initial safety and outcomes of the human trials ongoing with AAV-SERCA2a will be extremely valuable to fuel the journey of other targets and keep the momentum going for HF gene therapy.
Acknowledgments
Sources of Funding
This research was supported, in part, by NIH grants HL56205, HL61690, and HL075443 (Project 2) (to W.J.K.) and Common-wealth of Pennsylvania Department of Health grant A75301 (to W.J.K). P.W.R. was supported by Deutsche Forschungsgemein-schaft grant RA 1668/1-1, and L.E.V was supported by a Fulbright Fellowship and the Norwegian Research Council, project number 170463/V40.
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/cgi/content/full/102/12/1458
Disclosures
None.
References
- 1.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 2.Williams ML, Koch WJ. Viral-based myocardial gene therapy approaches to alter cardiac function. Annu Rev Physiol. 2004;66:49–75. doi: 10.1146/annurev.physiol.66.032102.141555. [DOI] [PubMed] [Google Scholar]
- 3.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 4.Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB, Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 5.Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, Aitken ML. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 7.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 8.Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, Hajjar RJ. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 9.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci U S A. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell JM, Lewandowski ED. Efficient, cardiac-specific adenoviral gene transfer in rat heart by isolated retrograde perfusion in vivo. Gene Ther. 2005;12:958–964. doi: 10.1038/sj.gt.3302477. [DOI] [PubMed] [Google Scholar]
- 11.Hayase M, Del Monte F, Kawase Y, Macneill BD, McGregor J, Yoneyama R, Hoshino K, Tsuji T, De Grand AM, Gwathmey JK, Frangioni JV, Hajjar RJ. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am J Physiol Heart Circ Physiol. 2005;288:H2995–H3000. doi: 10.1152/ajpheart.00703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raake P, von Degenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S, Andrees M, Kupatt C, Schuler G, Boekstegers P. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol. 2004;44:1124–1129. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, Mariani JA, Pepe S, Chien KR, Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Svensson EC, Marshall DJ, Woodard K, Lin H, Jiang F, Chu L, Leiden JM. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 17.Piacentino V, III, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 18.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology. 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 19.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 21.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 22.Koss KL, Grupp IL, Kranias EG. The relative phospholamban and SERCA2 ratio: a critical determinant of myocardial contractility. Basic Res Cardiol. 1997;92 suppl 1:17–24. doi: 10.1007/BF00794064. [DOI] [PubMed] [Google Scholar]
- 23.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito K, Yan X, Feng X, Manning WJ, Dillmann WH, Lorell BH. Transgenic expression of sarcoplasmic reticulum Ca(2+) atpase modifies the transition from hypertrophy to early heart failure. Circ Res. 2001;89:422–429. doi: 10.1161/hh1701.095522. [DOI] [PubMed] [Google Scholar]
- 27.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Schultz Jel J, Glascock BJ, Witt SA, Nieman ML, Nattamai KJ, Liu LH, Lorenz JN, Shull GE, Kimball TR, Periasamy M. Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity. Am J Physiol Heart Circ Physiol. 2004;286:H1146–H1153. doi: 10.1152/ajpheart.00720.2003. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, Lenoir C, Heimburger M, Choqueux C, Gellen B, Riou B, Michel JB, Franz WM, Mercadier JJ. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch JC, Borton AR, Albayya FP, Russell MW, Ohye RG, Metzger JM. Comparative analysis of parvalbumin and SERCA2a cardiac myocyte gene transfer in a large animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2004;286:H2314–H2321. doi: 10.1152/ajpheart.01137.2003. [DOI] [PubMed] [Google Scholar]
- 30a.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, Ly H, Kawase Y, Wagner K, Borow K, Jaski B, London B, Greenberg B, Pauly D, Patten R, Starling R, Mancini D, Jessup M. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008 doi: 10.1016/j.cardfail.2008.02.005. In press. [DOI] [PubMed] [Google Scholar]
- 31.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, II, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pattison JS, Waggoner JR, James J, Martin L, Gulick J, Osinska H, Klevitsky R, Kranias EG, Robbins J. Phospholamban overexpression in transgenic rabbits. Transgenic Res. 2008;17:157–170. doi: 10.1007/s11248-007-9139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haghighi K, Schmidt AG, Hoit BD, Brittsan AG, Yatani A, Lester JW, Zhai J, Kimura Y, Dorn GW, II, MacLennan DH, Kranias EG. Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem. 2001;276:24145–24152. doi: 10.1074/jbc.M102403200. [DOI] [PubMed] [Google Scholar]
- 35.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y, Kiriazis H, Yatani A, Schmidt AG, Hahn H, Ferguson DG, Sako H, Mitarai S, Honda R, Mesnard-Rouiller L, Frank KF, Beyermann B, Wu G, Fujimori K, Dorn GW, II, Kranias EG. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J Biol Chem. 2001;276:9392–9399. doi: 10.1074/jbc.M006889200. [DOI] [PubMed] [Google Scholar]
- 37.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 38.Kiriazis H, Sato Y, Kadambi VJ, Schmidt AG, Gerst MJ, Hoit BD, Kranias EG. Hypertrophy and functional alterations in hyperdynamic phospholamban-knockout mouse hearts under chronic aortic stenosis. Cardiovasc Res. 2002;53:372–381. doi: 10.1016/s0008-6363(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 39.Cross HR, Kranias EG, Murphy E, Steenbergen C. Ablation of PLB exacerbates ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol. 2003;284:H683–H690. doi: 10.1152/ajpheart.00567.2002. [DOI] [PubMed] [Google Scholar]
- 40.Delling U, Sussman MA, Molkentin JD. Re-evaluating sarcoplasmic reticulum function in heart failure. Nat Med. 2000;6:942–943. doi: 10.1038/79592. [DOI] [PubMed] [Google Scholar]
- 41.Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, Iaccarino G, Koch WJ, Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janczewski AM, Zahid M, Lemster BH, Frye CS, Gibson G, Higuchi Y, Kranias EG, Feldman AM, McTiernan CF. Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model. Cardiovasc Res. 2004;62:468–480. doi: 10.1016/j.cardiores.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Song Q, Schmidt AG, Hahn HS, Carr AN, Frank B, Pater L, Gerst M, Young K, Hoit BD, McConnell BK, Haghighi K, Seidman CE, Seidman JG, Dorn GW, II, Kranias EG. Rescue of cardiomyocyte dysfunction by phospholamban ablation does not prevent ventricular failure in genetic hypertrophy. J Clin Invest. 2003;111:859–867. doi: 10.1172/JCI16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu G, Kranias EG. Functional interplay between dual site phospholambam phosphorylation: insights from genetically altered mouse models. Basic Res Cardiol. 2002;97 suppl 1:I43–I48. doi: 10.1007/s003950200028. [DOI] [PubMed] [Google Scholar]
- 45.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Jr, Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 46.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 49.Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW, II, MacLennan DH, Kremastinos DT, Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, II, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 52.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, Hanske G, Schmitz W, Neumann J. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem. 2004;279:40827–40834. doi: 10.1074/jbc.M405770200. [DOI] [PubMed] [Google Scholar]
- 54.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 55.Yamada M, Ikeda Y, Yano M, Yoshimura K, Nishino S, Aoyama H, Wang L, Aoki H, Matsuzaki M. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 2006;20:1197–1199. doi: 10.1096/fj.05-5299fje. [DOI] [PubMed] [Google Scholar]
- 56.Eberhard M, Erne P. Calcium and magnesium binding to rat parvalbumin. Eur J Biochem. 1994;222:21–26. doi: 10.1111/j.1432-1033.1994.tb18836.x. [DOI] [PubMed] [Google Scholar]
- 57.Szatkowski ML, Westfall MV, Gomez CA, Wahr PA, Michele DE, DelloRusso C, Turner, Hong KE, Albayya FP, Metzger JM. In vivo acceleration of heart relaxation performance by parvalbumin gene delivery. J Clin Invest. 2001;107:191–198. doi: 10.1172/JCI9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- 59.Coutu P, Metzger JM. Optimal range for parvalbumin as relaxing agent in adult cardiac myocytes: gene transfer and mathematical modeling. Biophys J. 2002;82:2565–2579. doi: 10.1016/S0006-3495(02)75599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Most P, Remppis A, Pleger ST, Katus HA, Koch WJ. S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R568–R577. doi: 10.1152/ajpregu.00075.2007. [DOI] [PubMed] [Google Scholar]
- 61.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 62.Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Borries M, Niroomand F, Pieske B, Janssen PM, Eschenhagen T, Karczewski P, Smith GL, Koch WJ, Katus HA, Remppis A. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci U S A. 2001;98:13889–13894. doi: 10.1073/pnas.241393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remppis A, Greten T, Schafer BW, Hunziker P, Erne P, Katus HA, Heizmann CW. Altered expression of the Ca(2+)-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 64.Remppis A, Most P, Loffler E, Ehlermann P, Bernotat J, Pleger S, Borries M, Reppel M, Fischer J, Koch WJ, Smith G, Katus HA. The small EF-hand Ca2+ binding protein S100A1 increases contractility and Ca2+ cycling in rat cardiac myocytes. Basic Res Cardiol. 2002;97 suppl 1:I56–I62. doi: 10.1007/s003950200031. [DOI] [PubMed] [Google Scholar]
- 65.Most P, Remppis A, Pleger ST, Loffler E, Ehlermann P, Bernotat J, Kleuss C, Heierhorst J, Ruiz P, Witt H, Karczewski P, Mao L, Rockman HA, Duncan SJ, Katus HA, Koch WJ. Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem. 2003;278:33809–33817. doi: 10.1074/jbc.M301788200. [DOI] [PubMed] [Google Scholar]
- 66.Volkers M, Loughrey CM, Macquaide N, Remppis A, DeGeorge BR, Jr, Wegner FV, Friedrich O, Fink RH, Koch WJ, Smith GL, Most P. S100A1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium. 2007;41:135–143. doi: 10.1016/j.ceca.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Du XJ, Cole TJ, Tenis N, Gao XM, Kontgen F, Kemp BE, Heierhorst J. Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol Cell Biol. 2002;22:2821–2829. doi: 10.1128/MCB.22.8.2821-2829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr, Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 69.Most P, Pleger ST, Volkers M, Heidt B, Boerries M, Weichenhan D, Loffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 71.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 72.Packer M. The development of positive inotropic agents for chronic heart failure: how have we gone astray? J Am Coll Cardiol. 1993;22:119A–126A. doi: 10.1016/0735-1097(93)90474-f. [DOI] [PubMed] [Google Scholar]
- 73.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 74.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 75.Brodde OE. β1-and β2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- 76.Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005;106:39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg SF. The molecular basis for distinct SF β-adrenergic receptor subtype actions in cardiomyocytes. Circ Res. 1999;85:1101–1111. doi: 10.1161/01.res.85.11.1101. [DOI] [PubMed] [Google Scholar]
- 78.Hata JA, Williams ML, Koch WJ. Genetic manipulation of myocardial β-adrenergic receptor activation and desensitization. J Mol Cell Cardiol. 2004;37:11–21. doi: 10.1016/j.yjmcc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 79.Engelhardt S, Grimmer Y, Fan GH, Lohse MJ. Constitutive activity of the human β1-adrenergic receptor in β1-receptor transgenic mice. Mol Pharmacol. 2001;60:712–717. [PubMed] [Google Scholar]
- 80.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 81.Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW., II Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 82.Du XJ, Gao XM, Jennings GL, Dart AM, Woodcock EA. Preserved ventricular contractility in infarcted mouse heart overexpressing β2-adrenergic receptors. Am J Physiol Heart Circ Physiol. 2000;279:H2456–H2463. doi: 10.1152/ajpheart.2000.279.5.H2456. [DOI] [PubMed] [Google Scholar]
- 83.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. β-arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 85.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maurice JP, Hata JA, Shah AS, White DC, McDonald PH, Dolber PC, Wilson KH, Lefkowitz RJ, Glower DD, Koch WJ. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary β2-adrenergic receptor gene delivery. J Clin Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah AS, Lilly RE, Kypson AP, Tai O, Hata JA, Pippen A, Silvestry SC, Lefkowitz RJ, Glower DD, Koch WJ. Intracoronary adenovirus-mediated delivery and overexpression of the β2-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation. 2000;101:408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- 88.Kypson A, Hendrickson S, Akhter S, Wilson K, McDonald P, Lilly R, Dolber P, Glower D, Lefkowitz R, Koch W. Adenovirus-mediated gene transfer of the β2-adrenergic receptor to donor hearts enhances cardiac function. Gene Ther. 1999;6:1298–1304. doi: 10.1038/sj.gt.3300940. [DOI] [PubMed] [Google Scholar]
- 89.Tevaearai HT, Eckhart AD, Walton GB, Keys JR, Wilson K, Koch WJ. Myocardial gene transfer and overexpression of β2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation. 2002;106:124–129. doi: 10.1161/01.cir.0000020220.79105.fd. [DOI] [PubMed] [Google Scholar]
- 90.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 91.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 92.Iaccarino G, Koch WJ. In vivo adenoviral-mediated gene transfer of the βARKct to study the role of Gβγ in arterial restenosis. Methods Mol Biol. 2004;237:181–192. doi: 10.1385/1-59259-430-1:181. [DOI] [PubMed] [Google Scholar]
- 93.Akhter SA, Eckhart AD, Rockman HA, Shotwell K, Lefkowitz RJ, Koch WJ. In vivo inhibition of elevated myocardial β-adrenergic receptor kinase activity in hybrid transgenic mice restores normal β-adrenergic signaling and function. Circulation. 1999;100:648–653. doi: 10.1161/01.cir.100.6.648. [DOI] [PubMed] [Google Scholar]
- 94.Urasawa K, Yoshida I, Takagi C, Onozuka H, Mikami T, Kawaguchi H, Kitabatake A. Enhanced expression of β-adrenergic receptor kinase 1 in the hearts of cardiomyopathic Syrian hamsters, BIO53.58. Biochem Biophys Res Commun. 1996;219:26–30. doi: 10.1006/bbrc.1996.0175. [DOI] [PubMed] [Google Scholar]
- 95.Akhter SA, Skaer CA, Kypson AP, McDonald PH, Peppel KC, Glower DD, Lefkowitz RJ, Koch WJ. Restoration of β-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1997;94:12100–12105. doi: 10.1073/pnas.94.22.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a α-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson KM, Eckhart AD, Willette RN, Koch WJ. The myocardial β-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension. 1999;33:402–407. doi: 10.1161/01.hyp.33.1.402. [DOI] [PubMed] [Google Scholar]
- 98.Vinge LE, Oie E, Andersson Y, Grogaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and β-arrestin isoforms in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 2001;281:H2490–H2499. doi: 10.1152/ajpheart.2001.281.6.H2490. [DOI] [PubMed] [Google Scholar]
- 99.Drazner MH, Peppel KC, Dyer S, Grant AO, Koch WJ, Lefkowitz RJ. Potentiation of β-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J Clin Invest. 1997;99:288–296. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial β-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac βARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a β-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 103.Eckhart AD, Koch WJ. Expression of a β-adrenergic receptor kinase inhibitor reverses dysfunction in failing cardiomyocytes. Mol Ther. 2002;5:74–79. doi: 10.1006/mthe.2001.0508. [DOI] [PubMed] [Google Scholar]
- 104.Tevaearai HT, Eckhart AD, Shotwell KF, Wilson K, Koch WJ. Ventricular dysfunction after cardioplegic arrest is improved after myocardial gene transfer of a β-adrenergic receptor kinase inhibitor. Circulation. 2001;104:2069–2074. doi: 10.1161/hc4201.097188. [DOI] [PubMed] [Google Scholar]
- 105.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted β-adrenergic receptor kinase (βARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 106.Dorn GW, II, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of β2-adrenergic receptors differentially affect cardiac hypertrophy and function in Gαq-overexpressing mice. Proc Natl Acad Sci U S A. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eckhart AD, Fentzke RC, Lepore J, Lang R, Lin H, Lefkowitz RJ, Koch WJ, Leiden JM. Inhibition of βARK1 restores impaired biochemical β-adrenergic receptor responsiveness but does not rescue CREB(A133) induced cardiomyopathy. J Mol Cell Cardiol. 2002;34:669–677. doi: 10.1006/jmcc.2002.2007. [DOI] [PubMed] [Google Scholar]
- 108.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., II Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and β-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 109.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 110.Du XJ, Autelitano DJ, Dilley RJ, Wang B, Dart AM, Woodcock EA. β2-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation. 2000;101:71–77. doi: 10.1161/01.cir.101.1.71. [DOI] [PubMed] [Google Scholar]
- 111.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 113.Phan HM, Gao MH, Lai NC, Tang T, Hammond HK. New signaling pathways associated with increased cardiac adenylyl cyclase 6 expression: implications for possible congestive heart failure therapy. Trends Cardiovasc Med. 2007;17:215–221. doi: 10.1016/j.tcm.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., II Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, Hammond HK. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 116.Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation. 1999;99:3099–3102. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 117.Rebolledo B, Lai NC, Gao MH, Takahashi T, Roth DM, Baird SM, Hammond HK. Adenylylcyclase gene transfer increases function of the failing heart. Hum Gene Ther. 2006;17:1043–1048. doi: 10.1089/hum.2006.17.1043. [DOI] [PubMed] [Google Scholar]
- 118.Tepe NM, Liggett SB. Transgenic replacement of type V adenylyl cyclase identifies a critical mechanism of β-adrenergic receptor dysfunction in the Gαq overexpressing mouse. FEBS Lett. 1999;458:236–240. doi: 10.1016/s0014-5793(99)01147-3. [DOI] [PubMed] [Google Scholar]
- 119.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 121.Tepe NM, Lorenz JN, Yatani A, Dash R, Kranias EG, Dorn GW, II, Liggett SB. Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: enhanced basal catalytic activity and function without increased cardiomyocyte β-adrenergic signalling. Biochemistry. 1999;38:16706–16713. doi: 10.1021/bi991619k. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 123.Lai NC, Roth DM, Gao MH, Fine S, Head BP, Zhu J, McKirnan MD, Kwong C, Dalton N, Urasawa K, Roth DA, Hammond HK. Intra-coronary delivery of adenovirus encoding adenylyl cyclase VI increases left ventricular function and cAMP-generating capacity. Circulation. 2000;102:2396–2401. doi: 10.1161/01.cir.102.19.2396. [DOI] [PubMed] [Google Scholar]
- 124.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 125.Bos JM, Ommen SR, Ackerman MJ. Genetics of hypertrophic cardiomyopathy: one, two, or more diseases? Curr Opin Cardiol. 2007;22:193–199. doi: 10.1097/HCO.0b013e3280e1cc7f. [DOI] [PubMed] [Google Scholar]
- 126.Karkkainen S, Peuhkurinen K. Genetics of dilated cardiomyopathy. Ann Med. 2007;39:91–107. doi: 10.1080/07853890601145821. [DOI] [PubMed] [Google Scholar]
- 127.Michele DE, Albayya FP, Metzger JM. Thin filament protein dynamics in fully differentiated adult cardiac myocytes: toward a model of sarcomere maintenance. J Cell Biol. 1999;145:1483–1495. doi: 10.1083/jcb.145.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muntoni F. Cardiomyopathy in muscular dystrophies. Curr Opin Neurol. 2003;16:577–583. doi: 10.1097/01.wco.0000093100.34793.81. [DOI] [PubMed] [Google Scholar]
- 129.Rodino-Klapac LR, Chicoine LG, Kaspar BK, Mendell JR. Gene therapy for Duchenne muscular dystrophy: expectations and challenges. Arch Neurol. 2007;64:1236–1241. doi: 10.1001/archneur.64.9.1236. [DOI] [PubMed] [Google Scholar]
- 130.Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- 131.Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, Danos O. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- 132.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, de Kimpe SJ, Ekhart PF, Venneker EH, Platenburg GJ, Verschuuren JJ, van Ommen GJ. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]