Abstract
Based on a wide variety of data, it is now clear that the brains of birds and teleost (bony) fish possess a core “social behavior network” within the basal forebrain and midbrain that is homologous to the social behavior network of mammals. The nodes of this network are reciprocally connected, contain receptors for sex steroid hormones, and are involved in multiple forms of social behavior. Other hodological features and neuropeptide distributions are likewise very similar across taxa. This evolutionary conservation represents a boon for experiments on phenotypic behavioral variation, as the extraordinary social diversity of teleost fish and songbirds can now be used to generate broadly relevant insights into issues of brain function that are not particularly tractable in other vertebrate groups. Two such lines of research are presented here, each of which addresses functional variation within the network as it relates to divergent patterns of social behavior. In the first set of experiments, we have used a sexually polymorphic fish to demonstrate that natural selection can operate independently on hypothalamic neuroendocrine functions that are relevant for 1) gonadal regulation and 2) sex-typical behavioral modulation. In the second set of experiments, we have exploited the diversity of avian social organizations and ecologies to isolate species-typical group size as a quasi-independent variable. These experiments have shown that specific areas and peptidergic components of the social behavior network possess functional properties that evolve in parallel with divergence and convergence in sociality.
Keywords: sociality, aggression, sexual behavior, communication, vocalization, arginine vasopressin, arginine vasotocin, isotocin, mesotocin, oxytocin, fish, bird, bed nucleus of the stria terminalis, amygdala, lateral septum, anterior hypothalamus, ventromedial hypothalamus, preoptic area, periaqueductal gray, nucleus intercollicularis, c-fos, egr-1, Zenk
The research program described below is designed to address two major goals. The first of these goals is to elucidate evolutionary themes in the neuroendocrine, functional, and connectional organization of brain circuits that regulate social behavior. This will provide a phylogenetically broad framework for examining the neural and neuroendocrine mechanisms of behavior, and will allow detailed and meaningful comparisons to be made across the major vertebrate classes. Building upon this foundation, the second primary goal is to capitalize on the extraordinary behavioral diversity of birds and teleost (bony) fish to elucidate the ways that neural and neuroendocrine mechanisms are adjusted over evolutionary time to produce intraspecific and interspecific variation in behavior. If conducted within a well-characterized, comparative framework, these findings obtained in birds and fish should yield solid predictions for other vertebrates as well. Thus, our work addresses evolutionary themes, as described in the first section below, and variations on those themes, as presented in the second section.
Evolutionary Themes and the Concept of a Vertebrate Social Behavior Network
As originally suggested for mammals (Newman, 1999), the brain's social behavior network is comprised of six nodes – the extended medial amygdala (i.e., the medial amygdala and the medial bed nucleus of stria terminalis, BSTm), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH) and the midbrain (Fig. 1 and Table 1). The relevant midbrain areas include the periaqueductal gray (PAG) and various areas of the tegmentum that link forebrain regions with motoneuron pools of the hindbrain. The other five nodes lie within the basal (“limbic”) forebrain. Newman (1999) proposed that these areas comprise a social behavior network based upon a few important criteria. First, each of the nodes has been implicated in the control of multiple forms of social behavior. These include aggression, appetitive and consummatory sexual behavior, various forms of communication, social recognition, affiliation, bonding, parental behavior and responses to social stressors (Kirkpatrick et al., 1994; Kollack-Walker and Newman, 1995; Bamshad and Albers, 1996; Coolen et al., 1997; Kollack-Walker et al., 1997; Wang et al., 1997; Lonstein et al., 1998; Morgan et al., 1999; Delville et al., 2000; Kalinichev et al., 2000; Gammie and Nelson, 2001; Heeb and Yahr, 2001; Sheehan et al., 2001; Ferguson et al., 2002; Cushing et al., 2003; Lim and Young, 2004). The nodes are also bidirectionally connected (Risold and Swanson, 1997b; Coolen et al., 1998; Coolen and Wood, 1998; Dong and Swanson, 2004), and each area contains sex steroid receptors that are essential for the sexual differentiation and temporal coordination of social behavior (Morrell and Pfaff, 1978; Commins and Yahr, 1985; Simerly et al., 1990; Wood and Newman, 1995). The mammalian brain obviously contains a large number of other areas that are relevant for social behavior (e.g., other basal forebrain areas that regulate stress and reward processes, and cortical areas that serve executive functions); thus Newman's network should be regarded as the “core” of the social brain, not the social brain in toto.
Figure 1.
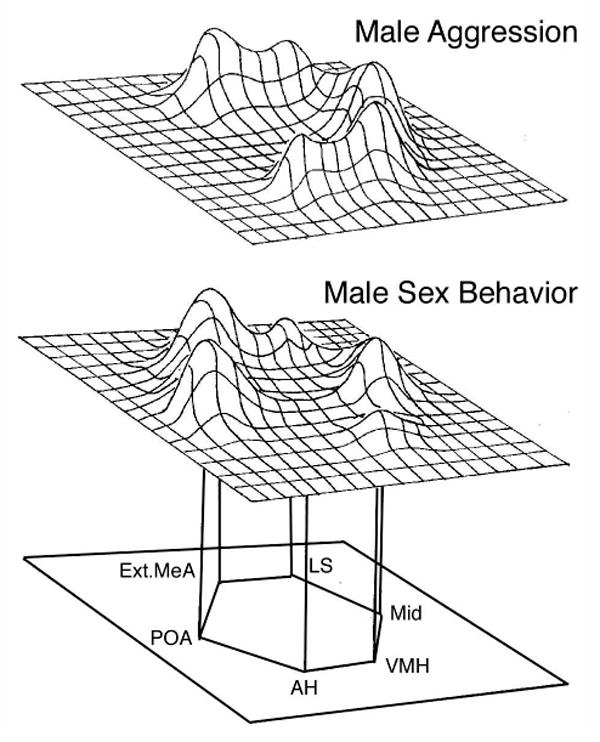
The social behavior network as originally suggested for mammals (schematics modified from Newman, 1999). The network is comprised of six nodes – the extended medial amygdala (i.e., the medial amygdala and the medial bed nucleus of stria terminalis, BSTm), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH) and various areas of the midbrain, including the periaqueductal gray. Each of the nodes binds sex steroid hormones and has been implicated in the control of multiple forms of social behavior. Newman (1999) proposes that this network does not contain segregated, linear systems for each kind of behavior. Rather, as shown in these schematic representations of immediate early gene data, each behavioral context is associated with a distinct pattern of activation across the nodes.
Table 1.
Components of the social behavior network as described for mammals (Newman, 1999) and proposed homologues in other vertebrate classes. Classes Chondrichthyes (cartilaginous fish) and Agnatha (jawless vertebrates) are excluded due to insufficient data.
| Class Mammalia | Class Aves | Class Reptilia | Class Amphibia | Class Osteichthyes (bony fish) |
|---|---|---|---|---|
| Extended medial amygdala: medial amygdala (MeA) and medial bed nucleus of the stria terminalis (BSTm) | nucleus taeniae and BSTm | MeA1 and BST2 | MeA (formerly the amygdala pars lateralis) and BST2 | supracommissural nucleus of the ventral telencephalon (amygdala homologue; subdivisions not known) |
| preoptic area (POA) | POA | POA | POA | POA3 |
| anterior hypothalamus (AH)4 | AH4 | AH4 | AH4 | AH (includes ventral tuberal region)4 |
| lateral septum (LS)5 | LS5 | LS5 | LS and dorsal septum | ventral nucleus of the ventral telencephalon (septum homologue; subdivisions not known) |
| ventromedial hypothalamus (VMH) | VMH | VMH | VMH | anterior tuberal nucleus (in part) 6 |
| Midbrain: periaqueductal or central gray (PAG/CG) 7 | CG (substantia grisea centralis) | PAG/CG | ? | PAG/CG |
The nucleus sphericus of squamate reptiles has also been compared to the mammalian MeA based upon vomeronasal inputs, but this structure is most likely homologous to the mammalian posteromedial cortical nucleus of the amygdala (Lanuza and Halpern, 1997).
The BST of reptiles and amphibians is not differentiated into medial and lateral subdivisions, but a portion of the nucleus contains AVT neurons (Stoll and Voorn, 1985; Smeets et al., 1990; Lowry et al., 1997; Marin et al., 1998), as found for the medial BST of most mammals and birds (De Vries et al., 1985; Caffe et al., 1989; Aste et al., 1998).
The POA of fish incorporates peptidergic neuron groups that are homologous to anterior hypothalamic populations in tetrapods (supraoptic and paraventricular nuclei) (Moore and Lowry, 1998).
This large area represents a transition zone between the POA and posterior hypothalamic zones, and contains multiple subdivisions in each class. Insufficient data are available to allow direct comparisons of these subdivisions.
Detailed comparisons of subdivisions can be made between rodents, songbirds and lizards (Font et al., 1995; Risold and Swanson, 1997b; Risold and Swanson, 1997a; Font et al., 1998; Goodson et al., 2004a).
As recently advanced by Forlano et al. (2005), this homology is suggested by connectivity, position and the distribution of steroid hormone receptors. However, sex steroid receptors are most dense in hypothalamic areas that are caudolaterally adjacent to the anterior tuberal nucleus (Fine et al., 1990, 1996; Forlano et al., 2005). This topography is similar to that observed in tetrapods for the VMH core and shell (or medial and lateral VMH, respectively), with most sex steroid receptors being found in the shell (e.g., Wood and Newman, 1995); thus much of the anterior tuberal nucleus may be comparable to the VMH core.
The midbrain also includes a variety of tegmental sites that are important for social behavior. These include a vocally-active tegmental field that lies lateral to the PAG and medial to the auditory torus/inferior colliculus (Phillips et al., 1972; Kennedy, 1975; Jürgens, 1994; Goodson and Bass, 2002) in all classes except Amphibia, in which a comparable vocal site is located at isthmal levels (Wetzel et al., 1985).
Other selected references: Northcutt, 1981; Bruce and Neary, 1995; Balthazart et al., 1996; Panzica et al., 1996; Lanuza et al., 1997; Wong, 1997; Coolen et al., 1998; Coolen and Wood, 1998; Lanuza and Halpern, 1998; Nieuwenhuys et al., 1998; Cheng et al., 1999; Goodson and Bass, 2000c; Dong et al., 2001; Petrovich et al., 2001; Goodson et al., 2003; Moreno and Gonzalez, 2003; Dong and Swanson, 2004; Goodson et al., 2004a; Rink and Wullimann, 2004; Wullimann and Mueller, 2004.
Newman (1999) proposes that this network of brain areas does not contain segregated, linear systems for the regulation of each kind of social behavior. Rather, each node of the network responds to a variety of stimuli, with each social context and behavioral response being associated with a distinct pattern of response across the nodes. For instance, some of the same areas show increases in immediate early gene (IEG) activity during male sexual behavior, female sexual behavior and male aggression, but the overall pattern is distinct for each behavioral context (Fig. 1). Although this model is in some ways simplistic (e.g., each area may have distinct neuronal populations with different response profiles), the idea is nonetheless compelling and supported by a good body of data (see references above).
Increasing evidence suggests that this network is present in all vertebrates. While most relevant findings have come from birds, consistent data are also available for amphibians, fish and reptiles (see Table 1 and the following two sections), and Newman's model has been explicitly applied as a conceptual framework for data in geckos (Eublepharis macularius) (Crews, 2003). Interestingly, data from geckos demonstrate that behavioral variations are correlated with distinct patterns of functional connectivity within the network (Sakata et al., 2000; Sakata and Crews, 2004), a finding that lends good empirical support to Newman's (1999) ideas about the significance of distributed activation patterns.
Our own research expands upon these studies in two important respects: First, our work on the vocal circuitry of the plainfin midshipman fish (Porichthys notatus) provides the first comprehensive mapping of the network's connections in a non-mammal, and has yielded strong evidence that the social behavior network arose in the earliest vertebrates (Goodson and Bass, 2002). As detailed below, the vocal system of the midshipman offers opportunities for systems-level experiments on the network that are not possible in most other animals (i.e., the whole brain can be exposed during analyses of social behavior patterning); thus understanding the comparative organization of the relevant circuitry in fish is particularly valuable. Secondly, our anatomical and behavioral experiments in both fish and birds demonstrate that many functional, structural and neuroendocrine features are exceptionally similar to those in mammals, thereby rendering detailed comparisons across classes much more feasible than we initially anticipated.
The social behavior network in a vocalizing fish
One difficulty of studying behavioral systems in most vertebrates is that naturalistic social behavior cannot be observed in a neurophysiological preparation that allows access to large amounts of the brain. However, the plainfin midshipman offers such an opportunity. These fish produce social vocalizations that are structurally simple (rhythmic), and the fundamental frequency and duration of actual vocalization relate in a one-to-one manner with the activity of the sonic motor nucleus in the medulla. The sonic motor neurons fire synchronously; thus their output (“fictive vocalizations”) can be neurophysiologically recorded to provide a very accurate depiction of patterned social “behavior” in an acute preparation (Fig. 2A; Bass and Baker, 1990; also see Goodson and Bass, 2000b). The vocal-motor system can be driven by low-amplitude electrical stimulation in many areas of the brain (Goodson and Bass, 2000a,b,c, 2002), and therefore offers the opportunity to study socially-relevant brain circuits at a level of detail that is not possible in most other animals (but see Kelley, 2004 for a comparable approach in frogs).
Figure 2.
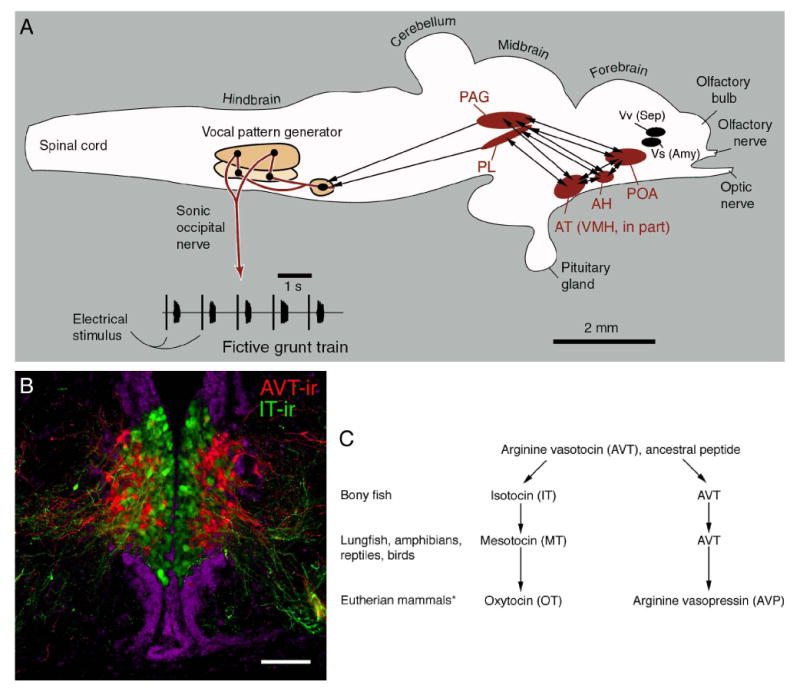
Connectional, functional and histochemical features of the social behavior network as exemplified in a vocalizing fish, the plainfin midshipman (Porichthys notatus). A. A schematic sagittal view of the midshipman brain. Electrical stimulation of numerous brain sites activates the hindbrain vocal pattern generator and elicits fictive vocal-motor output that precisely mimics natural vocalizations (shown here are fictive agonistic grunts). Tract tracings of vocally-active sites demonstrate that reciprocal connections exist between four areas (shown in red) that are homologous to nodes of the mammalian social behavior network (see Table 1). These areas are also reciprocally connected with nuclei of the basal telencephalon that are homologous to the septum and amygdala (shown in black; for clarity, connections are not shown). As with the six nodes of the mammalian social behavior network, these six areas express sex steroid receptors (e.g., Forlano et al., 2005). B. Neurons and fibers immunoreactive for arginine vasotocin (red) and isotocin (green) in the parvocellular preoptic area of the midshipman fish (DAPI counterstain is pseudocolored purple). These neurons are the evolutionary precursors of peptidergic neurons in the paraventricular hypothalamus of tetrapods (see panel C for evolution of the vasotocin-like peptide forms; schematic after Acher, 1972). In all vertebrates, these neurons project to components of the social behavior network, and in the midshipman, these projection systems modulate vocal-motor activity in a sex- and morph-specific manner (see text section on “Blending the sexes”). Scale bar in B =200 μm (modified from Goodson et al., 2003). Other abbreviations: AH, anterior hypothalamus; AT, anterior tuberal nucleus of the hypothalamus; PAG, periaqueductal gray; PL, paralemniscal tegmentum; POA, preoptic area; VMH, ventromedial hypothalamus; Vs, supracommissural nucleus of the ventral telencephalon; Vv, ventral nucleus of the ventral telencephalon.
In order to determine the afferent and efferent connections of vocally-active brain loci, we injected biotin compound tract tracers into neurophysiologically-identified sites, which were found distributed throughout the basal forebrain, midbrain tegmentum and hindbrain (Goodson and Bass, 2002). Other tracings were conducted concurrently on the ascending auditory circuitry (Bass et al., 2000; Goodson and Bass, 2002), and we confirmed the general pattern found for midshipman in a related vocal teleost, the gulf toadfish (Opsanus beta) (Goodson and Bass, 2002). Once completed, our mapping of vocal-acoustic circuitry in the midshipman and toadfish had outlined a network that is strikingly comparable to the social behavior network of mammals (Fig. 2A).
Forebrain vocal-acoustic sites were found in areas that are homologous to three of Newman's (1999) nodes: the AH, anterior tuberal nucleus (VMH homologue; see Table 1 and Forlano et al., 2005) and POA. Midbrain sites were located within the PAG and adjacent tegmentum (also corresponding to a node in Newman's network). As in the mammalian social behavior network, all of these forebrain and midbrain areas are reciprocally connected, and all are interconnected with basal forebrain areas that are homologous to the septum and amygdala (Northcutt, 1981). In addition, each of these six areas contains sex steroid receptors (Fine et al., 1990, 1996; Forlano et al., 2005). The topography of these sites is also generally comparable to that in other vertebrates. In fact, the midbrain topography is virtually identical to the topography of vocal sites in mammals (e.g., squirrel monkeys) (Jürgens, 1994), with vocally-active sites being found in the portion of the PAG that lies adjacent to the auditory torus (homologue of the inferior colliculus), and in a tegmental band that extends along the medial edge of the torus and lateral lemniscus (paratoral tegmentum and paralemniscal tegmentum). Finally, fish exhibit neuropeptide distributions throughout these areas that are highly similar to neuropeptide distributions in other vertebrate groups (e.g., Batten et al., 1990; Goodson and Bass, 2000c; Goodson et al., 2003; see Fig. 2B-C)
The social behavior network in birds
A wide variety of data have been collected on the neuroendocrine systems of birds, and the avian literature is growing rapidly (Goodson et al., submitted-b). On the whole, the social behavior network in birds exhibits extensive similarities with the mammalian network in its connections, histochemistry, and locations of sex steroid receptors (e.g., Watson and Adkins-Regan, 1989; Balthazart et al., 1994; Balthazart et al., 1996; Briganti et al., 1996; Panzica et al., 1996; Aste et al., 1998; Cheng et al., 1999; Atoji and Wild, 2004; Goodson et al., 2004a; Montagnese et al., 2004). The pattern of IEG induction that is observed in birds following agonistic interactions is also comparable to that observed in mammals, as are IEG responses to appetitive and consummatory sexual interactions (Kollack-Walker and Newman, 1995; Coolen et al., 1996, 1997; Kollack-Walker et al., 1997; Meddle et al., 1997, 1999; Delville et al., 2000; Tlemcani et al., 2000; Maney and Ball, 2003; Davis and Marler, 2004; Goodson and Evans, 2004; Riters et al., 2004; Goodson et al., submitted-a),. These similarities in birds and mammals include sex differences in the response to copulation; thus the POA is more responsive in males whereas the VMH is more responsive in females. Finally, the IEG findings are well corroborated by lesion data on the neural substrates of appetitive and consummatory sexual behavior, as well as aggression (Kondo et al., 1990; Liu et al., 1997; Balthazart et al., 1998; Thompson et al., 1998; Goodson et al., 1999; Riters and Ball, 1999; Absil et al., 2002).
One node of the network that we have focused on extensively is the LS. Septal lesions and neuropeptide infusions influence aggression and social communication in a variety of avian and mammalian species (Fig. 4; Clarke and File, 1982; Ferris et al., 1990; Koolhaas et al., 1990; Goodson, 1998b; Goodson, 1998a; Goodson and Adkins-Regan, 1999; Goodson et al., 1999), and in birds, the effects of these manipulations vary in relation to species-typical social structure (see second subsection below). The LS receives the heaviest hippocampal input of any structure in the brain, and in both birds and mammals, this projection is topographically organized (Risold and Swanson, 1997b; Atoji and Wild, 2004). Hence, it is not surprising that the effects of septal manipulations are complex and context-dependent (e.g., Goodson, 1998a; Goodson et al., 1999). The organization of the LS is likewise daunting in its complexity, with 20 chemoarchitectonic zones having been described for rats (Risold and Swanson, 1997a). However, this architecture is remarkably conserved: By using antibodies against ten neuropeptides and enzymes, we have recently identified virtually all of these zones (or their conglomerates) in songbirds (Goodson et al., 2004a; see Fig. 3).
Figure 4.
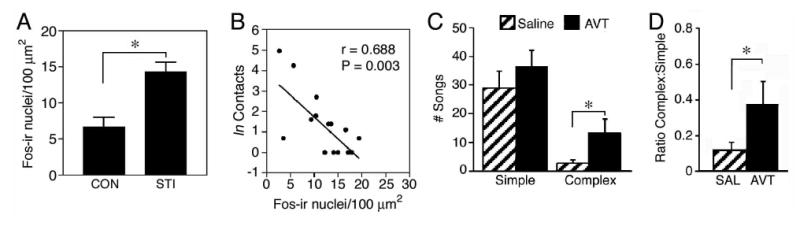
Functional themes of the lateral septum (LS) as exemplified by findings in wild-caught, territorial male song sparrows (Melospiza melodia; A-B) and field sparrows (Spizella pusilla; C-D). Similar to rodents, Fos-immunoreactive (-ir) cell counts in the ventrolateral LS are increased by an agonistic encounter (A; simulated territorial intrusion; STI), but the most aggressive animals show virtually no Fos induction (B; cf. Kollack-Walker and Newman, 1997). The aggression measure shown in B is based on contacts with the wire barrier that separated the subject from the intruder (data in A and B are from Goodson et al., submitted-a). Also similar to rodents, septal infusions of arginine vasotocin (AVT; homologue of arginine vasopressin) facilitate agonistic communication (C-D; cf. Irvin et al., 1990). Data shown in C-D are from field sparrows housed on semi-natural territories (field-based flight cages placed in natural habitat; modified from Goodson, 1998a). C. The number of simple and complex songs given spontaneously during the dawn singing period; these song types are multipurpose and strictly agonistic, respectively (see Nelson and Croner, 1991). D. The ratio of complex to simple songs, showing an increase in the agonistic content of song following intraseptal infusion of 100 ng AVT. *p<0.05, unpaired t-test (A) or Wilcoxon signed ranks (C-D).
Figure 3.
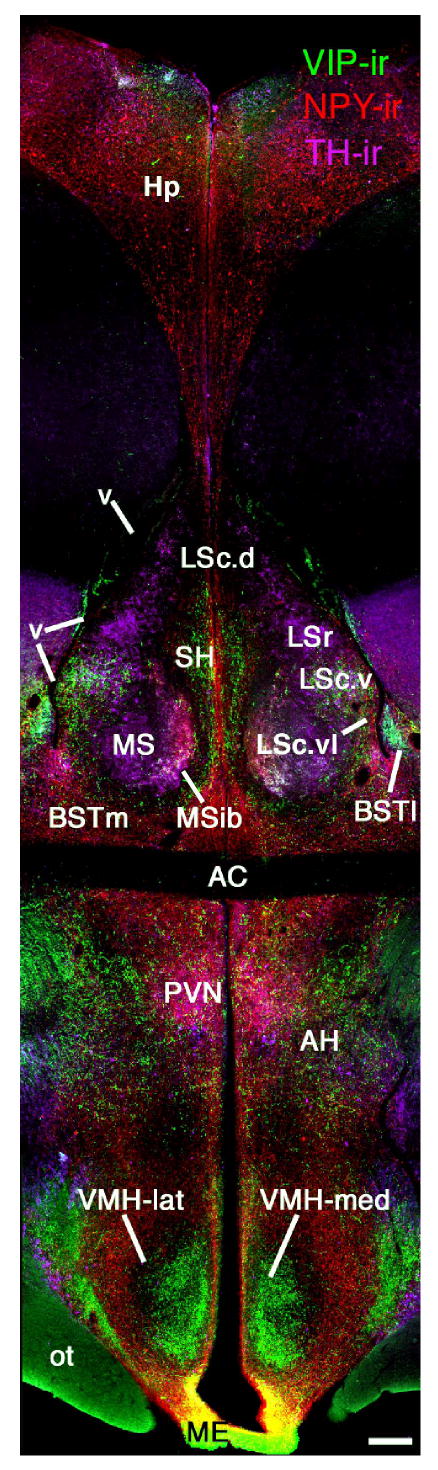
Chemoarchictural themes of the social behavior network as exemplified in songbirds (photomontage of a female zebra finch brain at the level of the anterior commissure; AC). Immunohistochemical triple-labelling for vasoactive intestinal polypeptide (VIP; Alexa Fluor 488, green), neuropeptide Y (NPY; Alexa Fluor 594, red) and tyrosine hydroxylase (TH; Alexa Fluor 350, pseudocolored purple) shows the location of multiple zones of the lateral septum (LS) and bed nucleus of the stria terminalis (BST) that are now known to be extensively comparable to mammals (see Aste et al., 1998; Goodson et al., 2004a; Richard et al., 2004). This labeling also clearly shows the medial and lateral divisions of the ventromedial hypothalamus (i.e., VMH core and shell, respectively), which exhibit a similar topography across the vertebrate taxa (see Table 1). Scale bar = 200 μm. Modified from Goodson et al., 2004a. Other abbreviations: AH, anterior hypothalamus; BSTm, medial bed nucleus of the stria terminalis; BSTl, lateral bed nucleus of the stria terminalis; Hp, hippocampus; LSc, caudal division of the lateral septum (dorsal, ventrolateral, lateral and ventral zones denoted as LSc.d, LSc.vl, LSc.l and LSc.v, respectively); LSr, rostral division of the lateral septum; ME, median eminence; MS, medial septum; MSib, internal band of the medial septum; ot, optic tract; OM, occipitomesencephalic tract; PVN, paraventricular nucleus of the hypothalamus; SH, septohippocampal septum; v, lateral ventricle.
These anatomical studies have provided a detailed framework for analyzing IEG data, and we have therefore produced data on LS function that are on a relatively fine spatial scale (Goodson and Evans, 2004; Goodson et al., 2005; Goodson et al., submitted-a). Recent IEG studies show that the ventrolateral zones of the caudal LS are the most responsive to social stimuli, a finding that matches well to data from mammals. Interestingly, LS responses to agonistic encounters are greater in subordinant male hamsters (Mesocricetus auratus) than in dominant animals (Kollack-Walker et al., 1997), and we have likewise found that IEG responses of the LS are negatively correlated with aggressive behavior in male song sparrows (Melospiza melodia; Fig. 4A-B). In both hamsters and sparrows, this effect is particularly pronounced in the ventrolateral LS.
Variations on the Themes: Evolutionary Modifications Associated with Phenotypic Diversity
While the network described above appears to be fundamental to the expression of social behavior in all vertebrate species, it must also express functionally labile features that underlie phenotypic variation in behavior. As described below, we have identified several network features that provide some of this functional diversity. For instance, we have demonstrated that various neuropeptides exhibit morph- and species-specific effects on behavior (Goodson, 1998a,b; Goodson and Adkins-Regan, 1999; Goodson and Bass, 2000a), and have additionally shown that network responses to social stimuli are divergently patterned in songbird species that differ selectively in sociality (Goodson et al., 2005). Ongoing work suggests that these species-specific network responses are related to divergence in peptidergic neuron activity, and species differences in neuropeptide receptor distributions. That is, peptidergic variables may coordinate the species differences in network response.
A number of these studies have focused on the vasotocin family of neuropeptides, which includes arginine vasotocin (AVT; found in non-mammals), its mammalian homologue arginine vasopressin (AVP), and the oxytocin-like peptides (isotocin, found in fish; mesotocin, found in lungfish and non-eutherian tetrapods; and oxytocin, found in eutherian mammals) (Acher, 1972; see Fig. 2B-C). More so than any other class of neurochemicals, these peptides have been found to be axes of behavioral plasticity and social diversity, and the features of these systems are associated with seasonal variation, sex differences, and species divergence in behavior (reviews: De Vries and Boyle, 1998; Foran and Bass, 1999; Insel and Young, 2000; Goodson and Bass, 2001; Panzica et al., 2001; Keverne and Curley, 2004; Lim et al., 2004a). Despite these aspects of variation, the locations of AVT/AVP neurons and fibers have been strongly conserved during vertebrate evolution (Moore and Lowry, 1998; Goodson and Bass, 2001), and the AVT/AVP system is an integral component of the social behavior network in all vertebrate groups.
Blending the sexes: Peptidergic neuromodulation in a sexually polymorphic fish
Given that 1) the plainfin midshipman exhibits strong sex and morph differences in the contextual use of vocalizations (Brantley and Bass, 1994), 2) peptides of the vasotocin family influence communication behaviors across a wide range of vertebrate taxa (Goodson and Bass, 2001), and 3) peptides of the vasotocin family occasionally exhibit sex-specific effects (see below), we conducted a series of neurophysiological experiments in the midshipman to determine the neuromodulatory effects of isotocin and AVT on fictive social vocalizations (Goodson and Bass, 2000a). We hypothesized that neuropeptide manipulations would produce sex-specific effects, given that AVT and AVP are more strongly implicated in male-typical behaviors (although this is not ubiquitous), while oxytocin is more strongly associated with a female-typical functions (Bluthe and Dantzer, 1990; Insel and Hulihan, 1995; Carter and Altemus, 1997; Francis et al., 2002; Goodson et al., 2004b; Keverne and Curley, 2004).
The plainfin midshipman exhibits three reproductive phenotypes (type I males, type II males and females) and this species therefore offers a relatively novel system for the examination of sex-specific peptide functions. Type I males excavate nests in the intertidal zone and vocally court females with a long-duration hum that can last up to two hours. These hums are exhibited solely by the type I males, as are territorial growls. Given that females visit nests only to spawn, the type I males are also the sole providers of parental care. Type II males are morphologically feminized and likewise visit nests only to spawn (by sneak spawning or satellite spawning). However, while the type II males are feminized in their appearance and much of their behavior, they are gonadally hypermasculinized and possess gonad:body size ratios that are ∼9 times that of the type I males (Brantley and Bass, 1994). All three of the morphs exhibit agonistic grunts -- short-duration calls (∼50 ms) that are distinguished from hums primarily by their duration and contextual use. Grunts are the only vocalization exhibited by females and type II males (for a review of midshipman behavioral biology, see Bass, 1996).
We found that fictive grunting elicited by stimulation of the AH in female midshipman is 1) inhibited by local delivery of isotocin in a dose-dependent manner, 2) dramatically facilitated an oxytocin antagonist, and 3) relatively insensitive to AVT and an AVP antagonist. In contrast, type I males show an opposite pattern of sensitivity, being sensitive to AVT and its antagonist, but not isotocin or its antagonist. Interestingly, the type II males exhibit a pattern of peptide sensitivity that is virtually identical to females (Goodson and Bass, 2000a), despite the fact that the site of peptide delivery is located in a brain region (the POA-AH) that regulates the pituitary gonadal axis of type II males in a hypermasculine manner. Hence, these data indicate that regulatory mechanisms for sex-typical behavior and gonadal physiology can be uncoupled from each other within the same general brain areas. Indeed, this uncoupling may occur at the level of individual neurons, as other data from teleosts show that IT and gonadotropin releasing hormone are produced by the same cells (Maejima et al., 1994).
Patterns of peptidergic modulation and network response reflect species-typical group size
AVT also exerts behavioral effects that are species-specific. For instance, AVT inhibits overt aggression when infused into the septum of territorial songbird species, but facilitates aggression in a colonial species (Goodson, 1998b; Goodson, 1998a; Goodson and Adkins-Regan, 1999). These experiments employed two species that independently evolved territoriality, and controlled for multiple aspects of behavior, ecology and phylogeny. However, results in male field sparrows (Spizella pusilla) indicate that these effects may not reflect direct effects on agonistic behavior per se, given that AVT facilitates the use of a strictly agonistic song type during spontaneous dawn singing (Fig. 4C-D), but inhibits overt aggression directed at a male intruder (Goodson, 1998a). Such findings suggest that AVT may primarily modulate anxiety or stress-related processes, with subsequent effects on agonistic behavior being context-dependent. Indeed, by combining AVT receptor blockade with presentation of an intense non-social stressor and/or simulated territorial challenge, we have shown that the release of endogenous AVT into the LS primarily modulates responses to stress (as determined by examining immunoreactivity for the IEG Zenk) (Goodson and Evans, 2004). These effects of AVT are generally opposite of those produced by intraseptal infusions of vasoactive intestinal polypeptide (VIP) (Goodson, 1998b; Goodson, 1998a; Goodson and Adkins-Regan, 1999). Interestingly, these findings are paralleled by data showing that VIP- and AVT-immunoreactive (-ir) fibers within the LS are differentially sensitive to castration and testosterone replacement in male quail (i.e., castration produces increases in VIP-ir and decreases in AVT-ir, with effects being reversed by treatments with testosterone) (Aste et al., 1997).
The experiments described above suggest that neurooendocrine processes related to stress may play an important role in differentiating the behavior of animals that live in large groups from those that live as territorial pairs. Data from mammals are consistent with this idea, as neuropeptide manipulations that influence affiliation also modulate anxiety (e.g., Pitkow et al., 2001). However, evolutionary shifts in species-typical group size (“sociality”) could reflect natural selection on several motivational processes in addition to anxiety and stress, including social arousal, approachavoidance, reward, and dominance. In fact, evolutionary divergence in reward processes has been clearly linked to evolution in another aspect of social structure – mating system. Monogamous mammals exhibit higher levels of vasopressin and oxytocin receptors within the ventral pallidum and nucleus accumbens than do non-monogamous species, and these species-specific features play an important role in the expression of bonding (Pitkow et al., 2001; Lim et al., 2004b; Lim and Young, 2004). However, while these findings show that reward-related processes are linked to affiliation and bonding, they do not make clear whether reward processes evolve divergently in relation to sociality, when sociality is uncoupled from mating system.
Thus, in order to gain a broader understanding of how neuro-motivational systems evolve in relation to species-typical group size, we recently examined Fos and Zenk (egr-1) responses to a same-sex conspecific in songbird species that differ in sociality. Analyses were focused on components of the social behavior network and related structures that are involved in stress and reward processes. In order to isolate sociality as a quasi-independent variable, we used four estrildid species (both sexes) that differ selectively in their species-typical group sizes (Goodson et al., 2005). These species are the territorial violet-eared waxbill, Uraeginthus granatina (group size = 2 excluding dependent young; i.e., male-female pair), the moderately gregarious Angolan blue waxbill (group size = 8-40), and two colonial species, the zebra finch, Taeniopygia guttata, and the spice finch, Lonchura punctulata (group sizes = 90-300). In choosing these species, we sought to control for multiple aspects of behavior and ecology that may influence how an animal responds to social stimuli. The species that we used are all monogamous (typically pair-bonding for life), exhibit biparental care, breed semi-opportunistically dependent upon rainfall and inhabit semi-arid and/or grassland scrub habitat (although the spice finch's range also includes more mesic habitats). These songbirds exhibit only limited conditional variability in grouping behavior such that the species differences in sociality are stable across seasons and contexts (Skead, 1975; Goodwin, 1982; Zann, 1996; also see references within).
Our paradigm for social exposure limited behavioral performance, thus species differences in IEG response should reflect divergence in motivational and/or perceptual processes. The significant results from this experiment are presented schematically in Figure 3. Within the extended medial amygdala (which is involved in appetitive approach, social arousal and avoidance; Thompson et al., 1998; Newman, 1999; Sheehan et al., 2000; Absil et al., 2002), we observed species differences in IEG response that are negatively graded in relation to sociality. In addition, the ventrolateral septum, anterior hypothalamus, and lateral subdivision of the ventromedial hypothalamus exhibited IEG responses that were significantly greater in the territorial species than in the three gregarious species (Goodson et al., 2005). These three areas are strongly responsive to social subjugation in hamsters (Kollack-Walker et al., 1997), thus the pattern of IEG response that distinguishes the territorial species may be reflective of social stress. This hypothesis is solidly supported by other IEG data in song sparrows: As shown in Figure 4A-B, the ventrolateral LS is strongly responsive to agonistic encounters, but Fos and Zenk responses correlate negatively with the subjects' aggressive behavior (similar to the difference between dominant and subordinant hamsters). The AH exhibits a similar profile to the ventrolateral LS (Goodson et al., submitted-a) and the lateral VMH is responsive to both handling/restraint stress and agonistic challenge (Goodson and Evans, 2004).
Importantly, the responses of other stress-related areas (most zones of the LS, lateral BST and paraventricular nucleus of the hypothalamus; see Goodson and Evans, 2004) and reward areas (nucleus accumbens and ventral pallidum) do not correlate with sociality. These findings suggest that social evolution has been accompanied by selection on a relatively discrete suite of motivational systems.
We are currently completing multiple studies that are designed to elucidate neuroendocrine mechanisms that underlie these species differences in neural response. These recent experiments have added a second territorial species, the melba finch (Pytilia melba), and therefore include two species that have independently evolved territoriality (melba finch and violet-eared waxbill), two species that independently evolved coloniality (spice finch and zebra finch), and one intermediate species (Angolan blue waxbill). We have found that receptor distributions for corticotropin releasing factor, VIP and AVT are all species-specific, with multiple species differences showing good correspondence to species-typical group size (J. L. Goodson, Y. Wang and A. K. Evans, unpubl. obs.). In addition, an extensive series of experiments is focused on activation of the AVT neurons in the extended medial amygdala (BSTm). By immunocytochemically double-labeling for AVT and IEGs (Fos and Zenk), we have found that the activity of these AVT neurons reflects both reproductive state and species-typical group size (J. L. Goodson and A. K. Evans, unpubl. obs.), and we are now more fully characterizing the stimulus parameters that elicit IEG responses within this neuronal population.
Conclusions
Based on a variety of behavioral, connectional and histochemical findings, it is now clear that the brain circuits that regulate social behavior in non-mammalian vertebrates are extensively similar to those in mammals. These observations strongly support the proposal that the social behavior network (as first described for mammals; Newman, 1999) is a fundamental and evolutionarily conserved feature of the vertebrate brain. Given this, it should be possible to select species for study based upon their behavioral biology, not their taxonomic class, and yet generate insights that are potentially relevant for all vertebrate classes. However, research is often (even typically) conducted within a taxon-specific framework, even when relevant data are available in other vertebrate groups. As our understanding of behavioral neuroendocrinology becomes more advanced, such an approach will unnecessarily limit us as to what we can understand. Studies of sociality provide a good example: Even in the most socially diverse taxa, species differences in group size are typically confounded with other important variables, including mating system, patterns of parental care, habitat preferences, and/or the seasonal expression of behavior. Aggregation in many species is also highly conditional. Thus, for most animals, controlled species comparisons of grouping behavior are virtually impossible to conduct. Fortunately, a limited number of songbird groups exhibit stable patterns of aggregation that can be investigated independently of other important variables. These species therefore offer a unique opportunity to understand how neural, neuroendocrine and motivational processes evolve in relation to species-typical group size, and in response to ecological variables that ultimately select for relevant behavioral variations.
Figure 5.
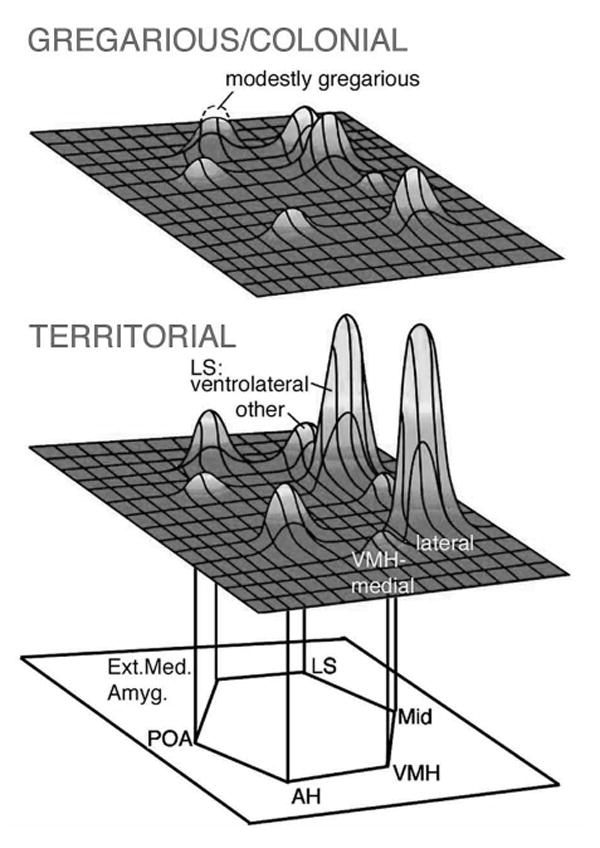
A schematic representation of immediate early gene responses (Fos and/or Zenk) within the social behavior network following exposure to a same-sex conspecific in four estrildid songbird species that differ selectively in their species-typical group sizes (two colonial species, one modestly gregarious species and one territorial species; both males and females were examined). Exposure was conducted in a paradigm that limits overt behavioral performance, thus species differences should reflect differences in motivational and/or perceptual processes. Modified from Goodson et al., 2005.
Acknowledgments
I would like to thank my graduate advisor, Elizabeth Adkins-Regan, and my postdoctoral advisor, Andrew H. Bass, for their strong and continuing support; Sarah Newman for kindly allowing me to adapt her ideas and figures; and numerous other collaborators, assistants and colleagues, including Richmond Thompson, Andrew Evans, Matthew Weeg, Jessica McKibben, Margaret Marchaterre, Kiran Soma, Yiwei Wang, Ben Mines, Jon Sakata, Laura Lindberg, Camryn Allen, Bess Choy, Richard Eibach, Bob and Joan Johnston, Sunny Boyd and Larry Young. I also thank my wife, Marcy Kingsbury (an outstanding developmental neuroscientist) for being my sounding board, troubleshooter, and field assistant. Finally, I would like to acknowledge the large number of SBN members who have been exceptionally generous and patient with me during my training as a scientist. In many ways, I have been group-taught by the SBN membership, and I am deeply grateful for their support and consideration for the Frank Beach Award. Training fellowships for JLG have been provided by Cornell University, NSF and NIH. Other funding was provided by NIH grants MH62656 (JLG), F32 NS-0443 and DC00092 (AHB), and NSF grant IBN 9421319 (AHB).
References
- Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav Evol. 2002;60:13–35. doi: 10.1159/000064119. [DOI] [PubMed] [Google Scholar]
- Acher R. Endocrinology. American Physiological Society; Washington, D.C.: 1972. Chemistry of the neurohypophysial hormones: an example of molecular evolution; pp. 119–130. [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. [PubMed] [Google Scholar]
- Aste N, Viglietti-Panzica C, Balthazart J, Panzica GC. Testosterone modulation of peptidergic pathways in the septo-preoptic region of male Japanese quail. Poult Avian Biol Rev. 1997;8:77–93. [Google Scholar]
- Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- Ball GF, Tlemcani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2770. doi: 10.1097/00001756-199709080-00032. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball Gregory F. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tissue Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus) J Comp Neurol. 1996;369:252–263. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bass A, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363. [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Batten TFC, Cambre ML, Moons L, Vandesande F. Comparative distribution of neuropeptide-immunoreactive systems in the brain of the green molly Poecilia latipinna. J Comp Neurol. 1990;302:893–919. doi: 10.1002/cne.903020416. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. Periaqueductal gray. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 173–182. [Google Scholar]
- Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Briganti F, Beani L, Panzica GC. Connections of the dorsomedial part of the nucleus intercollicularis in a male non-songbird, the grey partridge: A tract-tracing study. Neurosci Lett. 1996;221:61–65. doi: 10.1016/s0304-3940(96)13261-4. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Neary TJ. Afferent projections to the ventromedial hypothalamic nucleus in a lizard, Gekko gecko. Brain Behav Evol. 1995;46:14–29. doi: 10.1159/000113255. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Van Ryen PC, Vand Der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann NY Acad Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53:243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviours. Pharmacol Biochem Behav. 1982;17:623–628. doi: 10.1016/0091-3057(82)90334-3. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J Comp Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience. 1997;77:1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: A combined Fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Crews D. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol. 2003;43:1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. C-FOS changes following an aggressive encounter in female California mice: A synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM. The central mesencephalic grey in birds: nucleus intercollicularis and substantia grisea centralis. Brain Res Bull. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in golden hamsters. J Comp Neurol. 1990;293:476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Fine ML, Chen FA, Keefer DA. Autoradiographic localization of dihydrotestosterone and testosterone concentrating neurons in the brain of the oyster toadfish. Brain Res. 1996;709:65–80. doi: 10.1016/0006-8993(95)01275-3. [DOI] [PubMed] [Google Scholar]
- Fine ML, Keefer DA, Russel-Mergenthal H. Autoradiographic localization of estrogen-concentrating cells in the brain and pituitary of the oyster toadfish. Brain Res. 1990;536:207–219. doi: 10.1016/0006-8993(90)90027-9. [DOI] [PubMed] [Google Scholar]
- Font C, Hoogland Piet V, Van Der Zee Eefke V, Perez CJ, Martinez-Garcia F. The septal complex of the telencephalon of the lizard Podarcis hispanica: I. Chemoarchitectonical organization. J Comp Neurol. 1995;359:117–130. doi: 10.1002/cne.903590108. [DOI] [PubMed] [Google Scholar]
- Font C, Lanuza E, Martinez-Marcos A, Hoogland PV, Martinez-Garcia F. Septal complex of the telencephalon of lizards: III. Efferent connections and general discussion. J Comp Neurol. 1998;401:525–548. [PubMed] [Google Scholar]
- Foran CM, Bass AH. Preoptic GnRH and AVT: Axes for sexual plasticity in teleost fish. Gen Comp Endocrinol. 1999;116:141–152. doi: 10.1006/gcen.1999.7357. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distriubtion of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Rhythmic midbrain-evoked vocalization is inhibited by vasoactive intestinal polypeptide in the teleost Porichthys notatus. Brain Res. 2000b;865:107–111. doi: 10.1016/s0006-8993(00)02232-0. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost, Porichthys notatus. J Comp Neurol. 2000c;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;98:167–180. [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004a;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004b;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Soma KK. Fos and Zenk responses to aggressive challenge correlate with behavior in male song sparrows. doi: 10.1097/01.wnr.0000183898.47160.15. Submitted-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: Insights from studies on birds. Horm Behav. doi: 10.1016/j.yhbeh.2005.04.005. Submitted-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Estrildid finches of the world. Cornell University Press; Ithaca, NY: 1982. [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–700. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behaviour. Behav Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Vocal communication in frogs. Curr Opin Neurobiol. 2004;14:751–757. doi: 10.1016/j.conb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kennedy M. Vocalization elicited in a lizard by electrical stimulation of the midbrain. Brain Res. 1975;91:321–325. doi: 10.1016/0006-8993(75)90556-9. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shinoda A, Yamanouchi K, Arai Y. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm Behav. 1990;24:421–434. doi: 10.1016/0018-506x(90)90019-t. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Moor E, Hiemstra Y, Bohus B. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behavior of male rats. In: Jaard S, Jamison R, editors. Vasopressin. INSERM/Libbey; Paris: 1990. pp. 213–220. [Google Scholar]
- Lanuza E, Font C, Martinez-Marcos A, Martinez-Garcia F. Amygdalo-hypothalamic projections in the lizard Podarcis hispanica: A combined anterograde and retrograde tracing study. J Comp Neurol. 1997;384:537–555. doi: 10.1002/(sici)1096-9861(19970811)384:4<537::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Halpern M. Afferent and efferent connections of the nucleus sphericus in the snake Thamnophis sirtalis: Convergence of olfactory and vomeronasal information in the lateral cortex and the amygdala. J Comp Neurol. 1997;385:627–640. doi: 10.1002/(sici)1096-9861(19970908)385:4<627::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Halpern M. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the snake Thamnophis sirtalis. Brain Behav Evol. 1998;51:1–22. doi: 10.1159/000006525. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. J Neuroendocrinol. 2004a;16:325–332. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004b;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: Differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Richardson CF, Zoeller TR, Miller LJ, Muske LE, Moore FL. Neuroanatomical distribution of vasotocin in a urodele amphibian (Taricha granulosa) revealed by immunohistochemical and in situ hybridization techniques. J Comp Neurol. 1997;385:43–70. doi: 10.1002/(sici)1096-9861(19970818)385:1<43::aid-cne3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Maejima K, Oka Y, Park Min K, Kawashima S. Immunohistochemical double-labeling study of gonadotropin-releasing hormone (GnRH)-immunoreactive cells and oxytocin-immunoreactive cells in the preoptic area of the dwarf gourami, Colisa lalia. Neuroscience Research. 1994;20:189–193. doi: 10.1016/0168-0102(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Marin O, Smeets WJ, Gonzalez A. Basal ganglia organization in amphibians: chemoarchitecture. J Comp Neurol. 1998;392:285–312. [PubMed] [Google Scholar]
- Meddle SL, Foidart A, Wingfield JC, Ramenofskyand M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Szekely AD, Adam A, Csillag A. Efferent connections of septal nuclei of the domestic chick (Gallus domesticus): an anterograde pathway tracing study with a bearing on functional circuits. J Comp Neurol. 2004;469:437–456. doi: 10.1002/cne.11018. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. Hodological characterization of the medial amygdala in anuran amphibians. J Comp Neurol. 2003;466:389–408. doi: 10.1002/cne.10887. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long lasting effects of electrical simulation of the medial preoptic area and medial amygdala on maternal behavior in female rats. Behav Brain Res. 1999;99:61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Pfaff DW. A neuroendocrine approach to brain function: Localization of sex steroid concentrating cells in vertebrate brains. Am Zool. 1978;18:447–460. [Google Scholar]
- Nelson DA, Croner LJ. Song categories and their functions in the field sparrow (Spizella pusilla) Auk. 1991;108:42–52. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The Central Nervous System of Vertebrates. Springer-Verlag; New York: 1998. [Google Scholar]
- Northcutt RG. Evolution of the telencephalon in nonmammals. Ann Rev Neurosci. 1981;4:301–330. doi: 10.1146/annurev.ne.04.030181.001505. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: Behavioral implications. Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietta-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Phillips RE, Youngren OM, Peek FW. Repetitive vocalizations evoked by electrical stimulation of avian brains I: Awake chickens (Gallus gallus) Anim Behav. 1972;20:689–705. doi: 10.1016/s0003-3472(72)80141-6. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Martinez-Garcia F, Lanuza E, Davies DC. Distribution of corticotropin-releasing factor-immunoreactive neurons in the central nervous system of the domestic chicken and Japanese quail. J Comp Neurol. 2004;469:559–580. doi: 10.1002/cne.11023. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Connections of the ventral telencephalon (subpallium) in the zebrafish (Danio rerio) Brain Res. 2004;1011:206–220. doi: 10.1016/j.brainres.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Rev. 1997a;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997b;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav Evol. 2000;55:139–51. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Crews D. Developmental sculpting of social phenotype and plasticity. Neurosci Biobehav Rev. 2004;28:95–112. doi: 10.1016/j.neubiorev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–352. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Skead DM. Ecological studies of four Estrildines in the central Transvaal. Ostrich supp. 1975;11:1–55. [Google Scholar]
- Smeets WJAJ, Sevensma JJ, Jonker AJ. Comparative analysis of vasotocin-like immunoreactivity in the brain of the turtle Pseudemys scripta elegans and the snake Python regius. Brain Behav Evol. 1990;35:65–84. doi: 10.1159/000115857. [DOI] [PubMed] [Google Scholar]
- Stoll CJ, Voorn P. The distribution of hypothalamic and extrahypothalamic vasotocinergic cells and fibers in the brain of a lizard Gekko gecko presence of a sex difference. J Comp Neurol. 1985;239:193–204. doi: 10.1002/cne.902390206. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Tlemcani O, Ball GF, D'Hondt E, Vandesande F, Sharp PJ, Balthazart J. Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior. Brain Res Bull. 2000;52:249–262. doi: 10.1016/s0361-9230(00)00233-1. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Watson JT, Adkins-Regan E. Neuroanatomical localization of sex steroid-concentrating cells in the Japanese quail (Coturnix japonica): Autoradiography with [3H]-testosterone, [3H]-estradiol, and [3H]-dihydrotestosterone. Neuroendocrinology. 1989;49:51–64. doi: 10.1159/000125091. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Haerter UL, Kelly DB. A proposed neural pathway for vocaliztion in the South American clawed frog, Xenopus laevis. J Comp Physiol. 1985;157:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- Wong CJH. Connections of the basal forebrain of the weakly electric fish, Eigenmannia virescens. J Comp Neurol. 1997;389:49–64. [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]


