Abstract
We have shown that nanoparticles (NPs) conjugated to trans-activating transcriptor (TAT) peptide bypass the efflux action of P-glycoprotein and increases the transport of the encapsulated ritonavir, a protease inhibitor (PI), across the blood-brain-barrier (BBB) to the central nervous system (CNS). A steady increase in the drug parenchyma/capillary ratio with time without disrupting the BBB integrity suggests that TAT-conjugated NPs are first immobilized in the brain vasculature prior to their transport into parenchyma. Localization of NPs in the brain parenchyma was further confirmed with histological analysis of the brain sections. The brain drug level with conjugated NPs was 800-fold higher than that with drug in solution at two weeks. Drug clearance was seen within four weeks. In conclusion, TAT-conjugated NPs enhance the CNS bioavailability of the encapsulated PI and maintained therapeutic drug level in the brain for a sustained period that could be effective in reducing the viral load in the CNS which acts as a reservoir for replicating HIV-1 virus.
1. Introduction
The central nervous system (CNS) serves as one of the principal anatomical reservoirs for the replicating HIV-1 virus [1]. The virus enters the CNS at an early stage of infection and reinfects the peripheral tissues resulting in reactivation of the infection [2]. A spectrum of CNS complications ensues due to residence of HIV within the CNS, including progressive dementia, memory loss, HIV-mediated encephalopathy and cerebrovascular complications [3]. These neurological dysfunctions contribute toward significant morbidity and mortality amongst HIV-infected individuals [4].
The U.S. FDA-approved classes of drugs currently used in the treatment of HIV are nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs). PIs are an important class of anti-retroviral drugs that block the protease enzyme required by the virus in order to replicate. A number of biopharmaceutical limitations such as poor absorption, extensive protein binding and first-pass effect contribute toward the poor transport of these drugs across the blood-brain-barrier (BBB) [5]. In addition, most of the FDA-approved PIs are known substrates of the human multidrug-resistance gene (MDR1) transporter, P-glycoprotein (P-gp), which limits their transport across the BBB to the CNS [6]. Approaches such as developing potent PI derivatives [7], prodrugs (e.g., azidothymidine [8, 29] or using P-gp inhibitors [10] have been investigated to enhance the CNS bioavailability of anti-HIV drugs. However, developing new drugs or prodrugs could be an extensive undertaking with uncertain future. Given the wide expression of P-gp in various vital organs, use of P-gp inhibitors could also affect the pharmacokinetics of other drugs such as co-administered xenobiotics that the HIV-infected individual may be on simultaneously [6]. Hence, there is a need to design an alternative approach that can overcome the efflux action of the P-gp transporter and improve the permeability of PIs across the BBB.
Recently, nanocarrier systems such as micelles, liposomes, nanoparticles (NPs), dendrimers, etc. have been under investigation for delivery of therapeutic agents to the CNS [11]. To increase the efficiency of uptake, certain modifications have been investigated, including conjugation of NPs to specific ligands such as thiamine [12] or transferrin [13], or coating with surfactant [14]. However, there remains the issue of efficiency of drug uptake and drug retention in the brain. In recent times, the properties of cell-penetrating peptides (CPPs) have been explored to further enhance the cellular permeability of drug carrier systems as well as macromolecular therapeutics such as proteins and peptides [15]. The HIV-1 trans-activating transcriptor (TAT) peptide is one of the most widely used molecular beacons for cellular drug delivery [16]. Certain regions of the TAT-peptide known as protein transduction domains (PTDs), which consist of 9-16 amino acids, can pass through biological membranes by a mechanism that is independent of transporters and receptor-mediated endocytosis [17]. Fusion of β-galactosidase to TAT-peptide demonstrated the passage of the conjugated biomacromolecule across the BBB in mice [18].
We hypothesized that anti-HIV drugs loaded in NPs could bypass the efflux action of P-gp and TAT-conjugation would enhance their transport across the BBB, thereby enhancing the CNS bioavailability of anti-HIV drugs. To test this hypothesis, ritonavir, which is also the substrate for P-gp, was used as a model PI for encapsulation in biodegradable NPs [19]. The uptake and transport of ritonavir, either in solution, unconjugated NPs or TAT-conjugated NPs, were determined in MDCK wild-type (wt) and the P-gp over-expressing multidrug resistant (MDR1) MDCK cell lines. The CNS bioavailability and biodistribution of the drug were determined following intravenous injection into P-gp intact FVB/Ntac mice over four weeks. The drug distribution in the brain endothelium and parenchyma was also analyzed with time. Our results demonstrated that despite larger size (∼300 nm, hydrodynamic diameter) than the opening of the BBB (∼81 Å), TAT-conjugated NPs are transported across to the parenchyma and maintain therapeutic drug level for a sustained period of time.
2. Materials and Methods
2. 1. Materials
Poly (L-lactide) (PLA, inherent viscosity = 0.4 dl/g, molecular weight 40,000) was purchased from Birmingham Polymers, Inc. (Birmingham, AL). Ritonavir (ChemPacific Corporation, Baltimore, MD), tritium-labeled (3H) ritonavir and 14C-labeled mannitol (Moravek Biochemicals, Brea, CA) were purchased. TAT-peptide of the sequence Tyr-Gly-Arg-(Lys)2-(Arg)2-Gln-(Arg)3 (molecular weight 1917) was custom synthesized by Invitrogen Corporation (Carlsbad, CA). Denacol® EX-521 (Pentaepoxy, molecular weight 742) was a gift from Nagase Chemicals Ltd (Tokyo, Japan). Soluene®-350 and the scintillation cocktail, ScintiVerse were obtained from PerkinElmer, Inc. (Waltham, MA). Zinc tetrahydrofluroborate hydrate, poly(vinyl alcohol) (PVA, average molecular weight 30,000 – 70,000), dextran, boric acid, and ethanol were obtained from Sigma Chemical Co. (St. Louis, MO). Chloroform was obtained from Fisher Scientific (Pittsburgh, PA).
2. 2. Formulation of ritonavir-loaded NPs
3H-ritonavir-loaded NPs were formulated using an emulsion-solvent evaporation technique. Briefly, 12 mg of unlabeled ritonavir, 100 μCi of 3H-ritonavir and 48 mg of PLA polymer were dissolved in 2 mL of chloroform. PLA of the above molecular weight was selected based on the solid-state solubility of the drug in polymers of different molecular weight PLA and PLGA (poly dl-lactide co-glycolide) with different ratios of lactide to glycolide polymers as described in our previous study [20]. Polymers that show greater solid-state solubility have been shown to form NPs with higher drug encapsulation. The ritonavir and polymer solution was then emulsified into 12 mL of 2% aqueous PVA, using a probe sonicator set at 55 W energy output (XL 2015 Sonicator ultrasonic processor, Misonix Inc., Farmingdale, NY) for 2 min over an ice bath to form an oil/water emulsion. The resulting emulsion was kept stirring overnight at 4 °C to evaporate the chloroform. NPs were recovered by ultracentrifugation at 30,000 rpm for 20 min at 4 °C (Beckman Optima LE-80K, Beckman Instruments Inc., Palo Alto, CA) and washed thrice with distilled water to remove PVA and unencapsulated ritonavir. The pellet was resuspended in water, sonicated for 30 sec, and centrifuged at 1,000 rpm for 10 min at 4 °C. The supernatant was collected, frozen at -70 °C and lyophilized for 48 h to form a dry powder (Freeze Dryer, VirTis Inc., Gardiner, NY).
2. 3. Ritonavir loading in NPs and in vitro release
Ritonavir loading in NPs was determined indirectly by analyzing the supernatant and washings collected from the NPs formulation. The washings were analyzed for ritonavir content using a liquid scintillation counter (LSC) (Packard, Downers Grove, IL) and this amount was subtracted from the total drug added to the formulation to calculate the encapsulation efficiency and drug loading in NPs.
Drug release from NPs in vitro was carried out in double diffusion chambers separated by a Millipore® hydrophilic membrane of 0.1 μm porosity (Millipore Co., Bedford, MA). Each donor chamber was filled with 5 mL of NP suspension in phosphate-buffered saline (PBS) (154 mM, pH 7.4) containing 0.1% (w/v) Tween-80 (Sigma), while the receiver chamber contained the buffer without NPs. Diffusion cells were placed on an Environ orbital shaker (LabLine, Melrose Park, IL) rotating at 110 rpm at 37 °C. At several predetermined time intervals, the entire content of each receiver chamber was removed and replaced with fresh PBS. The amount of ritonavir that diffused into the receiver chamber was quantified by HPLC [13].
2. 4. Conjugation of TAT-peptide to NPs
The short sequence of TAT-peptide used in our study has been previously demonstrated to increase cellular internalization of cargoes more effectively as compared to longer peptide sequences [21]. A minimum 11-amino acid backbone is required for transduction. TAT-peptide was conjugated to NPs by using an epoxy conjugation method as described previously in our studies for transferrin conjugation to NPs [22]. In the initial step, NP surface was activated by the epoxy compound, Denacol®, followed by conjugation of the activated NPs to TAT-peptide in the second step. Briefly, 60 mg of NPs were sonicated in 4 mL of borate buffer (50 mM, pH 5.0) for 30 s on an ice-bath. This was followed by the addition of 40 mg of Denacol® that was also dissolved in an equal volume of buffer and the reaction was allowed to take place for 30 min at 37 °C with gentle stirring in the presence of 50 mg of zinc tetrahydrofluroborate hydrate, which acted as a catalyst. NPs were separated by ultracentrifugation at 30,000 rpm at 4 °C for 20 min followed by three washings with borate buffer to remove any unreacted Denacol®. To conjugate TAT-peptide, epoxy activated NPs were resuspended in 4 mL of borate buffer and mixed with a solution containing 200 μg of TAT peptide in the same buffer. The reaction was allowed to proceed at 37 °C for 2 h with constant stirring (see Supporting Information, Figure S1). The unreacted peptide was then removed by ultracentrifugation as discussed above; the final NP suspension was lyophilized for 48 h. The amount of the peptide conjugated to NPs was determined by using fluorescein-conjugated TAT-peptide (Invitrogen Corporation). The washings from the peptide conjugation reaction were analyzed by fluorescence spectroscopy (LS55, PerkinElmer) to determine the amount of unattached TAT-peptide (λex = 488nm λem = 520 nm). This amount was then subtracted from the total amount of TAT-peptide initially used in the reaction to obtain the amount of peptide conjugated to NPs.
2. 5. Particle size analysis and zeta potential of NPs
The mean hydrodynamic diameter and zeta potential of unconjugated and TAT-conjugated NPs were measured using ZetaPlus™ particle size analyzer (Brookhaven Instrument Corp, Holtsville, NY). For particle size analysis by transmission electron microscopy (TEM) a drop of NP suspension was placed on a Formvar-coated copper TEM grid, air dried, counterstained with 2% (w/v) aqueous solution of uranyl acetate (Sigma), and air dried again prior to visualization using a Philips CM12 electron microscope (FEI Company, Hillsboro, OR). The NIH ImageJ software was used to calculate mean particle size from the TEM photomicrographs.
2. 6. Elemental analysis of NP surface
To confirm the presence of TAT-peptide on NP surface, the elemental composition of NPs prior to and after TAT-conjugation was determined by X-ray photo-electron spectroscopy (XPS). An XPS system (PHI-5600, PerkinElmer) equipped with a conventional dual anode X-ray source (Mg and Al) was used under high vacuum (with a pressure of less than 9 × 10−9 Torr) and with an inert gas sputtering source (PHI-04-303) for sample cleaning and depth profiling. The following sequence of spectra was recorded: C1s, O1s, N1s. The binding energies (eV) were calculated and elemental concentrations were expressed as atomic percentage.
2. 7. Cell culture
Madine Darby canine kidney cells overexpressing P-gp (MDCK-MDR1) and non-P-gp-expressing MDCK wild type (MDCK-wt) cells were obtained as a kind gift from the laboratory of Dr. Donald Miller (Department of Pharmaceutical Sciences, University of Nebraska Medical Center, Omaha, NE). MDCK cells are capable of polarized growth with the formation of tight junctions which, along with the expression of P-gp, makes these cells a suitable model for BBB transport and permeability studies [23]. The MDCK-MDR1 cells were cultured in Dulbecco's Modified Eagle's Medium containing 1% lglutamine, 10% fetal bovine serum, 1% penicillin/streptomycin and supplemented with 80 ng/mL of colchicine in order to maintain high levels of P-gp expression. The transfected cells were incubated in 5% CO2 at 37 °C wherein media was replaced every other day. The MDCK-wt cells were cultured similarly to the MDCK-MDR1 cells except that colchicine was not added to the culture media.
2. 8. Drug transport and uptake studies
To determine whether the drug encapsulated in NPs bypasses the efflux action of membrane-bound P-gp, the transport studies were carried out in MDCK-MDR1 and MDCK-wt cells. The cells were seeded on PET cell culture inserts (3.0 μm pore size, 4.2 cm2 growing area) at a seeding density of 250,000 cells/insert in 6-well plates (BD Biosciences, Franklin Lakes, NJ). Cell culture media was added to the apical and basolateral chambers and media was changed every other day. During the culture period, the cells grown on insert membranes were tested for monolayer integrity by measuring their transepithelial electrical resistance (TEER) using an Epithelial Voltohmmeter (EVOM™, World Precision, Inc., Sarasota, FL). Experiments were conducted only with cells that had a TEER of > 200 ω-cm2 after correcting for the resistance obtained in control blank insert membranes. Prior to the beginning of transport experiments, the cell culture media was replaced by assay buffer containing 150 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM HEPES, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, and 0.4 mM K2HPO4 adjusted to pH 7.4. Monolayers of MDCK cells grown on insert membranes were then sandwiched between the donor and receiver compartments of a Side-Bi-Side diffusion cell (Crown Bio Scientific Inc., Somerville, NJ). The chambers were maintained at 37 °C.
To perform the apical-basolateral transport studies, the donor side of the chamber was filled with 3 mL of either ritonavir solution, ritonavir-loaded NPs or TAT-conjugated ritonavir-loaded NPs (5 μM ritonavir concentration) dispersed in assay buffer. The receiver side of the chamber was filled with an equal amount of assay buffer. The entire solution from the receiver compartment was withdrawn at different time points and replaced with fresh assay buffer to maintain sink conditions. 14C-labeled mannitol was used as a paracellular marker during the transport studies to ensure cellular membrane integrity. The collected samples were then transferred to scintillation vials containing 5 mL of scintillation cocktail and radioactivity was analyzed using a LSC. Standard plots with different concentrations of ritonavir solution, and TAT-conjugated and unconjugated NPs dispersed in the assay buffer were simultaneously constructed under similar conditions to determine the amount of ritonavir transported across the cell monolayer.
2. 8. 1. Drug permeability studies
Permeability of the drug was determined by calculating the apparent permeability coefficient (Papp) of ritonavir using equation (1):
| (1) |
where dQ/dT is the rate of transport of the drug, A (cm2) is the surface area of the cell monolayer available for drug transport, and C0 is the donor concentration of drug [24]. To correct for the permeability of drug across the insert membrane itself, the Papp of the drug across the cell monolayer was adjusted using equation (2):
| (2) |
where Pe is the drug permeability across the cell monolayer, Pt is the drug permeability across both the cell monolayer and the insert membrane, and Pf is the drug permeability across the insert membrane alone [25].
2. 8. 2. Cellular uptake of ritonavir
MDCK-wt and MDCK-MDR1 cells were seeded on 6-well plates at a seeding density of 250,000 cells/well. The cells were allowed to attach for 24 h and the culture media was then changed every other day until confluency was attained (3-4 days post-seeding). Prior to the experiment, the conditioned media in each well was replaced with either 3H-ritonavir solution, or drug-loaded conjugated or unconjugated NPs (5 μM ritonavir concentration) in 3 mL assay buffer and incubated for 5 h at 37 °C. After the incubation period, the cells were washed thrice with fresh assay buffer to remove any uninternalized drug. The cells were lysed by the addition of 0.5 mL trypsin (Invitrogen Corporation) followed by a series of alternate freeze and thaw cycles. The cell lysate (0.4 mL) was lyophilized for 48 h and analyzed for drug levels using LSC. The remaining cell lysate (0.1 mL) was used to determine the protein content of cells using a Bradford protein assay (Bio-Rad, Hercules, CA). A standard plot with different concentrations of drug-loaded NPs was constructed simultaneously to determine the amount of drug in cell lysates and the levels were normalized to protein content.
2. 9. CNS uptake and biodistribution of drug
This set of studies was performed to determine whether drug loaded into TAT-conjugated NPs results in greater CNS delivery of ritonavir as compared to that with unconjugated NPs or drug in solution. For this purpose, P-gp intact (wild type) mice (male 1 a (+/+ mice (FVB/Ntac) weighing 20 to 30 g (Taconic, Germantown, NY) were used as an animal model. Experiments were carried out as per the guidelines of the Institutional Animal Care and Use Committees and the Offices of Chemical and Radiation Safety at the University of Nebraska Medical Center and the Cleveland Clinic.
The mice were divided into four groups consisting of i) saline control, ii) unconjugated, drug-loaded NPs, iii) TAT-conjugated, drug-loaded NPs, and iv) drug in solution. 3H-ritonavir was used to analyze drug levels in different tissues and blood. The desired dose of drug (45 mg/kg), either loaded in NPs or present in solution, was injected in 100 μL of saline via the tail-vein. This dose of ritonavir has been found to be effective in the treatment of T-lymphocyte activity in mice [26]. At predetermined time intervals, mice were euthanized with intraperitoneal injection of sodium pentobarbital (100 mg/Kg) and blood was collected by cardiac puncture. The mice were then subjected to transcardiac perfusion by injecting 1 mL of heparinzed-saline (containing 0.9% NaCl and 10 U/mL heparin, Sigma) into the left ventricle and flushing out the blood for at least 2 min. Brain, heart, liver, lungs, spleen and kidneys were harvested at different time points following ritonavir injection, were washed with sterile PBS, blotted dry with paper towel and collected in pre-weighed glass vials to obtain the wet-tissue weight. The tissues were homogenized in 100 mM Tris-HCl buffer to prepare a 10% (w/v) tissue homogenate, which was subsequently digested with 0.5-0.7 mL of tissue solubilizer (Soluene®) at 50 °C for 24 h prior to drug analysis. After samples were cooled to room temperature, 0.2 mL of 30% hydrogen peroxide (Acros Organics, Morris Plains, NJ) was added to each sample for the sample decolorization. Scintillation cocktail (5 mL) was then added to each solubilized tissue sample for determination of ritonavir levels by LSC. A standard plot for 3H-ritonavir was simultaneously prepared using tissues from untreated mice that were injected with saline. Each organ from untreated mice was homogenized in a similar manner and different concentrations of drug either in solution or encapsulated in NPs were added to these tissue homogenates and the resulting tracer levels were measured by LSC. The tissue ritonavir levels were normalized to wet tissue weight. To analyze drug levels in the blood, a mixture of Soluene® and isopropyl alcohol (1 mL of 1:1 ratio solution) was added to 0.2 mL of each blood sample with constant stirring, followed by incubation at 60 °C for 2-5 h until the samples were completely digested. Radioactivity was measured after treating each sample with hydrogen peroxide as above.
2. 10. Drug distribution between blood capillaries and parenchyma
The distribution of ritonavir between brain capillaries and parenchyma was assessed by capillary depletion analysis [27]. Further, the amounts of ritonavir available in the free form and encapsulated in NPs were also determined. For this purpose, homogenized brain tissue samples were further homogenized with 2 mL of 26% aqueous dextran solution and centrifuged at 5,400 × g at 4 °C for 20 min to separate capillaries from parenchyma. The pellet consisted of ritonavir either bound to or taken up by brain capillaries, and the supernatant was comprised of ritonavir that had crossed the BBB. The data were represented as a fraction of the total drug in the brain that was present in capillaries and parenchyma. The brain parenchyma obtained above was further ultracentrifuged at 30,000 rpm for 20 min at 4 °C to separate the encapsulated ritonavir (pellet) from the free ritonavir (supernatant). The free ritonavir obtained in the supernatant represents the ritonavir released from the NPs.
2. 11. Localization of NPs in the brain
To determine the localization of NPs within the brain, 6-coumarin (Polysciences Inc., Warrington, PA)-loaded NPs were used. The loaded dye acts as a probe for NPs and can be visualized using fluorescence microscopy. 6-coumarin-loaded NPs were formulated as described in our previous studies [28]. Mice were injected with 250 mg/Kg of dye-loaded unconjugated or TAT-conjugated NPs via the tail vein. This dose of NPs used was the same dose as that of the ritonavir-loaded NPs used in the biodistribution study. After 24 h, the mice were sacrificed and their brains were harvested as described earlier. To determine whether NPs crossed the BBB, an anti-CD31 marker specific to the endothelial blood vessels was used. The mice brains were rinsed with saline and cryofrozen in TissueTec®OCT compound (Sakura Finetek Inc., Torrance, CA). The frozen brains were then cut using a microtome into coronal sections of approximately 8 μm thickness. The sections were then fixed with 2% formaldehyde in Sorensen's buffer for 20 min. After three washes of PBS for 10 min each, the sections were blocked with 2% goat serum in PBS for 30 min at room temperature. Excess blocking buffer was blotted off and the sections were incubated with rabbit anti-CD31 antibody (Santacruz Biotechnology Inc., Santacruz, CA) diluted in blocking buffer (1:250) at 4 °C overnight. The slides were washed at least three times in order to remove excess primary antibody. The sections were then incubated with goat anti-rabbit secondary antibody conjugated to AlexaFluor 568 (Molecular Probes, Eugene, OR) diluted to 1:1000 in blocking buffer and incubated for 1 h. After subsequent washing with PBS, the sections were stained with a drop of Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA) to visualize the nuclei. The sections were then visualized using a confocal laser scanning microscope equipped for fluorescence with FITC and Rhodamine filters under an oil lens at 40× magnification (Leica TCS-SP, Leica Microsystems Inc., Bannockburn, IL).
2. 12. Blood-brain-barrier integrity
Lastly, we evaluated the integrity of the BBB using the Evans Blue dye (Sigma) extravasation method [18]. In addition, the change in extravasation of the dye was also determined in other tissues (i.e., brain, heart, liver, lungs, spleen and kidneys). Mice were injected via tail vein as before, either with ritonavir in solution, or encapsulated in TAT-conjugated or unconjugated NPs. Twenty-four hours after the drug injection, Evans blue dye solution was injected (2 mL/Kg of 2% dye in 0.9% NaCl) intravenously through the tail vein. Animals were euthanized 2 h following injection of the dye, at which point they were perfused with heparinized saline until clear perfusate from the atrium was obtained. The brain and other organs were subsequently harvested. The dissected tissues were weighed and a volume of dimethylformamide (Sigma) two times the weight of the tissue was added to each sample. The tissues were homogenized and incubated at 50 °C for 24 h and then each sample was centrifuged for 20 min at 12,000 rpm. Dye levels in each tissue sample were determined using a UV-VIS spectrophotometer (Beckman DU 640B, Beckman-Coulter Inc.) at a wavelength of 620 nm, using a standard plot of the dye prepared in the tissue homogenates of control animals.
2. 13. Statistical Analysis
All data are expressed as mean ± standard error of means (s.e.m). Statistical analyses were performed using a Student's t-test. The differences were considered significant for p values < 0.05.
3. Results
3. 1. Characterization of NPs
NPs were in general spherical in shape with narrow particle size distribution (see Supporting Information, Figure S2b for TEM of NPs). The hydrodynamic diameter of NPs is greater than the TEM diameter because of the hydration of PVA associated with NPs [13]. TAT-conjugation changed the anionic zeta potential of NPs to slightly cationic (Table 1). Loading of ritonavir in NPs was 18.3% (w/w) (i.e., 100 mg of NPs contained 18.3 mg of ritonavir) with an encapsulation efficiency of 89.7%. About 80% of ritonavir was released from NPs over a period of 10 d (see Supporting Information, Figure S2a). Although, we have not determined the effect of conjugation on the release of the encapsulated drug, we do not anticipate that it would be significantly different than that from unconjugated NPs because the conjugation reaction occurs with the PVA associated with NPs at the interface without influencing the polymer matrix.
Table 1.
Physical characterization of ritonavir-loaded nanoparticles
| Formulation | Particle size (nm) | Zeta Potential (mV) |
|
|---|---|---|---|
| DLS*(PI) | TEM** | ||
| Unconjugated NPs | 300 (0.10) | 125 ± 6.3 | −19.3 ± 0.5 |
| TAT-conjugated NPs | 340 (0.14) | 157 ± 8.9 | +2.4 ± 0.3 |
Mean hydrodynamic diameter determined by dynamic light scattering (DLS). PI: Polydispersity index.
Particle size as measured by transmission electron microscopy (TEM), mean of 79 nanoparticles.
The peaks at binding energies of 280-305 eV, 396-416 eV, and 528-548 eV in XPS were ascribed to the elements C1s, N1s, and O1s, respectively (see Supporting Information, Figure S2c). The elemental composition percentages of carbon, oxygen, and nitrogen on the surface of unconjugated NPs were 61.8%, 38.2%, and 0%, respectively; while those on the surface of TAT-conjugated NPs were 63.9%, 35.6%, and 0.4%, respectively. Nitrogen atoms were not detected on the surface of unconjugated NPs, but were observed on the surface of TAT-conjugated NPs, thus confirming TAT-peptide conjugation to the NP surface. The estimated amount of TAT-peptide attached to NP surface was 0.23 μg per mg of NPs, corresponding to ∼2012 peptide molecules per NP, as calculated using the formula described in our previous study [13].
3. 2. Ritonavir transport and uptake in vitro
The cumulative transport of ritonavir in solution from the apical to basolateral sides of diffusion chambers was significantly lower than when the drug was encapsulated in either unconjugated or TAT-conjugated NPs in the MDCK-MDR1 cell line (see Supporting Information, Figure S3a). The Papp calculated from the transport studies in both MDCK-MDR1 and MDCK-wt demonstrated greater permeability of ritonavir when encapsulated in NPs conjugated to TAT peptide opposed to that of ritonavir in either solution or unconjugated NPs (Table 2). The transport of mannitol across the cell monolayer was less than 0.5% per h, indicating that monolayer integrity was maintained for the duration of the experiment.
Table 2.
Apparent permeability (Papp) and uptake of drug in MDCK-MDR1 and MDCK-wt cells.
| Cell Type | Papp (cm/sec) × 10−5 | Uptakea(μg/mg protein) | ||||
|---|---|---|---|---|---|---|
| Drug in Solution |
Unconjugated NPs |
TAT- conjugated NPs |
Drug in Solution |
Unconjugated NPs |
TAT- conjugated NPs |
|
| MDCK- MDR1 |
0.05 | 1.97 | 2.4 | 0.04 ± 0.01 | 0.59 ± 0.14 | 3.63 ± 0.75 |
| MDCK-wt | 0.60 | 0.81 | 3.0 | 0.29 ± 0.05 | 0.53 ± 0.02 | 0.83 ± 0.09 |
Papp was calculated from the transport studies (n=6; see transport figures in supporting information).
Data are mean ± s.e.m (n = 6).
p < 0.05 for the TAT-conjugated group between MDCK-MDR1 and MDCK-wt cells.
The uptake of ritonavir was greater in MDCK-wt cells than in MDCK-MDR1 cells with drug in solution whereas it was relatively the same in both of the cell lines with drug-loaded, unconjugated NPs (Table 2). TAT-conjugated NPs, however, showed four-fold greater uptake of ritonavir in MDCK-MDR1 cells than in MDCK-wt cells. The uptake of TAT-conjugated NPs was seen to increase by two-fold in the presence of free TAT-peptide in MDCK-MDR1 cells (2 ng/mL in culture media), but there was no change in the uptake of unconjugated NPs.
3. 3. CNS uptake and biodistribution of drug
The brain drug levels in animals which received drug in solution were higher up to 3 h following injection than in those animals which received either ritonavir-loaded unconjugated NPs or TAT-conjugated NPs (Figure 1). However, at later time points, the levels were higher in animals which received drug-loaded NPs, particularly in the animals which received TAT-conjugated NPs than in animals which received drug in solution. At the end of two weeks (14 d), the brain ritonavir levels were 0.1 ± 0.01 μg/g of tissue for the solution group, while they were 80.3 ± 5.6 and 12.2 ± 0.7 μg/g of tissue for TAT-conjugated and unconjugated NP groups, respectively. This represented an 800-fold higher brain ritonavir level with TAT-conjugated NPs than that with ritonavir in solution and about 7-fold higher than the same with unconjugated NPs. The initial drug biodistribution to different tissues varied with drug formulations (solution or unconjugated and TAT-conjugated NPs) and also changed with time. TAT-conjugation was seen to reduce the liver uptake of drug during the first day in contrast to that observed for unconjugated NPs or drug in solution. In general, increased drug levels were seen in the brain, heart, spleen and kidney 1 d after administration of TAT-conjugated and unconjugated NPs; the increase was greater in the spleen than in other tissues, particularly with TAT-conjugated NPs. The drug levels in the solution groups increased in all the organs up to 6 h after administration but the levels all declined slowly thereafter (Figure 1). At 28 d after administration, drug levels were detectable in the heart in animals which received TAT-conjugated NPs and in the spleen in those which received unconjugated and TAT-conjugated NPs but not in other organs. Blood ritonavir levels in animals which received drug in solution declined rapidly, whereas in animals that received NPs, the levels were relatively lower and remained detectable only up to 24 h after injection (Figure 2).
Figure 1.
Tissue distributions of ritonavir in mice injected intravenously with either ritonavir solution or ritonavir-loaded unconjugated or TAT-conjugated NPs at a drug dose of 45 mg/Kg. Data are represented as mean ± s.e.m. (n = 4). * p < 0.05 compared between the TAT-conjugated NPs group with the unconjugated NPs and solution groups.
Figure 2.
Blood levels of ritonavir in mice injected either with ritonavir solution, ritonavir-loaded unconjugated NPs, or TAT-conjugated ritonavir-loaded NPs. Data are represented as mean ± s.e.m. (n = 4). Blood drug levels in all groups were not detectable after 24 h. (Detection limit = 1 μg/mL).
3. 4. Ritonavir distribution in blood capillaries and parenchyma
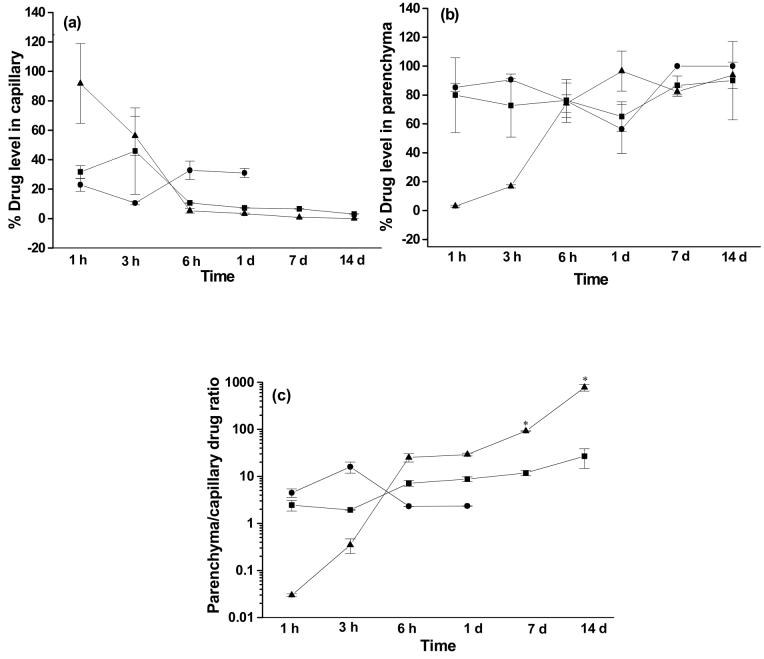
It was interesting to observe the dynamics of the change in drug distributions between blood capillaries and parenchyma with TAT-conjugated NPs. In this group, the fraction of the drug present in the capillaries decreased up to 6 h, while there was a concomitant increase in the parenchyma (Figure 3a and b). In the case of solution, the drug present in the brain beyond 1 d was localized in parenchyma and was undetectable in capillaries after 1 d. In the case of unconjugated NPs, the drug levels increased up to 3 h in capillaries, but the drug distribution between capillaries and parenchyma remained almost unchanged thereafter. The parenchyma/capillary (P/C) drug ratio was higher at later time points in animals that received drug in TAT-conjugated NPs compared to those which received drug in either unconjugated NPs or solution (Figure 3c).
Figure 3.
Changes in the fraction of drug distribution between brain capillaries and parenchyma over time following drug administration. Of the total brain drug, (a) the fraction present in brain capillaries, (b) the fraction present in parenchyma, and (c) the ratio of drug distribution between parenchyma/capillary in different groups. The capillary drug levels in the solution group were undetectable beyond 1 d. Data are represented as mean ± s.e.m. (n = 4). * p < 0.05 between TAT-conjugated and unconjugated NPs.
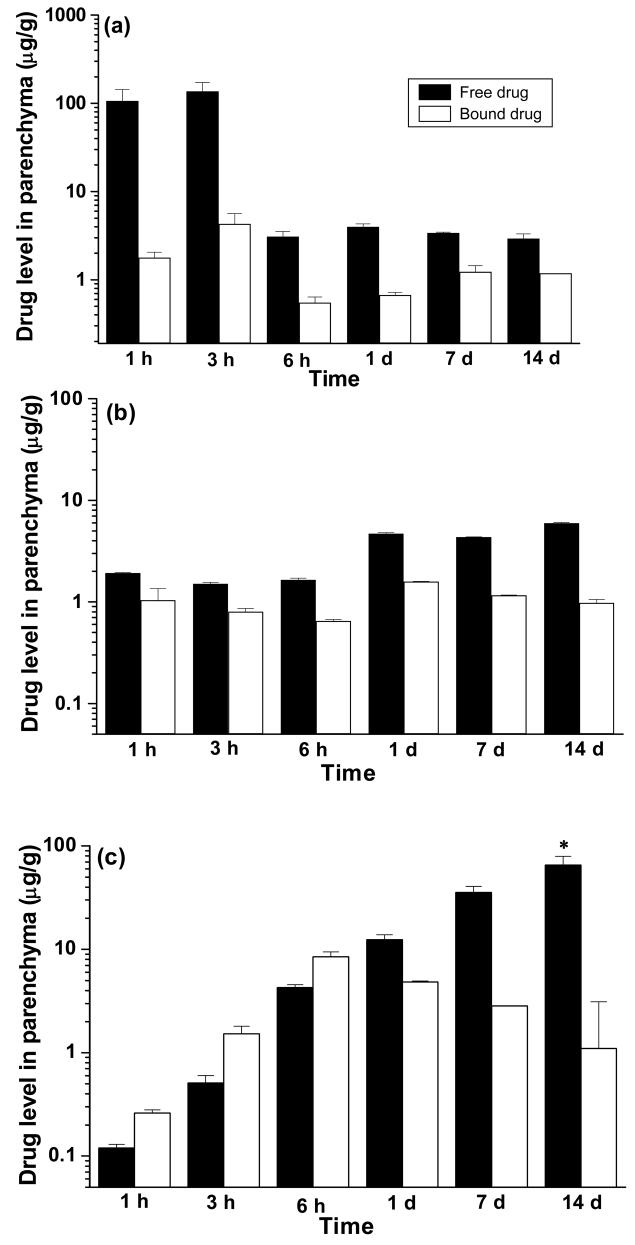
Analysis of free ritonavir and tissue-bound or NP-encapsulated ritonavir demonstrated the level of free drug was higher in animals that received drug in solution during the initial time point. However, it showed a rapid decline later, whereas the drug levels in animals receiving either unconjugated or TAT-conjugated NPs were higher at later time points (Figure 4). At the end of 14 d, the level of free ritonavir (unencapsulated) in the parenchyma of animals that received TAT-conjugated NPs was 11-fold higher than in animals that received unconjugated NPs, and 22-fold higher than in animals that received ritonavir in solution.
Figure 4.
Brain parenchymal drug levels compared in free (unencapsulated) and bound (NP-encapsulated or tissue-sequestered) forms among different treatment groups: (a) solution, (b) ritonavir-loaded unconjugated NPs, and (c) ritonavir-loaded TAT-conjugated NPs. Data are represented as mean ± s.e.m. (n = 3). * p < 0.05 between bound and free forms per treatment.
3. 5. Localization of NPs in the brain and BBB integrity
Fluorescence microscopic analysis of coronal sections of mouse brain revealed the staining pattern of CD31 cells (Figure 5a). Localization of TAT-conjugated NPs was observed in the brain cortex (Figure 5b). The confocal microscopic analysis showed NP localization within the ventricles, which possess an abundance of blood vessels (Figure 5 c-f). Enhanced accumulation of TAT-conjugated NPs within the narrow cavity of the third ventricle was observed as compared to unconjugated NPs (Figure 5f and d, respectively). The NPs appear to have crossed the BBB, as their localization was demonstrated within the parenchyma. There was no change in the extravasation of Evans blue in the brain or other organs in animals that received drug in either solution, unconjugated or TAT-conjugated NPs (see Supporting Information, Figure S4).
Figure 5.

Localization of 6-coumarin-loaded unconjugated NPs and TAT-conjugated NPs in mice brains, 1 d after intravenous injection of NPs at a dose of 250 mg/Kg. This dose of NPs is equivalent to the 45 mg/Kg dose of ritonavir used in the biodistribution study. All the sections were observed using a confocal microscope equipped for fluorescence at 40× magnification under an oil immersion lens. (a) Image of brain section demonstrating endothelial-cell staining with anti-CD31 antibody conjugated to AlexaFluor 568, (b) localization of TAT-conjugated NPs was observed within the cortex, (c) and (d) images demonstrating the localization of unconjugated NPs within the ventricles and parenchyma of the brain, respectively, and (e) and (f) images demonstrating that TAT-conjugated NPs localized within the lateral and third ventricles, respectively. Magnification bar = 25 μm. Representative data from studies in three mice per group. Blue fluorescence is due to DAPI-staining of the nuclei (N), red fluorescence is due to CD-31 antibody-staining the blood capillaries, and green fluorescence is due to the staining of NPs.
4. Discussion
Our results demonstrated enhanced and sustained brain delivery of ritonavir with TAT-conjugated NPs without influencing the integrity of the BBB, suggesting the transport of TAT-conjugated NPs could have occurred due to transcytosis across the endothelium of the brain vasculature. Several studies have reported transcytosis of TAT-conjugated cargoes across polarized epithelial cells. For example, Liang and Yang have shown transcytosis of insulin conjugated to TAT peptide across Caco-2 cell monolayer, which is used as model cell line for gastrointestinal epithelium [29]. Many theories have been proposed to explain the mechanism of cellular entry and the uptake of TAT-peptide. Recent studies indicate that binding of TAT-peptide to the cellular surface initiates the process of macropinocytosis, whereby the peptide enters the cell by forming an inverted micelle by temporary destabilization of the phospholipidic bilayer resulting in the internalization of conjugated cargo [17]. Many biophysical studies have shown that TAT conjugated to a cargo interacts favorably with the lipid bilayer [30]. Therefore, TAT-conjugated drug carrier systems have been extensively investigated to facilitate intracellular delivery of therapeutic agents, which has been shown to occur via non-endocytic pathways [15]
We observed greater uptake of ritonavir in both MDCK-wt and MDCK-MDR1 cells with TAT-conjugated NPs than with unconjugated NPs (Table 2). However, the drug transport across the monolayers of these same cells was similar for TAT-conjugated and unconjugated NPs (Figure S3a and b). This could be because of the lag phase in the transport of NPs, causing them to accumulate inside the cells. Koch et al. have also observed an initial lag phase in their transport study with TAT-FITC-conjugated CLIO NPs in CaCo-2 cells [31]. Our transport study could not be continued beyond 5 h because it affected the integrity of the cellular monolayer. However, higher Papp values and cellular uptake was observed with either TAT-conjugated or unconjugated NPs than with drug in solution in both the cell lines. This can be attributed to the combined effect of TAT-peptide and the P-gp-bypassing mechanism of NPs (Table 2). The higher uptake of drug in MDCK-MDR1 cells with TAT-conjugated NPs could be related to a greater affinity of TAT-peptide to these cells over MDCK-wt cells, potentially resulting from differences in their lipid composition [32]. MDCK-MDR1 cells display elevated levels of cell surface lipids, which can significantly affect drug uptake and the transport phenomenon as compared to that in MDCK-wt counterparts [33].
The total brain levels of ritonavir in mice in the solution group peaked at 1 h after injection but the levels dropped steadily thereafter, indicating the P-gp-mediated efflux of the drug (Figure 1). Similar diffusion of the drug from other tissues was also observed in the solution group (Figure 1). In the case of NPs, the drug levels were lower than that compared to solution at early time points, but they were higher at later time points, indicating sustained retention of the drug with NPs. Administration of ritonavir via TAT-conjugated NPs demonstrated approximately an 800-fold higher level of drug in the brain when compared to that with drug in solution at two weeks, thus standing testament to the greater efficacy of TAT-conjugated NPs to transport the drug across the brain endothelium. The blood drug levels were lower in the NP groups than in the solution group during the initial time points because of the uptake of NPs in circulating macrophages and their subsequent clearance by the reticuloendothelial system (Figure 2). The increase in the parenchyma-to-capillary drug ratio (P/C) after the initial lag phase suggests that TAT-conjugated NPs are first mobilized in the brain vasculature which are then transported to the parenchyma (Figure 3c). The NPs localized in the brain parenchyma released the drug and thus maintained the drug levels. Santra et al. have observed that only the Qdots conjugated to TAT-peptide migrated to the brain parenchyma beyond the endothelial cells [34], further signifying the role of TAT-peptide in the transport process across the endothelium.
It is interesting to note that the distribution of drug in different tissues changes with exposure time in the NP-injected animals (Figure 1). This observation could be attributed to a possible redistribution of NPs from the other tissues such as lymph, muscle and adipose tissue. At the end of 1 d, about 76% of the injected dose of ritonavir is estimated to be present in tissues other than the brain in the unconjugated NP group and 87% in the TAT-conjugated NP group This was calculated from subtraction of the amount of the drug recovered from the analyzed tissues from the total dose injected at the initial time point, assuming that drug elimination was minimum in 1 day in NP groups. For example, spleen and kidney showed increased ritonavir levels 1 d after administration of NPs. This could be because the phagocytic cells possibly engulfed the drug-loaded NPs and carried them to the excretory system of the body, i.e., kidney and spleen [35, 36]. The uptake of NPs by the phagocytic cells also explains the fall in the blood drug levels, reaching below the detection limit beyond 1 d. Polyakov et al., with [(99m)Tc] TAT-peptide chelates [37], and Wunderbaldinger et al., with CLIO magnetic NPs conjugated to TAT-peptide, also observed changes in the biodistribution pattern of NPs over time [38]. Lee et al. observed that TAT-biotin was rapidly sequestered into organs upon intravenous administration following initial extravascular distribution [39], thus supporting our data. The drug appears to have been eliminated from most of the body compartments, except from the heart and the spleen, within 28 d.
To reduce the viral load in the CNS, it is essential that therapeutically relevant levels of free ritonavir are maintained within the brain parenchyma. Clinical trials have indicated that the dosage of ritonavir, resulting in below-detection levels of HIV-1-RNA (80 copies/mL) in the cerebrospinal fluids (CSF) of HIV-1-affected patients, was < 2 ng/mL [40]. This level translates to 37.5 ng of ritonavir for a mouse, assuming: (i) a brain to body weight ratio of 1:17, (ii) an average body weight of 25 g, and (iii) a tissue density of 1 g/mL. In our experiments using TAT-conjugated NPs, the brain ritonavir level achieved at the end of two weeks was 65.2 ± 14.0 μg/g of tissue; taking into account the average weight of a mouse brain (0.35g), this becomes 22.8 ± 4.9 μg, which is significantly higher than the estimated therapeutic level as noted above (37.5 ng). A significant fraction of the total drug in the brain was found in free form, particularly with TAT-conjugated NPs (Figure 4). This corresponds to the drug released from NPs that were localized in the brain. This implies that even a lesser dose of TAT-conjugated NPs can be administered to achieve therapeutic levels of ritonavir in the brain.
The presence of circulating TAT in the serum of HIV patients can affect the delivery of TAT-conjugated NPs due to the presence of a common domain. Therefore, we tested the uptake of TAT-conjugated NPs in the presence of free peptide on MDCK-MDR1 cells (2 ng/mL). This concentration was based on the level of TAT-protein (1-3 ng/mL) present in the serum of HIV patients [41]. We observed that the uptake of TAT-conjugated NPs increased by two-fold in the presence of free TAT but not that of unconjugated NPs, suggesting that the effect is not due to the change in the cellular permeability. The above phenomenon has been observed by others with TAT-conjugated macromolecular therapeutics. For example, Dizhe et al. have reported increased cellular uptake of TAT/DNA complex in the presence of excess free TAT-peptide, and has attributed to a stimulatory effect of excess TAT-peptide on the cellular entry of TAT/DNA conjugate [42].
For anti-retroviral therapy to be effective, not only is it important to have therapeutically significant levels of the drug present in the brain, but also to sustain these drug levels for a longer period of time [43]. It has been known that the endothelial cells of the BBB pose a great hindrance towards the transport of drugs to the brain. TAT-conjugated NPs not only overcome this barrier, but also enhance the uptake and sustain the retention of the drug in the CNS, which is critical for therapeutic efficacy of the anti-retroviral drug therapy (Figure 1). The localization of NPs within the brain ventricles suggests that NPs are retained in these cavities initially, followed by their diffusion to the cerebral cortex via secretion into CSF. The CSF is one of the chief reservoirs for HIV-1, and a high concentration of PIs within this compartment is essential for their therapeutic efficacy [44]. Similarly, Lu et al. have demonstrated a substantial ventricular and periventricular localization of 6-coumarin-loaded, cationic bovine serum albumin-conjugated NPs, following an intravenous administration in mice [45]. The localization of NPs within the ventricles can result in high concentrations of the encapsulated ritonavir within the CSF and thus may help to reduce the CNS viral replication.
In addition to the brain, other organs such as the spleen and lymph nodes act as a sanctuary to the virus and PIs are ineffective because of the same P-gp efflux action of the endothelial membrane. It can be anticipated that TAT-conjugated NPs can result in better delivery of PIs to these tissues than drug in solution. Furthermore, CD4+ T-lymphocytes act as a principal target for HIV-1 replication [46]. TAT-conjugated NPs may also be effective in delivery of anti-HIV drugs to CD4+ lymphocytes as well. Tripathi et al. have reported an increased uptake and subsequent antiviral efficacy of anti-TAR (anti-transactivator responsive region, present in the HIV-1 genome) polyamide nucleotide conjugated to TAT-peptide in CEM CD4+ lymphocyte cells [47]. Thus, the combined effect of sustained release properties and their ability to overcome the cellular and tissue barriers, indicate TAT-conjugated NPs could improve the therapeutic efficacy of PIs and other anti-HIV drugs having similar drug delivery issues. It would be important to determine how long the drug levels need to be maintained to completely eradiate the virus from the CNS and other body compartments. This can be determined from studies in animal models of HIV, which is the focus of our future studies. In general, it can be concluded from our studies that TAT-conjugated NPs increase the CNS bioavailability of anti-HIV drugs and can provide a better therapeutic treatment option for HIV patients than the currently used oral drug therapy.
5. Conclusions
Our study demonstrates that TAT-conjugated NPs can effectively overcome the BBB in the transport of PIs, and thus can be effectively used to achieve therapeutic dosing of the drug in the brain. The sustained release properties of NPs can maintain therapeutic drug levels that could be very effective in controlling viral replication in the CNS.
Supplementary Material
Acknowledgements
Grant support was provided by the National Institutes of Health, Mental Health (R21 MH06 7525) and Cleveland Clinic (to VL). KSR is supported by a Pre-doctoral Fellowship from the American Heart Association, Heartland Affiliate (Grant # 0710119Z). We are appreciative of Dr. Donald Miller for the kind gift of MDCK cell lines. We would like to thank Drs. Sivakumar Vijayaraghvalu and Jasmine P. Davda for their assistance with the in vivo studies and Dr. Sanjeeb Sahoo for conjugation chemistry. We are grateful to Drs. Judy Drazba and John Peterson of the imaging core facility at Cleveland Clinic for their assistance with microscopic techniques. We thank Ms. Melissa Jedlicka for proof-reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schrager LK, D'Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Freed EO, Wolf KM, Robinson SM, Franko M, Kumar VB. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. J Virol. 2001;75:4681–91. doi: 10.1128/JVI.75.10.4681-4691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letendre S, Ances B, Gibson S, Ellis RJ. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2007;15:32–9. [PubMed] [Google Scholar]
- 4.Patsalides AD, Wood LV, Atac GK, Sandifer E, Butman JA, Patronas NJ. Cerebrovascular disease in HIV-infected pediatric patients: neuroimaging findings. AJR Am J Roentgenol. 2002;179:999–1003. doi: 10.2214/ajr.179.4.1790999. [DOI] [PubMed] [Google Scholar]
- 5.Begley DJ. Understanding and circumventing the blood-brain barrier. Acta Paediatr Suppl. 2003;92:83–91. doi: 10.1111/j.1651-2227.2003.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Gottesman MM. HIV-1 protease inhibitors and the MDR1 multidrug transporter. J Clin Invest. 1998;101:287–8. doi: 10.1172/JCI2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheha MM, el-Koussi NA, Farag HH. Brain delivery of HIV protease inhibitors. Arch Pharm (Weinheim) 2003;336:47–52. doi: 10.1002/ardp.200390003. [DOI] [PubMed] [Google Scholar]
- 8.Brewster ME, Anderson WR, Webb AI, Pablo LM, Meinsma D, Moreno D, et al. Evaluation of a brain-targeting zidovudine chemical delivery system in dogs. Antimicrob Agents Chemother. 1997;41:122–8. doi: 10.1128/aac.41.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrence PF, Kinjo J, Khamnei S, Greig NH. Synthesis and pharmacokinetics of a dihydropyridine chemical delivery system for the antiimmunodeficiency virus agent dideoxycytidine. J Med Chem. 1993;36:529–37. doi: 10.1021/jm00057a002. [DOI] [PubMed] [Google Scholar]
- 10.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–60. [PubMed] [Google Scholar]
- 11.Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3:219–32. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- 12.Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Control Release. 2003;93:271–82. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112:335–40. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- 14.Chavanpatil MD, Khdair A, Gerard B, Bachmeier C, Miller DW, Shekhar MP, et al. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol Pharm. 2007;4:730–8. doi: 10.1021/mp070024d. [DOI] [PubMed] [Google Scholar]
- 15.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008 doi: 10.1002/bip.20989. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Deliv Rev. 2008;60:548–58. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Rao KS, Labhasetwar V. Trans-activating transcriptional activator (TAT) peptide-mediated brain drug delivery. J Biomed Nanotech. 2006;2:173–85. [Google Scholar]
- 18.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 19.Perloff MD, von Moltke LL, Fahey JM, Greenblatt DJ. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol. 2007;59:947–53. doi: 10.1211/jpp.59.7.0006. [DOI] [PubMed] [Google Scholar]
- 20.Panyam J, Williams D, Dash A, Leslie-Pelecky D, Labhasetwar V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J Pharm Sci. 2004;93:1804–14. doi: 10.1002/jps.20094. [DOI] [PubMed] [Google Scholar]
- 21.Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem. 2002;269:494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2:373–83. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Rager JD, Weinstein K, Kardos PS, Dobson GL, Li J, et al. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier. Int J Pharm. 2005;288:349–59. doi: 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Xin HW, Schwab M, Klotz U. Transport studies with 5-aminosalicylate. Eur J Clin Pharmacol. 2006;62:871–5. doi: 10.1007/s00228-006-0182-3. [DOI] [PubMed] [Google Scholar]
- 25.Franke H, Galla H, Beuckmann CT. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res Brain Res Protoc. 2000;5:248–56. doi: 10.1016/s1385-299x(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 26.Andre P, Lotteau V, Klenerman P, Zinkernagel R, Groettrup M. US Patent No. 6506555. Use of HIV protease inhibiting compounds. 2003 inventors.
- 27.Samii A, Bickel U, Stroth U, Pardridge WM. Blood-brain barrier transport of neuropeptides: analysis with a metabolically stable dermorphin analogue. Am J Physiol. 1994;267:E124–31. doi: 10.1152/ajpendo.1994.267.1.E124. [DOI] [PubMed] [Google Scholar]
- 28.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233:51–9. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 29.Liang JF, Yang VC. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. 2005;335:734–8. doi: 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- 30.Dennison SR, Baker RD, Nicholl ID, Phoenix DA. Interactions of cell penetrating peptide Tat with model membranes: a biophysical study. Biochem Biophys Res Commun. 2007;363:178–82. doi: 10.1016/j.bbrc.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 31.Koch AM, Reynolds F, Merkle HP, Weissleder R, Josephson L. Transport of surface-modified nanoparticles through cell monolayers. Chembiochem. 2005;6:337–45. doi: 10.1002/cbic.200400174. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Wan L, Pooyan S, Su Y, Gardner CR, Leibowitz MJ, et al. Quantitative assessment of the cell penetrating properties of RI-Tat-9: evidence for a cell type-specific barrier at the plasma membrane of epithelial cells. Mol Pharm. 2004;1:145–55. doi: 10.1021/mp034014y. [DOI] [PubMed] [Google Scholar]
- 33.Lavie Y, Fiucci G, Czarny M, Liscovitch M. Changes in membrane microdomains and caveolae constituents in multidrug-resistant cancer cells. Lipids. 1999;34(Suppl):S57–63. doi: 10.1007/BF02562229. [DOI] [PubMed] [Google Scholar]
- 34.Santra S, Yang H, Stanley JT, Holloway PH, Moudgil BM, Walter G, et al. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem Commun (Camb) 2005:3144–6. doi: 10.1039/b503234b. [DOI] [PubMed] [Google Scholar]
- 35.Bazile DV, Ropert C, Huve P, Verrecchia T, Marlard M, Frydman A, et al. Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials. 1992;13:1093–102. doi: 10.1016/0142-9612(92)90142-b. [DOI] [PubMed] [Google Scholar]
- 36.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 37.Polyakov V, Sharma V, Dahlheimer JL, Pica CM, Luker GD, Piwnica-Worms D. Novel Tat-peptide chelates for direct transduction of technetium-99m and rhenium into human cells for imaging and radiotherapy. Bioconjug Chem. 2000;11:762–71. doi: 10.1021/bc000008y. [DOI] [PubMed] [Google Scholar]
- 38.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–8. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Pardridge WM. Pharmacokinetics and delivery of Tat and Tat-protein conjugates to tissues in vivo. Bioconjug Chem. 2001;12:995–9. doi: 10.1021/bc0155061. [DOI] [PubMed] [Google Scholar]
- 40.Kravcik S, Gallicano K, Roth V, Cassol S, Hawley-Foss N, Badley A, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–5. [PubMed] [Google Scholar]
- 41.Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, et al. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93:1231–41. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- 42.Dizhe EB, Ignatovich IA, Burov SV, Pohvoscheva AV, Akifiev BN, Efremov AM, et al. Complexes of DNA with cationic peptides: Conditions of formation and factors affecting internalization by mammalian cells. Biochemistry (Moscow) 2006;71:1350–56. doi: 10.1134/s0006297906120108. [DOI] [PubMed] [Google Scholar]
- 43.Notermans DW, Goudsmit J, Danner SA, de Wolf F, Perelson AS, Mittler J. Rate of HIV-1 decline following antiretroviral therapy is related to viral load at baseline and drug regimen. Aids. 1998;12:1483–90. doi: 10.1097/00002030-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Kaddoumi A, Choi SU, Kinman L, Whittington D, Tsai CC, Ho RJ, et al. Inhibition of P-glycoprotein activity at the primate blood-brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35:1459–62. doi: 10.1124/dmd.107.016220. [DOI] [PubMed] [Google Scholar]
- 45.Lu W, Zhang Y, Tan YZ, Hu KL, Jiang XG, Fu SK. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J Control Release. 2005;107:428–48. doi: 10.1016/j.jconrel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Finzi D, Siliciano RF. Taking aim at HIV replication. Nat Med. 2000;6:735–6. doi: 10.1038/77444. [DOI] [PubMed] [Google Scholar]
- 47.Tripathi S, Chaubey B, Ganguly S, Harris D, Casale RA, Pandey VN. Anti-HIV-1 activity of anti-TAR polyamide nucleic acid conjugated with various membrane transducing peptides. Nucleic Acids Res. 2005;33:4345–56. doi: 10.1093/nar/gki743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.