Abstract
Neuroendocrine factors that produce species differences in aggregation behavior (“sociality”) are largely unknown, although relevant studies should yield important insights into mechanisms of affiliation and social evolution. We here focused on five species in the avian family Estrildidae that differ selectively in their species-typical group sizes (all species are monogamous and occupy similar habitats). These include two highly gregarious species that independently evolved coloniality; two territorial species that independently evolved territoriality; and an intermediate, modestly gregarious species that is a sympatric congener of one of the territorial species. Using males and females of each species, we examined binding sites for 125I- vasoactive intestinal polypeptide (VIP), 125I-sauvagine (SG; a ligand for corticotropin releasing factor, CRF, receptors) and a linear 125I-V1a vasopressin antagonist (to localize receptors for vasotocin, VT). VIP, CRF and VT are neuropeptides that influence stress, anxiety and/or various social behaviors. For numerous areas (particularly within the septal complex), binding densities in the territorial species differed significantly from binding in the more gregarious species, and in most of these cases, binding densities for the moderately greagarious species were either comparable to the two colonial species, or were intermediate to the territorial and colonial species. Such patterns were observed for 125I-VIP binding in the medial bed nucleus of the stria terminalis, medial septum, septohippocampal septum, and subpallial zones of the lateral septum; for 125I-SG binding in the infundibular hypothalamus, and lateral and medial divisions of the ventromedial hypothalamus; and for the linear 125I-V1a antagonist in the medial septum, and the pallial and subpallial zones of the caudal lateral septum. With the exception of 125I-SG binding in the infundibular hypothalamus, binding densitites are positively related to sociality.
Keywords: lateral septum, hypothalamus, vasotocin, vasopressin, corticotropin releasing factor, vasoactive intestinal polypeptide, evolution, sociality, songbird
Components of social organization can change rapidly during evolution, as suggested by the fact that many vertebrate groups exhibit substantial diversity in behavioral dimensions such as grouping, mating systems, patterns of parental care, philopatry and kin affiliation. Thus, a given form of behavior or social structure may have evolved independently many times in various vertebrate lineages. Somewhat inconveniently, the evolution of a given species-typical social structure could hypothetically be produced by more than one kind of evolutionary modification to the brain, reducing our ability to generalize across species. Species-typical group size (“sociality”) provides a good example: Natural selection on aggregation behavior could conceivably act on neural mechanisms that relate to anxiety, stress, aggression, reward, bonding, and/or simple arousal to social stimuli1. Therefore, in order to increase our confidence that a given mechanism is reliably associated with a given pattern of behavior, it is important to determine 1) whether evolutionary divergence in species-typical behavior is accompanied by evolutionary divergence in neural mechanisms, and 2) whether mechanistic convergence is observed when the behavior evolves independently.
To date, such analyses have been conducted primarily in relation to mating systems in mammals. Monogamous mice, voles and monkeys reliably exhibit higher densities of vasopressin (VP) V1a receptors in the ventral pallidum than do non-monogamous comparators (Insel, 1992; Insel et al., 1994; Wang et al., 1997; Young et al., 1997, 1999; Bester-Meredith et al., 1999; this area was identified as the diagonal band in some earlier reports; see Young et al., 2001), and a variety of functional studies clearly demonstrate that these receptors are key to the expression of selective partner preference (Pitkow et al., 2001; Lim et al., 2004; Lim and Young, 2004). In this case, then, divergence and convergence in behavior is reliably associated with divergence and convergence in an underlying neural mechanism. Interestingly, these studies have also shown that species-specific densities of V1a receptors in brain areas other than the ventral pallidum do not correlate with mating system, suggesting that adaptive receptor functions remain to be elucidated that are independent of mating system (review: Goodson and Bass, 2001). A similar observation is made for CRF receptors in voles (Lim et al., 2005). We hypothesize that these other adaptive functions include the regulation of sociality, thus the present studies focus on the evolution of species-typical group size and its relationship to neuropeptide receptor densities.
The proximate neural and endocrine mechanisms of that influence sociality, independently of other behavioral dimensions, are almost wholly unexplored (but see Goodson et al., 2005a). This may reflect the fact that due to potential confounds, most vertebrate groups do not provide good opportunities for comparative studies of sociality. For instance, even within the most socially diverse groups of mammals (e.g., rodents of the Peromyscus and Microtus genera), species differences in aggregation are confounded with other important variables, including mating system, patterns of parental care, habitat preferences, and seasonal or facultative expression of behavior (for an introduction to these genera, see King, 1968; Tamarin, 1985). In contrast, the behavioral and ecological diversity of birds (Konishi et al., 1989) offers at least a few good opportunities for highly controlled, comparative studies of sociality (Goodson et al., 2005c). Given that the neurobehavioral systems of birds are very similar to other vertebrates, including mammals, avian studies on this topic should also be broadly relevant (Ball and Balthazart, 2001; Goodson, 2005).
Interestingly, manipulations that influence pair-bonding in monogamous prairie voles (Microtus ochrogaster) also influence non-sexual affiliation (Pitkow et al., 2001). This finding suggests that evolution in sociality and evolution in mating system may involve coordinated modifications of at least some neural mechanisms. However, in contrast to the prairie vole, which is both monogamous and gregarious, the vast majority of highly gregarious mammals are polygamous (e.g., herd ungulates and many primates), and many gregarious songbirds are also polygamous. Conversely, many monogamous birds are highly territorial (i.e., fairly asocial), as are some monogamous mammals (Clutton-Brock, 1989; Bennett and Owens, 2002). Hence, there is much to be learned about sociality that can be dissociated from mechanisms related to mating system, although mating system may constrain or otherwise influence the evolution of neural systems related to sociality. The present findings suggest that evolution in sociality may relate primarily to processes that lie outside of the brain areas that are most strongly implicated in the evolution of mating system, such as the nucleus accumbens and ventral pallidum (Young and Wang, 2004).
For the present experiments, we used five species of estrildid finches and waxbills that are all monogamous (typically bonding for life), exhibit biparental care, breed semi-opportunistically dependent upon rainfall, and inhabit semi-arid and/or grassland scrub habitat (Immelmann, 1965; Skead, 1975; Goodwin, 1982; Zann, 1996). These include two territorial species that independently evolved territoriality (the melba finch, Pytilia melba, and the violet-eared waxbill, Uraeginthus granatina); two strongly gregarious species that independently evolved a high level of coloniality (the spice finch, Lonchura punctulata, and the zebra finch, Taeniopygia guttata), and one moderately gregarious species (the Angolan blue waxbill, Uraeginthus angolensis). The social structures of these species are temporally stable, thus the species differences in sociality are observed at all times of the year. Additional details on the social evolution of these birds are provided in the Methods (“Convergent and divergent social evolution”).
Previous data from four of these species suggest that evolutionary shifts in sociality may be associated with divergence in appetitive processes (approach-avoidance), social stress and related aversion processes (Goodson et al., 2005a), so we have here examined binding sites for three neuropeptides (CRF, VT and VIP) that are known to influence those functions (general reviews: Lovejoy and Balment, 1999; Goodson and Bass, 2001; Bale and Vale, 2004; Landgraf and Neumann, 2004) (songbirds: Maney et al., 1997; Castagna et al., 1998; Goodson, 1998a,b; Maney and Wingfield, 1998; Goodson and Adkins-Regan, 1999; Goodson and Evans, 2004; Goodson et al., 2004b).
Methods
Animals
A total of 40 subjects from five species (melba finch, violet-eared waxbill, Angolan blue waxbill, spice finch and zebra finch) were employed in the present investigations. We examined four males and four females of each species. The species-typical group size for the territorial species is 2, excluding dependent young (i.e., a male-female pair). Group sizes for the moderately gregarious Angolan blue waxbill are approximately 8-40. Species-typical group sizes for the two colonial species are approximately 100, with much larger flocks occasionally observed (Immelmann, 1965; Skead, 1975; Goodwin, 1982; Zann, 1996).
We collected melba finches, violet-eared waxbills and Angolan blue waxbills by mist net in northwestern South Africa, vicinity of Madikwe game reserve (24° 45′ 26° 30′ E). Wild spice finches were collected by a commercial supplier. We used wild-caught individuals that had been in captivity for 7-12 months, with the exception of four melba finches (two male-female pairs), which were captive-raised from wild parents. Zebra finches (native to Australia) were of domestic stock. Importantly, detailed ethological analyses have failed to demonstrate the presence of behavioral differences between wild-caught and domestic zebra finches (Morris, 1958). Collections and procedures were conducted legally and humanely under all applicable federal and state permits and in compliance with all applicable institutional and federal guidelines, including the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Housing, testing and euthanization procedures were designed to minimize subject stress, and were approved by the Institutional Animal Care and Use Committee.
Convergent and divergent social evolution
The strength of the present comparative approach depends in large part upon the assertion that we have employed 1) two colonial species that independently evolved coloniality, 2) two territorial species that independently evolved territoriality, and 3) an intermediate, modestly gregarious species (Uraeginthus angolensis) that is a sympatric congener of one of the territorial species (U. granatina). These species are distributed across three estrildid tribes – the Poephilini, Estrildini, and Lonchurini, which diverged approximately 14 million years ago (Christidis, 1987a,b; also see Delacour, 1943; Mayr, 1968). A review of behavior in the approximately 139 estrildids suggests that the common ancestor was modestly gregarious (based largely on Goodwin, 1982; also Harrison, 1962; Immelman, 1965; Skead, 1975)2, an hypothesis which is consistent with the behavior of presumably basal estrildids (Sorenson et al., 2004). Of the approximately 15 Poephilini species, only the zebra finch is highly colonial (with the chestnut-eared finch being considered a subspecies of zebra finch). Average species-typical group sizes for the zebra finch are approximately 100 (Zann, 1996); the next most social species in the Poephilini, Taeniopygia bichenovii, is found in parties of 4-20 individuals. Similarly, only three of the approximately 56 Lonchurini species have evolved a level sociality comparable the zebra finch, including the spice finch. These combined observations suggest that a high degree of coloniality has evolved independently in the spice finch and zebra finch.
Our remaining subjects are in the tribe Estrildini, which contains approximately 68 species. Of the 11 species in the closely related genera Uraeginthus (5 species), Pyrenestes (3 species) and Spermophaga (3 species; relationships based on Sorenson et al., 2004), all form small parties or flocks during the nonbreeding season except the violet-eared waxbill (U. granatina). Most species distribute loosely for breeding. Only the violet-eared waxbill (U. granatina), the closely related U. ianthinogaster, and Spermophaga ruficapilla aggressively maintain exclusive breeding territories. Immelmann (cited in Goodwin, 1982) reports that the territories of U. granatina are several hundred meters square and that intruders are promptly driven out. Although U. ianthinogaster appears to be much the same, this species is encountered in small parties during the non-breeding season (as is S. ruficapilla; Goodwin, 1982), whereas adult pairs of U. granatina do not associate with other pairs (B.M. Mines and J.L. Goodson, unpubl. obs.). These observations suggest that the highly asocial behavior of the violet-eared waxbill is a derived condition within the Uraeginthus-Pyrenestes-Spermophaga complex, and has evolved independently of that in the melba finch, which is one of only three species in the Estrildini tribe to exhibit a behavioral profile matching that of the violet-eared waxbill. In contrast, the Angolan blue waxbill (U. angolensis) is the only species of the Uraeginthus-Pyrenestes-Spermophaga complex to remain social during the breeding season, nesting in a semi-colonial manner and forming small feeding flocks (Goodwin, 1982; Skead, 1975).
Housing and experimental conditions
In order to obtain tissue from the various species under equivalent circumstances, brains were collected at a time of the year that falls within the non-breeding season for all four wild-caught species (September). Subjects were housed outdoors for eight weeks at the University of California's Elliot Field Station (San Diego, CA) on a natural, declining photoperiod. Male-female pairs of each species were housed in flight cages (1 m W × 2 m D × 2 m H) and were not placed in flights adjacent to conspecifics. Finch seed mix, oyster shell and water were provided ad libitum. Water was provided only via sipper tubes, because access to open water can stimulate reproductive behavior and physiology in these species. No measurable precipitation occurred during the subjects' eight weeks outdoors.
Avian neuropeptide receptors and selection of iodinated ligands
A single VIP receptor has been cloned in turkeys and chickens that exhibits 55-65% sequence identity with VIP1 and VIP2 receptors in mammals (Kansaku et al., 2001; You et al., 2001). Using the same 125I-VIP employed here (human/porcine/rat; Perkin-Elmer Life Sciences, Boston MA), it has been shown that specific binding sites in turkey telencephalon exhibit a high affinity for both VIP and pituitary adenylate cyclase-activating polypeptide (PACAP) (Zawilska et al., 2004). These data are consistent with the known affinities of VIP receptors: Avian and mammalian VIP receptors exhibit a high affinity for both VIP and PACAP (hence these receptors are sometimes referred to as VPAC receptors), whereas the single PACAP receptor in birds and mammals is highly selective for PACAP (Vaudry et al., 2000; Zawilska et al., 2003).
The CRF peptide family includes the various vertebrate forms of CRF, and a second clade of peptides that are evolutionarily derived from urotensin I (found in fish); this clade includes sauvagine (SG) and the various forms of urocortin. In mammals, the CRF1 receptor is non-selective, binding all peptides of the CRF and urotensin I clades with approximately equal affinity, whereas the CRF2 receptor is strongly selective for the urotensin I-like peptides and binds CRF with a lower affinity (Primus et al., 1997; Lovejoy and Balment, 1999). Very little information is available on CRF1- and CRF2-like receptors in birds, and CRF receptor distributions in the brain have not been described. Despite the fact that it shares 87-88% sequence identity with the CRF1 receptor of human, mouse and rat, the chicken CRF1-like receptor does not exhibit the non-selectivity of the mammalian CRF1, and binds urotensin I with an affinity 20 times higher than for CRF (Yu et al., 1996). Directly comparable data are not available for the avian CRF2-like receptor, but it binds urocortin III (a selective agonist for mammalian CRF2) and antisauvagine-30 (a selective antagonist for mammalian CRF2) with high affinity (De Groef et al., 2003). Thus, the avian CRF2-like receptor exhibits properties that are typical of the mammalian CRF2 receptor. Given the profiles of these receptors, we here employed 125I-SG (SG is a member of the urotensin clade) (Perkin-Elmer) to localize both receptor types in the brain, and conducted cold competition studies with both SG and antisauvagine-30.
Two VT receptors have been cloned in birds. The avian VT2 receptor is homologous to the mammalian V1b receptor and is expressed only in the pituitary (Cornett et al., 2003). The VT1 receptor is most similar to the mammalian V1a receptor. Although its sequence identity with the mammalian V1a is not particularly high (51.9% with the rat V1a; Tan et al., 2000), the avian VT1 receptor contains amino acid residues that are important for the ligand selectivity of the mammalian V1a receptor, and the pharmacology of the avian VT1 is therefore more similar to the mammalian V1a than are V1a-like receptors in other non-mammalian taxa (see functional studies in Acharjee et al., 2004). Thus, we here used an iodinated, linear V1a antagonist (Phenylacetyl1, 0-Me-D-Tyr2, [125I-Arg6]-) (Perkin-Elmer) that has a very high affinity for mammalian V1a receptors, and a much more modest affinity for V1b receptors (Barberis et al., 1995; Young et al., 1997).
Quantitative autoradiography
Subjects were euthanized by isoflurane vapor. Brains were rapidly removed and immersed in isopentane cooled to −20° C. Five series of 20 μm coronal sections were cut on a cryostat and collected onto chrom-alum subbed slides. Following 2 hr in a cooled vacuum chamber, slides were stored at −80° C. Three of the tissue series were used for quantitative species comparisons, using 125I-SG, 125I-VIP and linear 125I-V1a antagonist. Remaining tissue was used for experiments with cold competitor peptides (SG, VIP, VT, and mesotocin) or antisauvagine-30. All cold competitors were employed at a concentration of 1 μM; SG and antisauvagine-30 were additionally used at concentrations of 0.01 and 0.1 μM.
We labeled tissue from all subjects (for a given ligand) in a timeframe of 5-7 days. Processing order was randomized across sexes and species, and representative tissue from all species and both sexes was labeled on each day of processing. After warming to room temperature, slides were immersed for 1 min in 0.2% paraformaldehyde in Tris-MgCl2 buffer (50 mM Tris-HCl + 10 mM MgCl2). Following 2 × 5 min rinses in Tris-MgCl2 buffer, sections were incubated with radioactive ligand in Tris-MgCl2 buffer + 0.3% bovine serum albumin + 0.05% bacitracin. Incubation concentrations and durations for each peptide were: 125I-SG, 30 pM for 60 min; 125I-VIP, 50 pM for 90 min; and linear 125I-V1a antagonist, 50 pM for 75 min. Concentrations were selected through pilot testing using a range of concentrations from the literature, and are similar to those used in previous investigations (e.g., Hof et al, 1991; Young et al., 1999; Bakshi et al., 2002). For 125I-SG binding, slides were then rinsed 2 × 4 min in ice-cold Tris-MgCl2 buffer, dipped in distilled water and dried under a cool stream of air. For 125I-VIP and linear 125I-V1a antagonist binding, incubation was followed by 3 × 4 min rinses in ice-cold Tris-MgCl2 buffer, 1 min in 0.1% paraformaldehyde, and 20 min in 50 mM Tris-HCl + 50 mM MgCl2 at room temperature (to reduce background). Slides were then dipped in distilled water and dried under a cool stream of air. Slides were left overnight in a vacuum desiccator at room temperature and then apposed to Kodak MS-1 film in stainless steel cassettes for 2 days (125I-VIP) or 3 days (125I-SG and linear 125I-V1a antagonist). Each cassette contained two sheets of film and four brains (mixed sexes and species), plus a 125I plastic standard (Amersham Biosciences, Piscataway, NJ) for each sheet of film.
Data analysis
After development of the films, one tissue series from each subject was stained with cresyl violet in order to aid in the identification of brain areas. This yields a very light stain in autoradiographic material, even with concentrated stain. However, major nuclei and gross structure (ventricles, fiber tracts) can be visualized in this manner. Importantly, based on extensive histochemical studies of four estrildid finches and one sparrow species (Goodson et al., 2004a), we have been able to develop methods for localizing areas of interest (e.g., septal zones and major hypothalamic areas) on the basis of major structural landmarks, typically by considering relative distances between them. Once the area has been localized, templates are superimposed on digital images of the sections. We have extensively validated this method in other studies (including five species) by comparing our template positioning (onto sections that were labeled for immediate early genes) with the distribution of histochemical markers in adjacent sections (Goodson and Evans, 2004; Goodson et al., 2005a; also see Goodson et al., 2005b).
Photomicrographs were captured with a Macintosh G4 computer linked to an Optronics Magnafire digital camera, using the Northern Lights accessory package (Amersham Biosciences) for macro imaging. When required, templates were superimposed over the digital images using Photoshop 5.5. Binding was sampled bilaterally in 1-4 sections per area, dependent upon size. Optical densities were measured in NIH Image 1.63 (W. Rasband, National Institutes of Health) using Rodbard polynomials that were generated from the 125I standards. Nonspecific binding was assessed using cold competitor peptides in alternate sections of representative brains from each species. For quantification of specific binding, we selected an area that exhibited no specific binding in the competition studies, and then for each subject, background binding in that area was subtracted from the optical densities of each area measured. Statistical analyses (ANOVAs followed by Fisher's PLSD) were performed using Statview (SAS Institute, Inc., Cary, NC).
Nomenclature and abbreviations
Nomenclature for the avian brain has recently been revised, with a major focus on the telencephalon (Reiner et al., 2004). Where appropriate, we have adopted this new nomenclature, although the majority of structures discussed here lie within basal forebrain regions that did not receive new names with the recent revision. Our nomenclature for these sites is consistent with our previous investigations in songbirds (see Goodson and Evans, 2004 for area delineations; also Goodson et al., 2005a), and we have employed the nomenclature of Goodson et al. (2004a) for the various subdivisions of the septal complex.
Abbreviations employed in the tables and figures are as follows: Ac, nucleus accumbents; AH, anterior hypothalamus; AM, anteromedial hypothalamus; BSTm, medial bed nucleus of the stria terminalis; BSTl, lateral bed nucleus of the stria terminalis; CcS, caudocentral septum; CoS, commissural septal nucleus; DMP, dorsomedial posterior nucleus of the thalamus; E, entopallium; GP, globus pallidus; HA, apical part of the hyperpallium; Hab, habenula; HD, densocellular part of the hyperpallium; Hp, hippocampus; Inf, infundibular hypothalamus; LSc, caudal division of the lateral septum (dorsal, ventrolateral, and ventral zones denoted as LSc.d, LSc.vl, and LSc.v, respectively); LSr, rostral division of the lateral septum (medial and dorsolateral zones denoted as LSr.m and LSr.dl, respectively); LSt, lateral striatum; M, mesopallium; ME, median eminence; MS, medial septum; MSt, medial striatum; N, nidopallium; NC, caudal nidopallium; NDB, nucleus of the diagonal band; nPC, nucleus of the pallial commissure; ot, optic tract; PLH, posterolateral nucleus of the hypothalamus; POA, preoptic area; PVH, periventricular hypothalamus; PVN, paraventricular nucleus of the hypothalamus; RA, robust nucleus of the arcopallium; SH, septohippocampal septum; Teg, midbrain tegmentum; TeO, optic tectum; TnA, nucleus taeniae of the amygdala; VMH, ventromedial nucleus of the hypothalamus; VP, ventral pallidum.
Results
Overview of results
Binding sites for 125I –VIP and 125I –SG were widely distributed throughout the brain (Figs. 1 and 2), whereas binding sites for the linear 125I -V1a antagonist were more restricted (Fig. 3). For all three ligands, binding sites within the septal nuclei showed significant correspondence to sociality, as did 125I –VIP binding within the medial bed nucleus of the stria terminalis (BSTm), and 125I –SG binding within the ventromedial hypothalamus (VMH) and infundibular hypothalamus (Tables 1-3; Figs. 3 and 4). With the exception of binding in the infundibular hypothalamus, the gregarious subjects exhibited significantly greater binding than did the territorial subjects. In each case, the moderately greagarious Angolan blue waxbill exhibited binding that was similar to the colonial species, or that was intermediate to the territorial and colonial species.
Figure 1.
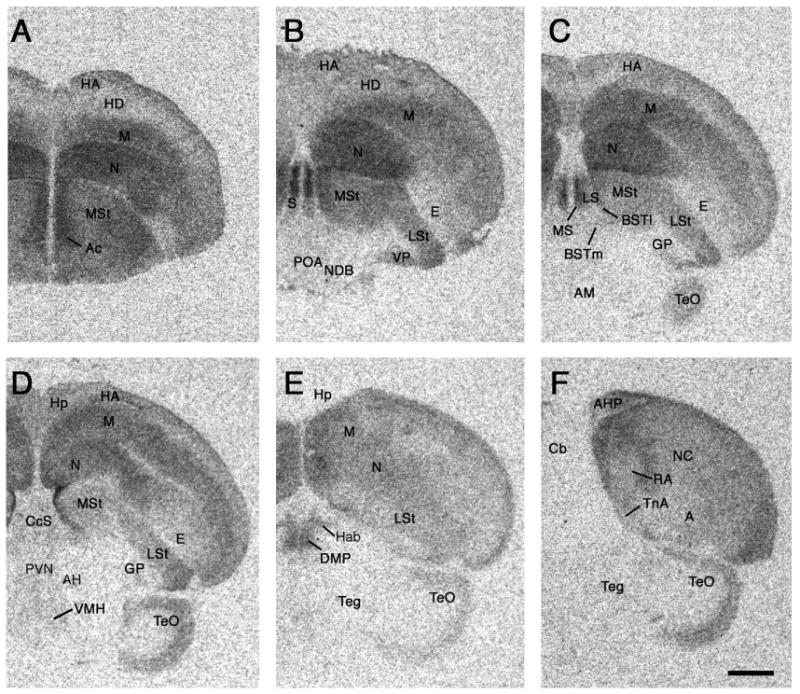
Photomicrographs of a rostral (A) to caudal (F) series showing representative binding of 125I-VIP in a female zebra finch. Quantitative results are shown in Table 1. Scale bar = 1 mm. See Methods for abbreviations (“Nomenclature and abbreviations”).
Figure 2.
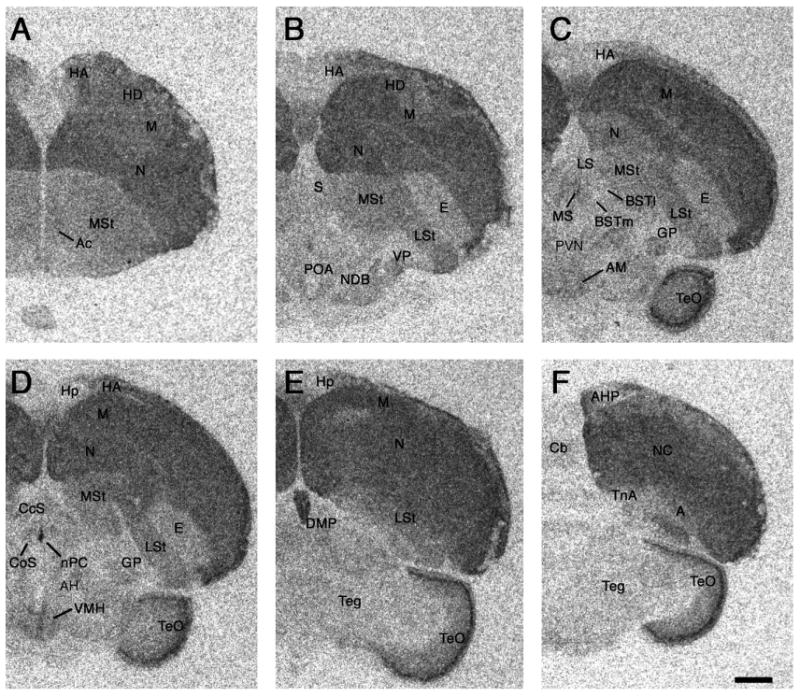
Photomicrographs of a rostral (A) to caudal (F) series showing representative binding of 125I-SG in a female zebra finch. Quantitative results are shown in Table 2. Scale bar = 1 mm. See Methods for abbreviations (“Nomenclature and abbreviations”).
Figure 3.
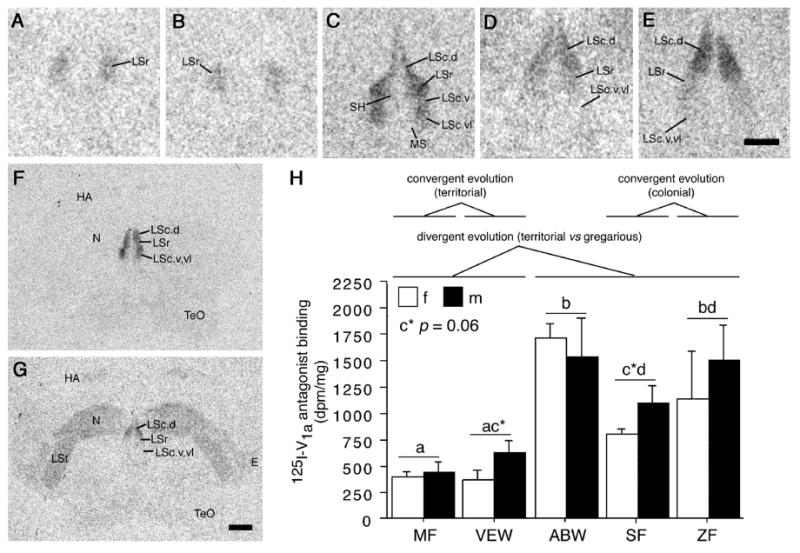
Species differences in linear 125I-V1a antagonist binding. (A-E) Representative binding in the septum of five monogamous species that differ selectively in their species-typical group sizes: Territorial melba finch (MF; A), territorial violet-eared waxbill (VEW; B), moderately gregarious Angolan blue waxbill (ABW; C), colonial spice finch (SF; D) and colonial zebra finch (ZF; E). (F-G) Representative sections for a male Angolan blue waxbill and male spice finch, respectively, showing species differences in binding for the nidopallium (N) and other areas of the forebrain. (H) Linear 125I-V1a antagonist binding in the dorsal (pallial) portion of the lateral septum (LS), shown as decompositions per minute/mg (dpm/mg; means ± SEM). Different letters above the error bars denote significant species differences (Fisher's PLSD following significant ANOVA; p < 0.05). Additional quantitative results are shown in Table 3. The scale bar in E corresponds to 500 μm in panels A-E; the scale bar in G corresponds to 1 mm in panels F-G. See Methods for abbreviations (“Nomenclature and abbreviations”).
Table 1.
Specific 125I-VIP binding (decompositions per minute/mg following substraction of nonspecific binding; means ± SEM) in forebrain areas of five songbird species that differ selectively in their species-typical group sizes1.
| Territorial | Gregarious | Highly Colonial | ||||
|---|---|---|---|---|---|---|
| Area2 | Melba Finch | Violet-Eared Waxbill | Angolan Blue Waxbill | Spice Finch | Zebra Finch | Significant Sociality Contrasts3 |
| Extended amygdala: | ||||||
| TnA | 1962 ± 128ac | 2183 ± 211ab | 2491 ± 145b | 1647 ± 188c | 2348 ± 105ab** | D* |
| BSTm | 509 ± 66a | 580 ± 80a | 639 ± 54a | 764 ± 98ab | 975 ± 153b* | A*,B** |
| BSTl | 1079 ± 109 | 1179 ± 189 | 941 ± 134 | 1038 ± 132 | 971 ± 137 | |
| Septal Complex: | ||||||
| LSr (LSr.m + LSr. dl) | 992 ± 93a | 1246 ± 154ab | 1610 ± 216bc | 1222 ± 102ab | 1884 ± 180c* | A***,B*,C* |
| LSc, pallial (LSc.dl + LSc.dm) | 2944 ± 308 | 3769 ± 249 | 3418 ± 269 | 3198 ± 135 | 4118 ± 385 | |
| LSc, subpallial (LSc.v + LSc.vl) | 851 ± 88a | 1025 ± 111ac | 1366 ± 167b | 1222 ± 68bc | 1870 ± 134d*** | A***,B***,C* |
| CcS | 649 ± 101 | 694 ± 79 | 666 ± 99 | 677 ± 139 | 796 ± 74 | |
| MS | 3385 ± 304a | 2835 ± 193a | 4617 ± 303b | 2800 ± 280a | 5939 ± 432c*** | A**,B*,C*** |
| SH | 1687 ± 194a | 1561 ± 71a | 3100 ± 369bc | 2380 ± 170ab | 3663 ± 680c** | A***,B**,C*** |
| Preoptic area/hypothalamus: | ||||||
| POA | 628 ± 130 | 603 ± 131 | 713 ± 130 | 555 ± 81 | 904 ± 180 | |
| AM | 231 ± 80a | 356 ± 82ab | 294 ± 70a | 633 ± 139b | 514 ± 79ab* | A*,B**,D* |
| AH | 479 ± 92 | 742 ± 169 | 429 ± 96 | 739 ± 149 | 790 ± 85 | D* |
| Inf | 575 ± 206 | 387 ± 75 | 357 ± 91 | 302 ± 104 | 361 ± 96 | |
| PLH | 1035 ± 187 | 1216 ± 134 | 752 ± 136 | 912 ± 128 | 1393 ± 260 | |
| PVH | 1370 ± 238 | 945 ± 218 | 984 ± 148 | 665 ± 104 | 1050 ± 143 | |
| VMH, medial | 470 ± 147 | 593 ± 157 | 451 ± 97 | 755 ± 102 | 680 ± 92 | D** |
| VMH, lateral | 825 ± 172 | 812 ± 169 | 599 ± 85 | 580 ± 101 | 935 ± 160 | |
| Other forebrain areas: | ||||||
| DMP | 2365 ± 199a | 2985 ± 252b | 3061 ± 281b | 1900 ± 137a | 1825 ± 96a*** | B***,D*** |
| Hab | 1283 ± 96a | 877 ± 153b | 1903 ± 174c | 687 ± 46b | 905 ± 112b*** | B*,C***,D*** |
| Hp, ventral | 2015 ± 207a | 2300 ± 226a | 1751 ± 178ab | 1464 ± 116b | 2049 ± 190a* | A* |
| Hp, dorsal | 2654 ± 304 | 2654 ± 235 | 2863 ± 170 | 2050 ± 187 | 2419 ± 318 | D* |
| N, medial | 3775 ± 314ac | 2566 ± 112b | 3223 ± 422ab | 2917 ± 309b | 4325 ± 267c*** | |
| VP | 1529 ± 143ab | 1452 ± 140ab | 1820 ± 156a | 1190 ± 146b | 2602 ± 214c*** | |
Different letters next to the means indicate significant species differences (Fisher's PLSD, p < .05) following significant ANOVA (* p < .05; ** p < .01; *** p < .001; sex × species; no main effects of sex were observed, thus male and female data are shown pooled). Four males and four females of each species were examined (total n = 40).
Data are not shown for target areas that exhibited no specific binding, including the CoS and nPC. See Methods for abbreviations (“Nomenclature and abbreviations”).
Sociality contrasts were conducted by pooling subjects of a given grade of sociality (e.g., by pooling territorial or colonial species). Letters indicate main effects of sociality (* p < .05; ** p < .01; *** p < .001 in a two-way ANOVA including sex as a factor; see Results for sex effects). Contrast A: territorial versus flocking (“flocking” includes all three gregarious/colonial species). Contrast B: territorial versus colonial, excluding the intermediate Angolan blue waxbill. Contrast C: territorial versus moderately gregarious (Angolan blue waxbill). Contrast D: moderately gregarious versus colonial.
Table 3.
Specific binding of linear 125I-V1a antagonist (decompositions per minute/mg following substraction of nonspecific binding; means ± SEM) in forebrain areas of five songbird species that differ selectively in their species-typical group sizes1.
| Territorial | Gregarious | Highly Colonial | ||||
|---|---|---|---|---|---|---|
| Area2 | Melba Finch | Violet-Eared Waxbill | Angolan Blue Waxbill | Spice Finch | Zebra Finch | Significant Sociality Contrasts3 |
| Septal Complex: | ||||||
| LSr (LSr.m + LSr. dl) | 770 ± 110a | 1368 ± 239ac | 2528 ± 315b | 1132 ± 102ac | 1733 ± 324c*** | A**,C***,D** |
| LSc, pallial (LSc.dl + LSc.dm) | 415 ± 49a | 493 ± 83ac | 1612 ± 186b | 963 ± 109cd | 1311 ± 273bd*** | A***,B***,C*** |
| LSc, subpallial (LSc.v + LSc.vl) | 421 ± 56a | 532 ± 70ab | 610 ± 66ab | 741 ± 115b | 726 ± 102b* | A**,B** |
| CcS | 185 ± 71 | 225 ± 90 | 540 ± 230 | 502 ± 117 | 439 ± 173 | A*,B* |
| MS | 499 ± 59 | 529 ± 53 | 752 ± 93 | 861 ± 165 | 879 ± 165 | A**,B**,C** |
| SH | 121 ± 42 | 296 ± 117 | 465 ± 141 | 322 ± 99 | 539 ± 167 | A* |
| Other forebrain areas: | ||||||
| N, medial | 666 ± 56a | 740 ± 69a | 560 ± 60a | 1474 ± 262b | 889 ± 91a*** | B**,D* |
| VP | 450 ± 72 | 375 ± 60 | 534 ± 149 | 605 ± 166 | 320 ± 115 | |
Different letters next to the means indicate significant species differences (Fisher's PLSD, p < .05) following significant ANOVA (* p < .05; ** p < .01; *** p < .001; sex × species; no main effects of sex were observed, thus male and female data are shown pooled). Four males and four females of each species were examined (total n = 40).
Data are not shown for target areas that exhibited no specific binding, including the Ac, AH, AM, BST, CoS, DMP, Hab, Hp, Inf, NDB, nPC, POA, and VMH. See Methods for abbreviations (“Nomenclature and abbreviations”).
Sociality contrasts were conducted by pooling subjects of a given grade of sociality (e.g., by pooling territorial or colonial species). Letters indicate main effects of sociality (* p < .05; ** p < .01; *** p < .001 in a two-way ANOVA including sex as a factor; see Results for sex effects). Contrast A: territorial versus flocking (“flocking” includes all three gregarious/colonial species). Contrast B: territorial versus colonial, excluding the intermediate Angolan blue waxbill. Contrast C: territorial versus moderately gregarious (Angolan blue waxbill). Contrast D: moderately gregarious versus colonial.
Figure 4.
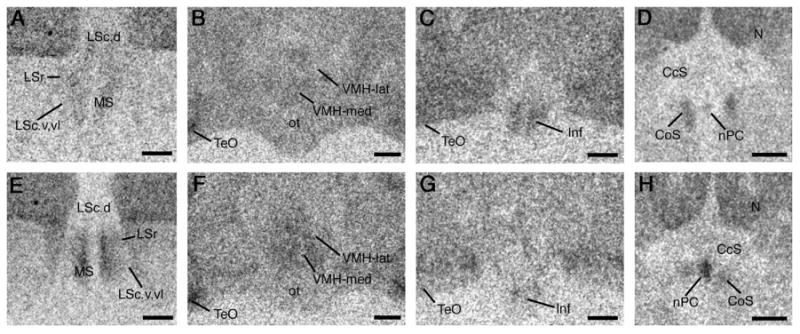
Photomicrographs of 125I-VIP and 125I-SG binding in several areas that show significant differences between territorial and colonial species (see Tables 1 and 2). Shown are photos from a female melba finch (territorial; A-D) and a female zebra finch (highly gregarious and colonial; E-H). Relative to territorial birds, colonial birds exhibit greater binding of 125I-VIP within the subpallial septum (A, E), and greater binding of 125I-SG within the ventromedial hypothalamus (VMH; B, F) and nucleus of the pallial commissure (nPC; D, H). Conversely, binding of 125I-SG is higher in the infundibular hypothalamus (Inf) of territorial birds (C, G). Scale bars = 500 μm. See Methods for abbreviations (“Nomenclature and abbreviations”).
Nonspecific binding and cold competitor comparisons
Binding was eliminated by addition of 1 μM cold SG (for 125I –SG), cold VIP (for 125I –VIP), or cold VT (for the linear 125I -V1a antagonist; similar reduction was not observed following competition with cold MT). Nonspecific binding for each ligand was homogenous through the basal forebrain, although portions of the pallium (central region of the nidopallium) and midbrain tegmentum showed higher levels of nonspecific binding than other brain areas, particularly in the material incubated with cold VT plus the linear 125I -V1a antagonist. These latter areas do not correspond to any regions that were analyzed in the present study. In addition to these assays, we incubated alternate control series with 0.01, 0.1 and 1 μM cold SG or with equivalent concentrations of antisauvagine-30. Both competitors produced comparable effects on binding throughout the brain. Complete elimination of binding was observed only with 1 μM cold competitor, although substantial reductions were observed at the two lower concentrations. Nonspecific binding for the linear 125I -V1a antagonist and 125I-VIP was low (286 ± 15 and 498 ± 23 decompositions per minute/mg, d.p.m./mg, respectively), whereas nonspecific binding for 125I –SG was modestly higher (742 ± 43 d.p.m./mg).
Given that we employed a single concentration of each radioligand, it remains possible that species differences in binding could reflect species differences in the affinity and/or maximal binding capacity of receptors. While we believe that such differences would likely be random or reflect phylogeny, a more comprehensive characterization of receptor pharmacology is required to fully resolve this.
General distributions of binding
We quantified binding in a variety of forebrain areas that are known to be involved in the regulation of social behavior, stress processes, and related physiological functions in birds and/or mammals. These areas include components of the medial extended amygdala (nucleus taeniae and BSTm), lateral BST, rostral and caudal divisions of the lateral septum (LSr and LSc, respectively), various other septal divisions (septohippocampal, medial and posterior septal nuclei), preoptic area, nucleus of the diagonal band, nucleus accumbens, ventral pallidum, numerous hypothalamic areas (anterior, AH; ventromedial, VMH; anteromedial; paraventricular; periventricular; posterolateral; and infundibular), habenula, dorsomedial posterior thalamus, and hippocampus. We also quantified binding within the medial nidopallium, as this area stands out based on its high levels of binding, particularly for 125I-VIP (Fig. 1).
Tables 1-3 show specific binding within these target areas for 125I-VIP, 125I-SG, and the linear 125I-V1a antagonist. Areas are not shown in the tables if sufficient levels of specific binding (2× background) were not detected in at least one of the five species. All data were analyzed by two-way ANOVA (sex × species). However, we observed no main effects of sex and the sexes were therefore pooled for presentation of the data in Tables 1-3. Only two sex × species interactions were observed. The first of these was for linear 125I-V1a antagonist binding in the septohippocampal septum (F4,1,29 = 3.294, p < 0.05), which was driven primarily by a large sex difference in zebra finches (females, 139 ± 102 d.p.m./mg; males, 940 ± 283 d.p.m./mg). The second interaction was observed for 125I-VIP binding in the medial VMH (F4,1,29 = 2.717, p < 0.05). This interaction was complex and marginally significant, but was largely produced by a sex difference in the Angolan blue waxbill (females, 680 ± 109 d.p.m./mg; males, 221 ± 154 d.p.m./mg)
In general, binding sites for 125I-VIP and 125I-SG were widely distributed throughout the brain, particularly the forebrain. Representative series from a female zebra finch are shown for each of these two ligands in Figures 1 and 2, respectively. For the areas quantified and shown in Tables 1-2, 125I-VIP binding densities were highest in the ventral pallidum, hippocampus, taenial amygdala, medial nidopallium, dosomedial posterior nucleus of the thalamus, and the various zones and nuclei of the septal complex. 125I-SG binding densities were highest in the structures of the posterior septum (nucleus of the pallial commissure and commissural septal nucleus), anteromedial and ventromedial hypothalamic nuclei, nucleus accumbens, ventral pallidum, and medial nidopallium.
Table 2.
Specific 125I-SG binding (decompositions per minute/mg following substraction of nonspecific binding; means ± SEM) in forebrain areas of five songbird species that differ selectively in their species-typical group sizes1.
| Territorial | Gregarious | Highly Colonial | ||||
|---|---|---|---|---|---|---|
| Area2 | Melba Finch | Violet-Eared Waxbill | Angolan Blue Waxbill | Spice Finch | Zebra Finch | Significant Sociality Contrasts3 |
| Extended amygdala: | ||||||
| BSTm | 772 ± 147 | 598 ± 134 | 640 ± 89 | 516 ± 136 | 827 ± 130 | |
| BSTl | 550 ± 110a | 392 ± 108a | 535 ± 106a | 446 ± 101a | 1015 ± 189b* | |
| Septal Complex: | ||||||
| CoS | 1915 ± 464a | 1972 ± 227a | 2692 ± 355a | 559 ± 263b | 1826 ± 336a** | D** |
| nPC | 489 ± 273a | 317 ± 316a | 450 ± 174a | 2167 ± 385b | 4720 ± 497c*** | A***,B***,D*** |
| Preoptic area/hypothalamus: | ||||||
| POA | 499 ± 143 | 401 ± 192 | 627 ± 133 | 359 ± 168 | 894 ± 226 | |
| AH | 723 ± 148 | 811 ± 164 | 1072 ± 126 | 710 ± 140 | 688 ± 79 | D* |
| AM | 1297 ± 212 | 1127 ± 181 | 1703 ± 145 | 1446 ± 245 | 1670 ± 181 | A*,C* |
| Inf | 855 ± 214a | 674 ± 156a | 647 ± 107a | 2 ± 139b | 389 ± 194ab* | A*,B** |
| PLH | 1755 ± 340 | 1449 ± 200 | 2033 ± 298 | 1035 ± 174 | 1668 ± 228 | |
| PVN | 394 ± 136 | 474 ± 209 | 761 ± 66 | 332 ± 105 | 588 ± 133 | |
| VMH-med | 1300 ± 204ab | 732 ± 185a | 1684 ± 179b | 1554 ± 231b | 1719 ± 281b* | A**,B*,C* |
| VMH-lat | 1075 ± 183 | 534 ± 187 | 1106 ± 149 | 1380 ± 206 | 1303 ± 309 | A*,B* |
| Other forebrain areas: | ||||||
| Ac | 1697 ± 206 | 1580 ± 280 | 2161 ± 541 | 1724 ± 442 | 2285 ± 467 | |
| DMP | 856 ± 244abc | 1220 ± 146ac | 493 ± 135b | 467 ± 115b | 1004 ± 184c* | A*,C* |
| Hab | 556 ± 236a | 605 ± 165a | 460 ± 105a | 1351 ± 114b | 509 ± 163a** | |
| Hp, ventral | 1018 ± 212ac | 1700 ± 170bd | 1564 ± 175cd | 866 ± 248a | 1383 ± 233ad* | |
| N, medial | 3388 ± 419 | 4443 ± 363 | 4094 ± 270 | 3531 ± 313 | 3676 ± 295 | |
| NDB | 356 ± 92 | 469 ± 185 | 683 ± 117 | 373 ± 128 | 811 ± 206 | |
| VP | 1372 ± 279 | 1439 ± 171 | 1661 ± 321 | 1531 ± 359 | 2191 ± 225 | |
Different letters next to the means indicate significant species differences (Fisher's PLSD, p < .05) following significant ANOVA (* p < .05; ** p < .01; *** p < .001; sex × species; no main effects of sex were observed, thus male and female data are shown pooled). Four males and four females of each species were examined (total n = 40).
Data are not shown for target areas that exhibited no specific binding, including the LSc, LSr, MS, SH, and TnA. See Methods for abbreviations (“Nomenclature and abbreviations”).
Sociality contrasts were conducted by pooling subjects of a given grade of sociality (e.g., by pooling territorial or colonial species). Letters indicate main effects of sociality (* p < .05; ** p < .01; *** p < .001 in a two-way ANOVA including sex as a factor; see Results for sex effects). Contrast A: territorial versus flocking (“flocking” includes all three gregarious/colonial species). Contrast B: territorial versus colonial, excluding the intermediate Angolan blue waxbill. Contrast C: territorial versus moderately gregarious (Angolan blue waxbill). Contrast D: moderately gregarious versus colonial.
In contrast, binding sites for the linear 125I-V1a antagonist were remarkably limited. As shown in Figure 3, specific binding was detected in the septal nuclei, nidopallium, lateral striatum, entopallium and in the apical hyperpallium near the lateral margin of the hippocampus. Binding in areas outside of the septum was particularly pronounced in the spice finch (Fig. 3G) and virtually or completely absent in other species (e.g., Fig. 3F). A very low level of specific binding was also observed in the ventral pallidum (Table 3).
Sociality contrasts: Binding densities and their relation to species-typical group size
In a few cases, the pattern of species differences presented in Tables 1-3 show clear correspondence to species-typical group sizes, based on overall significant ANOVAs and significant pairwise comparisons. The best example of this is obtained for linear 125I-V1a antagonist binding in the pallial (dorsal) LSc, as shown in Figure 3. Other strong species differences are observed for 125I-SG binding in the nucleus of the pallial commissure (a component of the posterior septum; Table 2), and 125I-VIP binding in the subpallial LSc (Table 1). In each of these cases, the colonial species exhibit higher levels of binding than do the territorial species. With the exception of 125I-SG binding in the nucleus of the pallial commissure, the modestly gregarious Angolan blue waxbill exhibits binding similar to the two colonial species (this is true for binding in most areas of the brain).
However, while the ANOVAs and post-hoc pairwise comparisons are sufficient to demonstrate the presence of significant species differences, they do not in all cases allow for a clear assessment of whether species differences in binding reflect species differences in sociality. This is because one species may depart modestly from an overall trend. Thus, despite the fact that three or four pairwise species comparisons may fit the trend, it remains difficult to conclude that binding in that area does or does not reflect species differences in sociality. 125I-SG binding in the medial VMH provides a good example of this (Table 2): The territorial violet-eared waxbill exhibits significantly lower levels of binding than do the three more gregarious species, but the territorial melba finch exhibits an intermediate binding density and does not differ significantly from any other species.
Therefore, in order to provide a more useful metric for determining whether binding density reflects sociality, we have conducted a series of four sociality contrasts and presented the significant findings in Tables 1-3. Sociality contrasts were conducted by pooling subjects of a given sociality grade (e.g., by pooling territorial or colonial species) and then conducting a two-way ANOVA (sociality × sex). In Contrast A, we compared the territorial subjects to all flocking subjects (“flocking” includes the three gregarious/colonial species). In Contrast B, we compared territorial versus colonial subjects, excluding the intermediate Angolan blue waxbill. The remaining contrasts allowed us to determine whether the moderately gregarious Angolan blue waxbill differed significantly from the territorial subjects (Contrast C) and/or from the colonial subjects (Contrast D). Given that the Angolan blue waxbill is more closely related to the two territorial species than to the colonial species (and is also sympatric with the territorial species and a congener of one them), these last two contrasts provide important assessments of whether binding densities reflect divergent and convergent evolution in sociality.
A pattern of significance on Contrasts A, B and C – but not D, provides strong evidence that evolutionary convergence and divergence accompanies evolutionary convergence and divergence in flocking. Thus, the territorial subjects differ from subjects that flock (Contrast A), and from flocking birds that breed in large colonies (Contrast B), while the moderately gregarious species differs significantly from its closest relatives (the territorial subjects; Contrast C), but not from the colonial subjects (Contrast D). This pattern was observed for 125I-VIP binding in the LSr, subpallial LSc, medial septum, and septohippocampal septum (Table 1); for 125I-SG binding in the medial VMH (Table 2); and for linear 125I-V1a antagonist binding in the pallial LSc and medial septum (Table 3). In each of these cases, sociality relates to binding density in a positive manner. Representative photos for territorial and colonial species are shown in Figure 3 (linear 125I-V1a antagonist binding in the septum) and Figure 4 (125I-VIP binding in the septum and 125I-SG binding in the VMH).
While the ABC pattern will be observed when the intermediate species has fully converged with the colonial species, we can interpret significance on Contrasts A and B (but not C or D) as providing evidence that binding reflects gradations in sociality – that is, if the moderately gregarious is intermediate to, but not significantly different from, the territorial and colonial subjects. This pattern was observed for 125I-VIP binding in the BSTm (Table 1); for 125I-SG binding in the lateral VMH and infundibular hypothalamus (Table 2); and for linear 125I-V1a antagonist binding in the subpallial LSc (Table 3). With the exception of 125I-SG binding in the infundibular hypothalamus, these binding densities are positively related to sociality. Figure 4 shows representative 25I-SG binding in the infundibular hypothalamus for a female melba finch (territorial; Fig. 4C) and a female zebra finch (colonial; Fig. 4G).
These significant contrast patterns of ABC and AB account for 11 of the 28 cases where significant sociality contrasts were obtained. Of the remaining cases, 10 are consistent with an interpretation based on sociality, and only 7 cases do not conform to a pattern that could reflect sociality. Included in those that are consistent with an interpretation based on sociality are 125I-VIP binding in the anteromedial hypothalamus, medial VMH, dorsomedial posterior nucleus of the thalamus, and both dorsal and ventral hippocampus (Table 1); 125I-SG binding in the nucleus of the pallial commissure and anteromedial hypothalamus (Table 2); and linear 125I-V1a antagonist binding in the LSr, caudocentral septum, and medial nidopallium (Table 3). These binding densities are positively related to sociality, with the exception of 125I-VIP binding in the dorsomedial posterior nucleus of the thalamus, and dorsal and ventral hippocampus, where binding was negatively related to sociality. Representative 25I-SG binding in the nucleus of the pallial commissure is shown for a female melba finch and a female zebra finch in Figure 4D and 4H, respectively.
As stated in the previous section, no main effects of sex were detected in the species × sex ANOVAs. Similarly, the sociality comparisons yielded only limited evidence for sex differences. A main effect of sex on Contrast C was observed for 125I-SG binding in the nucleus accumbens (F1,1,20 = 6.306, p < 0.05; males, 1997 ± 223 d.p.m./mg; females 1614 ± 211 d.p.m./mg), and a main effect of sex on Contrast C was observed for linear 125I-V1a antagonist binding in the ventral pallidum (F1,1,20 = 5.501, p < 0.05; males, 366 ± 98 d.p.m./mg; females 540 ± 109 d.p.m./mg). Two sex × sociality interactions were observed for each of the ligands, although most were not strong (and were not sufficiently robust to be detected by the sex × species analyses) and were not found for areas in which significant sociality contrasts were obtained. These data are therefore not presented. Worth noting, however, is a significant interaction that was obtained on Contrast D for the medial VMH. This interaction was somewhat stronger than the others (F1,1,20 = 13.544, p < 0.01) and is consistent with the sex × species interaction for the medial VMH described in the previous section. Both the sex × species and sex × sociality interactions were produced primarily by sex differences in the Angolan blue waxbill (see previous section for relevant data).
Discussion
Using five monogamous species that differ selectively in their species-typical group sizes, we here show that binding sites for three neuropeptides have evolved in relation to sociality, most strongly within the septal nuclei, but also in the BSTm and mediobasal hypothalamic nuclei. The present experiments additionally offer novel insights into the distributions of neuropeptide binding sites in birds. We first discuss the general issues of binding distributions and ligand sensitivities, and then consider the structural and functional properties of neuropeptide systems as they relate to sociality.
Neuropeptide receptors in songbirds and other vertebrate groups
CRF receptor distributions have not been previously described for birds. The present findings for 125I-SG demonstrate that putative CRF receptors in songbirds are distributed in a pattern very similar to mammals (Primus et al., 1997; Sanchez et al., 1999; Van Pett et al., 2000; Lim et al., 2005), with 125I-SG binding sites being found throughout the pallium, striatum, extended amygdala, and various regions of the hypothalamus. However, in contrast to mammals, binding in the septal nuclei was largely restricted to the posterior septum (commissural septal nucleus and nucleus of the pallial commissure). Also unlike mammals, we found no evidence that 125I-SG in any brain area could be differentially eliminated by cold competition with SG versus antisauvagine-30. At the three concentrations employed here, these competitors were equally effective in reducing or eliminating 125I-SG binding throughout the brain. In mammals, only the CRF2 receptor binds antisauvagine-30 with a high affinity (Bale and Vale, 2004), thus the present results suggest that the pharmacologies of avian and mammalian CRF receptors may be substantially different. If so, there may also be meaningful differences in the CRF receptor functions of birds and mammals, given that the mammalian CRF1 and CRF2 receptors play complex and distinct functional roles (Risbrough et al., 2004).
The distributions of 3H-VP and 125I-VIP binding sites have both been previously described for birds, although some differences are observed across studies. Similar to pigeons and doves (non-songbirds) and mammals (Dietl et al., 1990; Hof et al., 1991; Askew et al., 1997; Joo et al., 2004) we here found that 125I-VIP binding sites in songbirds are widely distributed throughout the forebrain (pallium, subpallium and numerous diencephalic areas), with high levels of binding being observed for the septal nuclei. However, we did not observe widespread sex differences in 125I-VIP binding as described for ring doves (Streptopelia risoria; Askew et al., 1997).
We additionally found that binding sites for the linear 125I-V1a antagonist are more restricted in distribution than are binding sites for 3H-VP in canaries (Serinus canaria; Voorhuis et al., 1988). Thus, while robust linear 125I-V1a antagonist binding is observed in the septum and specific regions of the pallium, we did not obtain positive results for multiple other areas that bind 3H-VP in canaries, particularly the hypothalamus. Given that we observed large species differences in the present study, it may be that the differences between our present data and those for canaries reflect actual species differences. However, it is also possible that birds possess two or more VT receptors in the brain, with one type being more similar to the mammalian V1a receptor (which would be selectively targeted by the linear 125I-V1a antagonist). Finally, as in the estrildid finches examined here, robust binding for the linear 125I-V1a antagonist is typically observed in the mammalian LS, with large differences in density occurring across species (Insel, 1992; Insel et al., 1994; Wang et al., 1997; Young et al., 1997, 1999; Bester-Meredith et al., 1999).
Neuropeptide binding densities and evolution in species-typical group size
A variety of findings, including the present binding data, suggest that the septal complex is a major focal point in avian social evolution. Species differences in binding densities are observed in all eight of the septal areas that we examined (including all three ligands), and in 11 of the 12 cases where significant sociality contrasts were obtained, the differences in binding densities either show clear correspondence to sociality (8 cases), or are consistent with such an interpretation (3 cases). In each of these contrasts, binding density relates to sociality in a positive manner. Throughout the septal complex, linear 125I-V1a antagonist binding was higher in the subjects from species that flock versus those that are territorial, and binding showed particularly clear correspondence to evolutionary convergence and divergence in sociality within the dorsal (pallial) LSc. In contrast, species differences in 125I-VIP binding were observed in the subpallial nuclei of the septum, but not in the pallial LSc. For both of these ligands, the moderately gregarious Angolan blue waxbill exhibits binding densities that are similar to the two highly gregarious/colonial species (spice finch and zebra finch). However, the Angolan blue waxbill also exhibits 125I-SG binding in the posterior septum (nucleus of the pallial commissure) that is virtually identical to the two territorial species (melba finch and violet-eared waxbill), and strongly different from the two colonial species (the only case in the septum where this was observed). Hence, the moderately gregarious species exhibits a pattern of receptor distributions in the septal complex that is a mixture of territorial-like and colonial-like traits, but overall, results for this species strongly favor the colonial-like condition.
Similar to these findings in the septum, we found numerous other instances where the territorial species differed significantly from the more gregarious species (Contrasts A and/or B), and in most of these cases, binding densities for the Angolan blue waxbill were either comparable to the two colonial species, or were intermediate to the territorial and colonial species. Importantly, the gregarious Angolan blue waxbill is a congener of the territorial violet-eared waxbill and is sympatric with both of the territorial species. Thus, its convergence with the colonial species, and divergence from its closest relatives, provides compelling evidence that receptor systems have evolved in relation to sociality. A total of 11 such results were obtained, plus 10 more cases where the pattern of significant contrasts is fully consistent with an interpretation based on species-typical group size. In addition to the findings for the septum already summarized, good correspondence with sociality was observed for 125I-VIP binding in the BSTm, and for 125I-SG binding in the medial VMH, lateral VMH, and infundibular hypothalamus. In each of these cases, with the exception of 125I-SG binding in the infundibular hypothalamus, sociality relates to binding density in a positive manner.
The present binding data strongly corroborate immediate early gene data, which suggest that the functional properties of the LS, VMH and BSTm vary in relation to sociality. Using four of the species employed here (i.e., all but the melba finch), we recently found that exposure to a same-sex conspecific induces Fos and Egr-1 (Zenk) responses in numerous areas of the basal forebrain and midbrain, and a subset of those responses vary in relation to species-typical group size (Goodson et al., 2005a). Thus, in comparison to three gregarious species, the territorial violet-eared waxbill exhibits significantly greater responses within a discrete suite of forebrain areas – the ventrolateral LS, medial extended amygdala (nucleus taeniae and BSTm), AH and VMH. This suite of areas likely coordinates behavioral responses to aversive social stimuli (for discussion, see Goodson, 2005; Goodson et al., 2005b; also Sheehan et al., 2001). As in the present study, results for the modestly gregarious Angolan blue waxbill were virtually identical to the highly gregarious/colonial spice finch and zebra finch, with one exception – the medial extended amygdala, where an intermediate pattern of response was obtained (Goodson et al., 2005a).
What are the behavioral functions of VIP, VT and CRF, and how might their receptor densites influence sociality? To date, group-size preferences have not been directly examined following manipulations of these neuropeptide systems, although a variety of other findings are likely relevant. In mammals, CRF and VP modulate a wide variety of behavioral processes, including anxiety-like behavior and aggression (reviews: Goodson and Bass, 2001; Bale and Vale, 2004; Landgraf and Neumann, 2004; also see Farrokhi et al., 2004), and aggressive experience in turn influences VP immunoreactivity and receptor binding (Delville et al., 1998; Cooper et al., 2005). CRF and VP also facilitate pair-bonding in male prairie voles, and VP promotes general affiliation (DeVries et al., 2002; Wang and Aragona, 2004; Young and Wang, 2004). Although VP's effects on male bonding are most extensively established for the ventral pallidum (an area where we find no species differences in binding), VP also influences bonding via actions within the LS (Liu et al., 2001). To our knowledge, relevant VIP data are not available for mammals.
Perhaps the most relevant data from mammals show that V1a receptors in the LS are both necessary and sufficient for social recognition, and promote non-aggressive social interaction. Social recognition is severely impaired in V1a receptor knockout mice (Bielsky et al., 2004) and re-expression of V1a receptors in the LS by viral vector completely restores social memory (Bielsky et al., 2005). Similarly, viral vector-mediated over-expression of septal V1a receptors improves social recognition in rats (Landgraf et al., 2003), and also facilitates active social interactions. We hypothesize that V1a-like receptors perform similar functions in birds, given that gregarious estrildids exhibit significantly higher densities of V1a-like receptors in the septal nuclei than do less social, territorial species.
VT/VP afferents to the ventral pallidum and LS likely arise predominantly from the BSTm (De Vries and Buijs, 1983; De Vries and Panzica, 2006). Therefore, as suggested by site-specific VP manipulations within the ventral pallidum and LS, the BSTm neurons appear to be important mediators of affiliative behaviors such as bonding, non-sexual contact, and social recognition (reviews: Landgraf and Neumann, 2004; Wang and Aragona, 2004; Young and Wang, 2004). Similarly, in songbirds, intraseptal VT administrations facilitate aggression in the context of appetitive courtship behavior (i.e., during mate competition), but decrease resident-intruder aggression in a territorial context (review: Goodson, 2005). Both effects in birds can be interpreted as facilitating affiliation.
If VT/VP neurons in the BSTm and V1a-like receptors in their projection zones facilitate affiliative behaviors, then it follows that the BSTm VT/VP neurons should respond to social stimuli that elicit affiliative behaviors, but not to stimuli that do not elicit such behaviors. Recent data for the songbird species examined here show that this is indeed the case: VT-immunoreactive neurons in the BSTm exhibit a significant Fos response to positive social stimuli (i.e., to stimulus animals that the subject would normally affiliate with), but not to negative social stimuli (i.e., to aggressive subjugation, or in the case of territorial subjects, a same-sex conspecific). Furthermore, the control levels of Fos expression in these neurons differ significantly across species and relate positively to species-typical group size (J.L. Goodson, A.K. Evans and Y. Wang, ubpubl. obs.). To our knowledge, IEG responses of the BSTm VP neurons have not been examined in mammals, but VP mRNA significantly increases in the BSTm of male prairie voles following cohabitation with a female (Wang et al., 1994).
Consistent with these findings, V1a–like receptor densities in areas targeted by BSTm projections (e.g., ventral pallidum and LS) also reflect affiliative dimensions of behavior. As reviewed above, more V1a receptors are expressed within the ventral pallidum of monogamous mammals than in non-monogamous comparators (reviews: Wang and Aragona, 2004; Young and Wang, 2004), and we here demonstrate that evolution in species-typical group size shows close correspondence to linear 125I-V1a antagonist binding in the LS. Rodents also exhibit significant species differences in V1a receptor density within the LS, but to date, it has not been possible to link these differences to a particular dimension of behavior (for a discussion, see Goodson and Bass, 2001). However, the rodent species employed (which were selected based largely on their mating systems and patterns of parental care) do not show reliable and/or strong differences along the dimension of species-typical group size.
Finally, as with V1a receptor densities, numerous species differences have been observed for CRF receptor densities in voles (Lim et al., 2005), but these differences do not show clear correspondence to an identified dimension of behavior. We found that in songbirds, numerous species differences in 125I-SG binding reflect sociality, although these areas do not include areas that exhibit species differences in voles. To date, socially-relevent species comparisons have not been conducted for VIP (VPAC) receptors in mammals.
Conclusions
Using five species of estrildid songbirds that exhibit divergent and convergent evolution in sociality, we have shown here that putative receptor distributions for CRF, VIP and VT evolve in relation to species-typical group size. Species differences in binding are observed for a number of basal forebrain sites that participate in the control of social behavior, reproductive physiology, and stress responses. These include the LS, BSTm, and VMH -- areas that may act in concert to coordinate approach-avoidance behaviors and aversion processes (review: Goodson, 2005; also see Sheehan et al., 2001). Given that these brain areas are evolutionarily conserved in their functional and anatomical features, our findings may be relevant for a wide range of vertebrates.
Acknowledgments
We thank Ben Mines and Marcy Kingsbury for assistance with collections in Africa; Renier van Heerden, Ralph Correira and the Booyseens family for logistical support in Africa; and Larry Young and Sunny Boyd for technical advice. Support provided by National Institutes of Health grant R01 MH62656.
Footnotes
Note that variation in sociality could also arise conditionally (e.g., aggregation patterns could vary with fluctuations in resource distributions), without heritable modifications to brain mechanisms that regulate affiliation. Thus, in the present studies we have employed species that exhibit minimal conditional variation.
Sociality information for the 139 estrildids has been compiled into a table, available from J.L.G.
References
- Acharjee S, Do-Rego JL, Oh da Y, Ahn RS, Choe H, Vaudry H, Kim K, Seong JY, Kwon HB. Identification of amino acid residues that direct differential ligand selectivity of mammalian and nonmammalian V1a type receptors for arginine vasopressin and vasotocin. Insights into molecular coevolution of V1a type receptors and their ligands. J Biol Chem. 2004;279:54445–54453. doi: 10.1074/jbc.M408909200. [DOI] [PubMed] [Google Scholar]
- Askew JA, Buntin JD, Georgiou G, Sharp PJ, Lea RW. Analysis of central VIP binding sites in the breeding and non- breeding dove: Effect of intracerebroventricular anti-VIP serum and VIP antagonist upon incubation behaviour. Biogenic Amines. 1997;13:491–508. [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Ethological concepts revisited: Immediate early gene induction in response to sexual stimuli in birds. Brain Behav Evol. 2001;57:252–270. doi: 10.1159/000047244. [DOI] [PubMed] [Google Scholar]
- Barberis C, Balestre MN, Jard S, Tribollet E, Arsenijevic Y, Dreifuss JJ, Bankowski K, Manning M, Chan WY, Schlosser SS, Holsboer F, Elands J. Characterization of a novel, linear radioiodinated vasopressin antagonist - an excellent radioligand for vasopressin V-1a receptors. Neuroendocrinology. 1995;62:135–146. doi: 10.1159/000126998. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Owens IPF. Evolutionary ecology of birds: Life histories, mating systems, and extinction. Oxford University Press; New York: 2002. [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Castagna C, Absil P, Foidart A, Balthazart J. Systemic and intracerebroventricular injections of vasotocin inhibit appetitive and consummatory components of male sexual behavior in Japanese quail. Behav Neurosci. 1998;112:233–250. doi: 10.1037//0735-7044.112.1.233. [DOI] [PubMed] [Google Scholar]
- Christidis L. Biochemical systematics within paleotropic finches (Aves: Estrildidae) Auk. 1987a;104:380–392. [Google Scholar]
- Christidis L. Phylogeny and systematics of estrildine finches and their relationships to other seed-eating passerines. Emu. 1987b;87:119–123. [Google Scholar]
- Clements JF. Birds of the world: a checklist. 5th. Pica Press; Sussex: 2000. [Google Scholar]
- Clutton-Brock TH. Mammalian mating systems. Proc R Soc Lond B Biol Sci. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Elliott Albers H. Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm Behav. 2005;48:545–551. doi: 10.1016/j.yhbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Cornett LE, Kirby JD, Vizcarra JA, Ellison JC, Thrash J, Mayeux PR, Crew MD, Jones SM, Ali N, Baeyens DA. Molecular cloning and functional characterization of a vasotocin receptor subtype expressed in the pituitary gland of the domestic chicken (Gallus domesticus): avian homolog of the mammalian V1b-vasopressin receptor. Regul Pept. 2003;110:231–239. doi: 10.1016/s0167-0115(02)00216-1. [DOI] [PubMed] [Google Scholar]
- De Groef B, Goris N, Arckens L, Kuhn ER, Darras VM. Corticotropin-releasing hormone (CRH)-induced thyrotropin release is directly mediated through CRH receptor type 2 on thyrotropes. Endocrinology. 2003;144:5537–5544. doi: 10.1210/en.2003-0526. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanims, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour J. A revision of the subfamily Estrildinae of the family Ploceidae. Zoologica. 1943;28:69–86. [Google Scholar]
- Delville Y, Melloni RH, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J Neurosci. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Dietl MM, Hof PR, Martin JL, Magistretti PJ, Palacios JM. Autoradiographic analysis of the distribution of vasoactive intestinal peptide binding sites in the vertebrate central nervous system a phylogenetic study. Brain Res. 1990;520:14–26. doi: 10.1016/0006-8993(90)91687-c. [DOI] [PubMed] [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77:465–469. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004a;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004b;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci. 2005a;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005b;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: Insights from studies on birds. Horm Behav. 2005c;48:461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Estrildid finches of the world. Cornell University Press; Ithaca, NY: 1982. [Google Scholar]
- Harrison CJO. An ethological comparison of some waxbills (Estrildini) and its relevance to their taxonomy. Proc Zool Soc Lond. 1962;139:261–282. [Google Scholar]
- Hof PR, Dietl MM, Charna YY, Martin JL, Bouras C, Palacios JM, Magistretti PJ. Vasoactive-intestinal-peptide binding-sites and fibers in the brain of the pigeon Columba-livia - an autoradiographic and immunohistochemical study. J Comp Neurol. 1991;305:393–411. doi: 10.1002/cne.903050304. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Australian finches in bush and aviary. Angus and Robertson; Sydney and London: 1965. [Google Scholar]
- Insel TR. Oxytocin - a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Ohkubo T, Saito N, Suzuki T, Matsuda Y, Zadworny D. Molecular cloning of chicken vasoactive intestinal polypeptide receptor complementary DNA, tissue distribution and chromosomal localization. Biol Reprod. 2001;64:1575–1581. doi: 10.1095/biolreprod64.5.1575. [DOI] [PubMed] [Google Scholar]
- King JA, editor. Biology of Peromyscus (Rodentia) American Society of Mammologists; Stillwater, MN: 1968. [Google Scholar]
- Konishi M, Emlen ST, Ricklefs RE, Wingfield JC. Contributions of bird studies to biology. Science. 1989;246:465–472. doi: 10.1126/science.2683069. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Comp Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- Maney DL, Wingfield JC. Neuroendocrine suppression of female courtship in a wild passerine: corticotropin-releasing factor and endogenous opioids. J Neuroendocrinol. 1998;10:593–599. doi: 10.1046/j.1365-2826.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Wingfield JC. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol. 1997;9:487–491. doi: 10.1046/j.1365-2826.1997.00635.x. [DOI] [PubMed] [Google Scholar]
- Mayr E. The sequence of genera in the Estrildidae (Aves) Breviora. 1968;287:1–14. [Google Scholar]
- Morris D. The comparative ethology of Grassfinches (Erythrurae) and Mannikins (Amadinae) Proc Zool Sci Lond. 1958;131:389–439. [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, Guturkun O. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sheehan TP, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Skead DM. Ecological studies of four Estrildines in the central Transvaal. Ostrich. 1975 11:1–55. [Google Scholar]
- Sorenson MD, Balakrishnan CN, Payne RB. Clade-limited colonization in brood parasitic finches (Vidua spp.) System Biol. 2004;53:140–153. doi: 10.1080/10635150490265021. [DOI] [PubMed] [Google Scholar]
- Tamarin RH, editor. Biology of new world Microtus. American Society of Mammologists; Stillwater, OK: 1985. [Google Scholar]
- Tan F, Lolait SJ, Brownstein MJ, Saito N, MacLeod V, Baeyens DA, Mayeux PR, Jones SM, Cornett LE. Molecular cloning and functional characterization of a vasotocin receptor subtype that is expressed in the shell gland and brain of the domestic chicken. Biol Reprod. 2000;62:8–15. doi: 10.1095/biolreprod62.1.8. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Voorhuis TAM, De Kloet ER, De Wied D. The distribution and plasticity of tritiated vasopressin-labelled specific binding sites in the canary brain. Brain Res. 1988;457:148–153. doi: 10.1016/0006-8993(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): Studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997;768:147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- You S, Hsu CC, Kim H, Kho Y, Choi YJ, El Halawani ME, Farris J, Foster DN. Molecular cloning and expression analysis of the turkey vasoactive intestinal peptide receptor. Gen Comp Endocrinol. 2001;124:53–65. doi: 10.1006/gcen.2001.7685. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Yu J, Xie LY, Abou-Samra AB. Molecular cloning of a type A chicken corticotropin-releasing factor receptor with high affinity for urotensin I. Endocrinology. 1996;137:192–197. doi: 10.1210/endo.137.1.8536612. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]
- Zawilska JB, Niewiadomski P, Nowak JZ. PAC1 receptors in chick cerebral cortex: characterization by binding of pituitary adenylate cyclase-activating polypeptide, [125I]-PACAP27. Neurosci Lett. 2003;338:155–158. doi: 10.1016/s0304-3940(02)01397-6. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Niewiadomski P, Nowak JZ. Receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in turkey cerebral cortex: characterization by [125I]-VIP binding and effects on cyclic AMP synthesis. Gen Comp Endocrinol. 2004;137:187–195. doi: 10.1016/j.ygcen.2004.03.007. [DOI] [PubMed] [Google Scholar]


