Abstract
Dopamine (DA) is produced in numerous brain areas and influences a wide variety of social behaviors, but very few data are available to establish the socially-relevant response properties of most DA populations, which comprise eight cell groups numbered A8-A15. Anatomically, these DA populations are evolutionarily conserved, and all have been identified in both birds and mammals. We now report the Fos responses of tyrosine hydroxylase-immunoreactive (TH-ir; putatively dopaminergic) neurons in the A8-A15 cell groups of male zebra finches following exposure to a control condition or one of six different social stimuli: A heterospecific male, conspecific male, fighting in a mate competition paradigm (which includes both male and female stimuli), a courtship interaction without physical contact, a courtship interaction with physical contact but no mounting, and a courtship interaction with mounting. We found that the DA cell groups exhibit distinctive profiles of responsiveness to social stimuli. Fos induction in A8, A9, A10 and midbrain A11 neurons increased significantly in response to a variety of conspecific stimuli, but not heterospecific stimuli. In contrast, Fos induction in the preoptic A14 neurons was observed specifically in response to sexual interactions, and Fos induction in hypothalamic A11 neurons appears to primarily reflect the performance of courtship singing. Infundibular A12 neurons, which may be involved in stress-related processes, showed the highest level of TH+Fos colocalization in control subjects. This colocalization decreased in response to all conspecific stimuli except fighting, and did not decrease following exposure to a heterospecific male.
Keywords: dopamine, forebrain, aggression, sexual behavior, tyrosine hydroxylase, Fos
Dopamine (DA) is known to influence a variety of social behaviors, particularly male sexual behavior, in multiple vertebrate groups (Warner et al., 1991; Pomerantz, 1992; Absil et al., 1994; Hull et al., 1995; Dominguez et al., 2001; Woolley et al., 2001; Melis et al., 2003; Charlier et al., 2005), and DA cells are distributed in homologous groups across the vertebrate classes (Bailhache and Balthazart, 1993; Bottjer, 1993; Gonzalez and Smeets, 1994; Reiner et al., 1994; Tillet, 1994; Appeltants et al., 2001; Adrio et al., 2002). This similarity across vertebrates is particularly clear when comparing mammals and birds, which each have dopaminergic neurons (immunopositive for tyrosine hydroxylase, TH, and immunonegative for dopamine β-hydroxylase) localized to eight populations of the basal forebrain and brainstem numbered A8 through A15 (Reiner et al., 1994; Tillet, 1994). However, it is largely unknown how these various DA populations respond to a broad range of social stimuli, so we have here examined DA neuronal responses to a variety of interactions with heterospecific and conspecific animals.
Extensive data suggest that the release of DA into the preoptic area (POA) controls male sexual behavior. Administrations of the DA agonist apomorphine into the medial POA facilitate sexual behavior in male rats (Hull et al., 1986; Dominguez et al., 2001), whereas injections of the DA antagonist cis-flupenthixol into the medial POA reduce ejaculations, copulation rate, erections and penile movements before copulation, and sexual motivation (Pehek et al., 1988; Warner et al., 1991). Extracellular DA in male rats is increased in the medial POA during copulation and exposure to a receptive female (Hull et al., 1995), and in the paraventricular nucleus of the hypothalamus during copulation (Melis et al., 2003). Additional DA effects on sexual behavior are likely exerted by the mesolimbic and nigrostriatal systems, which respectively influence motivation and motor functions (reviews: Hull et al., 2004; Dominguez and Hull, 2005).
Dopamine also influences copulatory behavior in a number of non-mammalian species. Intraperitoneal injections of DA receptor agonists into whiptail lizards (Cnemidophorus spp.) increase the number of mounts and decrease the latency to mount (Woolley et al., 2001). In male Japanese quail (Coturnix japonica), DA is involved in both appetitive and consummatory sexual behaviors, but these are differentially influenced by various agonists and antagonists, and differential behavioral effects are exerted via D1 and D2 receptors (Balthazart et al., 1997; Castagna et al., 1997).
In rats, the majority of dopaminergic input into the POA appears to arise from TH-ir neurons of the medial hypothalamus (e.g., A14 cell group) (see Dominguez and Hull, 2005 for a review). However, anatomical studies on this point are limited, and no systematic retrograde tracings have been conducted from the POA in conjunction with immunocytochemical labeling of dopaminergic neurons. To date, the only species for which such data are available is the Japanese quail (Balthazart and Absil, 1997). DA inputs to the POA in quail appear to arise from a variety of sources, mostly strongly from the ventromedial A14, but also from all midbrain groups (particularly the A9 and A10 populations). To date, however, it has not been experimentally confirmed that the A14 neurons are activated in response to copulation or any other social stimuli. In contrast, A11 neurons of the midbrain central gray (CG) and A10 neurons of the ventral tegmental area (VTA) are known to exhibit immediate early gene responses to sexual interactions in male Japanese quail (Charlier et al., 2005).
In addition to copulatory behavior, DA is known to influence other social behaviors in a variety of species. DA plays a major role in the maternal behavior of lactating rats (Giordano et al., 1990) and its actions in reward-related circuitry of the basal forebrain (e.g., within the ventral pallidum) are necessary for pair bond formation in monogamous prairie voles, Microtus orchrogaster (Aragona et al., 2003). DA agonists also facilitate defensive aggression in cats (Maeda et al., 1985). This effect is mediated at least in part by D2 receptors within the medial POA and anterior hypothalamus (Sweidan et al., 1991). In the weakly electric fish, Apteronotus leptorhynchus, DA injections modulate agonistic electrocommunicative chirping (Maler and Ellis, 1987), and in the Arctic charr (Salvelinus alpinus) oral administrations of L-DOPA (a DA precursor) increase social dominance (Winberg and Nilsson, 1992). Finally, in male zebra finches (Taeniopygia guttata), administrations of a DA antagonist during the period of song learning produce significant deficits in adulthood in courtship singing, courtship dancing, and copulatory behaviors (Harding, 2004).
These findings raise the question of which DA neuronal populations are involved in each form of behavior. Given that DA is produced and released in many behaviorally-relevant regions of the brain, it may the case that some DA neurons are involved in highly specific forms of behavioral regulation, whereas others exert more general modulatory influences. For instance, the A14 neurons of the POA and medial hypothalamus may selectively influence consummatory sexual behavior (i.e., copulation) whereas the A10 neurons of the ventral tegmentum, which play important roles in reward and incentive processes, may influence a wide variety of behaviors (Filipenko et al., 2001; Sharf et al., 2005). In the present experiments, we examined the expression of the immediate early gene protein Fos within TH-ir neurons of male zebra finches following a variety of sociosexual interactions, thereby providing an extensive framework for understanding the role that DA plays in the regulation of socially motivated behavior.
Experimental Procedures
Animals and housing
Thirty-five adult male zebra finches (Taeniopygia guttata) were used in the present experiments. All subjects were housed with same-sex conspecifics in wire cages 61 × 36 × 41 cm on a 14L:10D photoperiod. Finch seed mix and water were provided ad lib. All subjects were housed such that they could see and hear females across the room (approximately 2 m). Housing, testing and euthanization procedures were designed to minimize subject stress and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee.
Hormonal treatment of female stimuli
In order to maintain female sexual receptivity throughout behavioral testing, female stimulus animals were implanted with a 4 mm silastic tubule (1.96 mm outer diameter, 1.47 mm inner diameter) tightly packed with estradiol benzoate (Sigma Chemical Co., St. Louis, MO). The implant was placed subcutaneously along the lateral edge of the breast.
Behavioral testing conditions
During the afternoon on the day before the behavioral test and perfusions, subjects were individually moved into test cages 36 × 33 × 17 cm in a quiet room. The following morning subjects were exposed to one of the six experimental conditions as described below. For all conditions, lights were turned off, food dishes were removed and the stimulus bird(s) was placed in the cage with subject. Lights were turned back on and the experimenter observed the subject through a small window in a plastic curtain for 10 min. During observation, behaviors relevant for each condition were recorded, including number of mounts, number of attempted mounts, number of directed songs, and number of aggressive displacements. After 10 minutes, the room lights were turned off, the stimulus bird(s) was removed, food dishes were put back in the cage, the lights were turned back on, and the subject was left in testing cage until perfusion 90 min after initiation of behavioral testing.
Treatment groups were as follows:
Group 1 (CON): No stimulus control (n=4).
Group 2 (BCW): Heterospecific male stimulus (black-cheeked waxbill, Estrildae erythronotus; n=4).
Group 3 (MALE): Conspecific male stimulus (n=5).
Group 4 (FIGHT): Competition paradigm; conspecific male and female stimuli (paradigm described below; n=5).
Group 5 (COURT): Conspecific female, courting but no successful mounting (n=5).
Group 6 (MOUNT): Conspecific female, successful mounting observed (n=5).
Behavior of female stimuli was not quantified. However, a common pool of female stimuli was employed for FIGHT, COURT and MOUNT subjects, with any given stimulus being employed only once per day. Three of the females were mounted by MOUNT subjects, but not by COURT subjects, suggesting that differences in stimulus behavior likely did not produce group differences in TH+Fos double-labeling.
Given that subjects were self-selected into the MOUNT group (i.e., if they did not mount, they were included in the COURT group), any Fos differences between COURT and MOUNT groups may reflect a difference in sexual motivation. Thus, in order to further establish whether TH+Fos double-labeling reflects appetitive sexual motivation, we added a “directed song only” group (DS; n=7) in which males could sing to a female through a wire barrier, but could not physically interact and mount. For logistical purposes, it was necessary to test all DS subjects in a single morning, which was accomplished as follows: Subjects were housed together overnight in a quiet room, and lights remained off in this room in the morning to reduce nonspecific brain activation. Subjects were transferred individually to a testing cage as described above and allowed to sing to a female for 10 min. After testing, the DS male was placed back into the dark room with other DS males until perfusion. Note that these data were collected only for the purpose of correlating song with TH+Fos double-labeling, and were not statistically compared to data from the other treatment groups.
Competition paradigm (FIGHT condition)
Zebra finches are a colonial species and do not display aggressive behavior in standard resident-intruder tests. However, if housed in sexual isolation, a pair of same-sex individuals can be induced to fight by allowing them to simultaneously court an individual of the opposite sex (“competition paradigm”; Adkins-Regan and Robinson, 1993). Although some amount of aggression is elicited from most subjects in this paradigm, the aggressive behavior is often not exceptionally robust. Thus, in order to ensure that the subjects in the FIGHT group exhibited robust fight behavior, we initially screened all subjects for aggressive behavior using the competition paradigm for 5 min in the home cages, not in experimental cages. All remaining subjects were distributed randomly across groups. During the actual behavioral test, the number of displacements was counted, allowing us to correlate aggressive behavior with neural responses.
Tissue processing
Ninety minutes after testing began, each subject was deeply anesthetized with isoflurane vapor and intracardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde. Brains were removed, postfixed overnight, sunk in 30% sucrose and then frozen in mounting medium at -80°C until time of sectioning. Brains were sectioned on a cryostat into three series of 40 μm sections. Free-floating sections were collected into section cryoprotectant (FD NeuroTechnologies, Baltimore, MD) and stored at -80°C until brains from all subjects had been sectioned.
One of the three series was used for immunocytochemical labeling, which was performed as follows: Six 10-min rinses in PBS, 20 min in 10 mM sodium citrate (pH 9.5; well plates placed into a shallow water bath heated to 70°C), two 10-min rinses in PBS, and 1 hr in PBS + 5.0% bovine serum albumin (BSA) + 0.3% Triton-X. Tissue was incubated for 40-48 hours at 4°C in sheep anti-TH (Novus Biologicals, Littleton, CO) and rabbit anti-Fos (Santa Cruz Biotechnology, Santa Cruz, CA), each diluted 1:1000 in PBS + 2.5% BSA + 0.3% Triton-X + 0.05% sodium azide. This was followed by two 30-min rinses in PBS, 2 hr in donkey anti-rabbit secondary conjugated to Alexa Fluor 594 (5μl/ml) + donkey anti-sheep conjugated to Alexa Fluor 488 (3μl/ml) in PBS + 2.5% BSA + 0.3% Triton-X. Alexa Fluors were purchased from Molecular Probes (now Invitrogen, Eugene, OR). Sections were then rinsed extensively in PBS and transferred to PB before mounting. Tissue was mounted on chrom-alum subbed slides and coverslipped with SlowFade Light containing DAPI nuclear stain (Molecular Probes).
All tissue was immunocytochemically labeled in four large runs using common antibody lots. Representatives of each exerimental group were included in each run, thereby eliminating the possibility that group differences would be generated based upon differences in immunocytochemical runs.
Data Analysis
TH+Fos double-labeling was quantified within the eight dopaminergic cell groups A8-A15 as shown in Figure 1. These cell groups correspond to those identified by Reiner et al. (1994) for the pigeon (Columba livia) and by Appeltants et al. (2001) for the canary (Serinus canaria; A12 neurons not reported) and exhibit a similar topography to identically numbered, homologous cell groups in mammals. These groups are: A8, in the rostral locus coeruleus (this area may correspond to the retrorubral nucleus in mammals, but while the homology of the A8 group is established, retrorubral homology is not; see Reiner et al., 2004); A9, in the substantia nigra; A10, in the VTA; A11, in the caudal periventricular hypothalamus and midbrain CG; A12, in the tuberoinfundibular hypothalamus (arcuate nucleus of the hypothalamus in mammals); A13, in the zona incerta; A14, in the medial hypothalamus and POA; and A15, in the lateral regions of the hypothalamus.
Fig. 1.
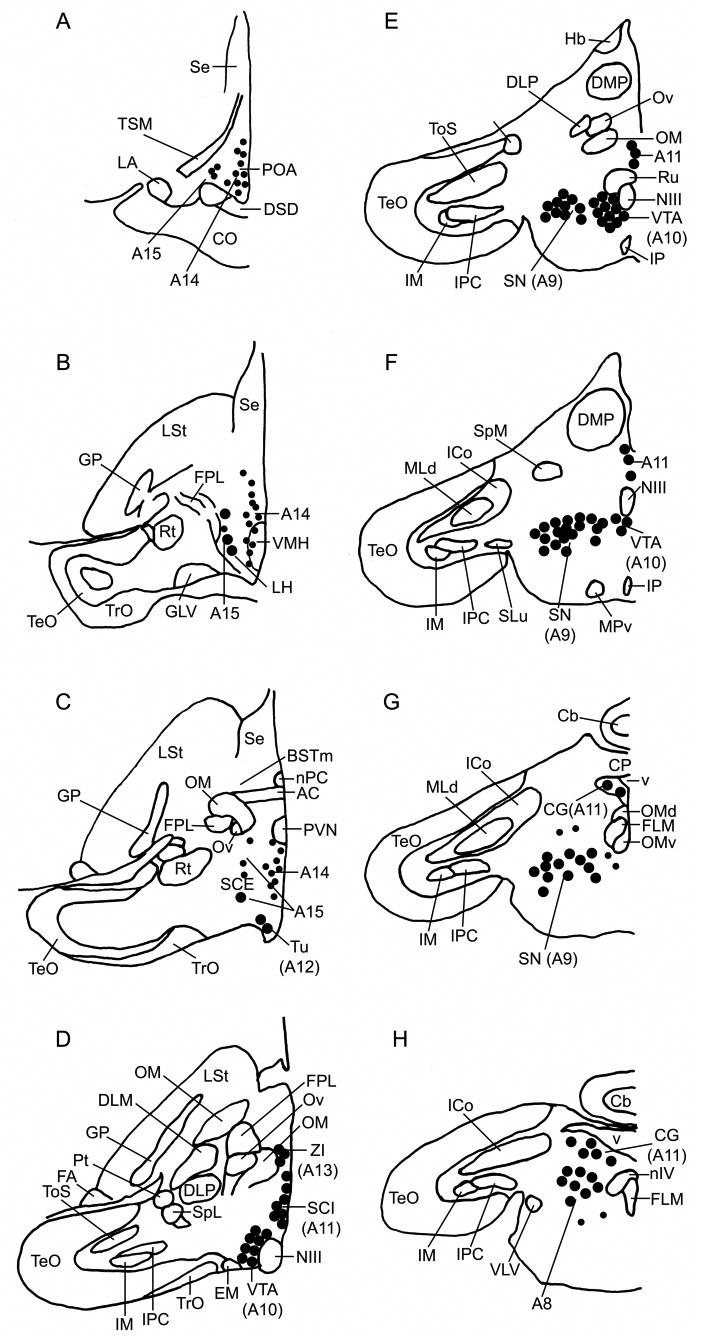
Distribution of A8-A15 TH-ir neurons (putatively dopaminergic) in the zebra finch brain. Small dots represent single neurons and large dots represent five neurons. The drawings are adapted from Mello et al. (1998). Panels A-H correspond respectively to planes A2.6, A2.2, A1.6, A0.8, A0.4, A0.2, P0.6 and P0.2 of the canary atlas (Stokes et al., 1974).
We took bilateral photomicrographs for analyses at six rostrocaudal levels of the A11 cell group (two hypothalamic and four in the midbrain CG), five levels of the A10 cell group, four levels of the A14 cell group (two preoptic A14 and two hypothalamic A14), three levels of the A9 cell group, two levels of the A12 and A15 cell groups, and one level of the A13 cell group. Photomicrographs were taken at 10× using a Zeiss Axioscop microscope and an Optronics Magnafire digital camera connected to a Macintosh G4 computer. Separate monochrome images were obtained for TH-ir and Fos-ir labeling in each area of interest. The number of TH-ir neurons and the number of TH-ir neurons that exhibited Fos-ir nuclei were counted for each image in Adobe Photoshop 7 by overlaying images of TH and Fos. Representative TH and TH+Fos double-labeling is presented in Figure 2.
Fig. 2.
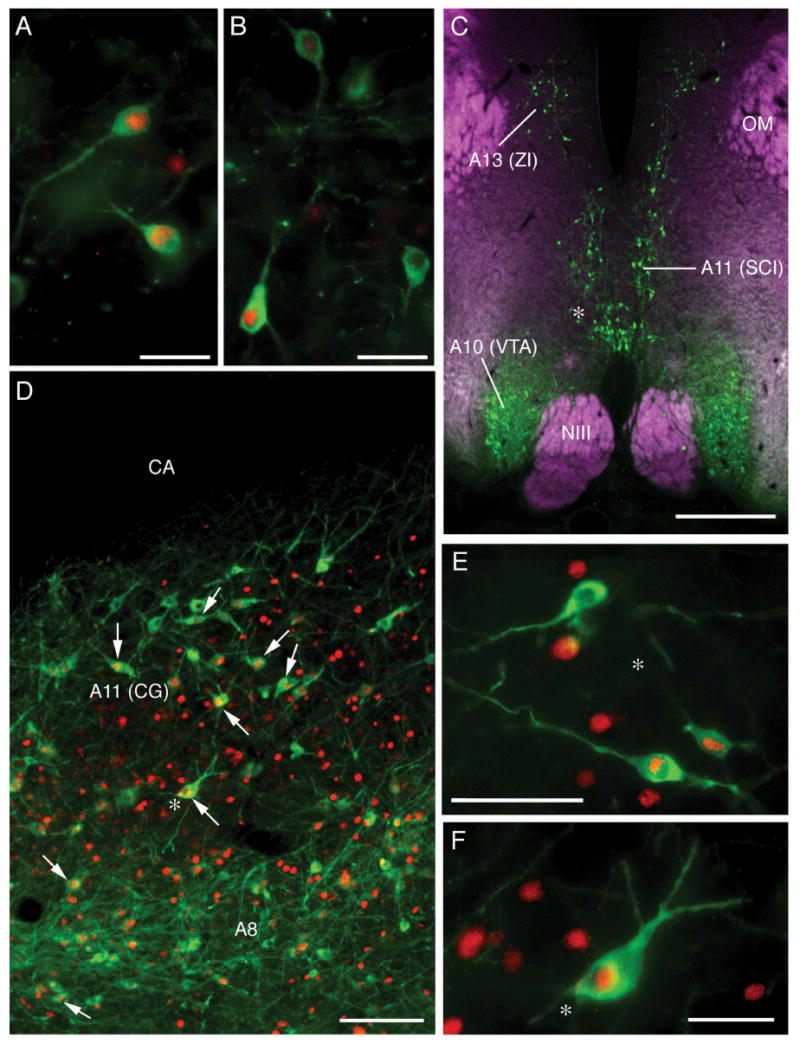
Immunocytochemical labeling of Fos-ir nuclei (red) and/or TH-ir neurons (green). (A) TH+Fos double-labeling of preoptic A14 neurons in a MOUNT subject. (C) Distribution of TH-ir neurons in the caudal diencephalon (A13 and rostral A11 subpopulation) and ventral tegmental area (VTA; A12) of a COURT subject. DAPI counterstain (quenched) is shown psuedocolored purple. The asterisk corresponds to the location of double-labeled neurons shown in panel E. (D) TH- and Fos-ir neurons in the caudal A11 subpopulation (midbrain central gray; CG) and A8 neurons in the rostral locus coeruleus of a COURT subject. The asterisk corresponds to the location of double-labeled neurons shown in panel F. Scale bars = 25 μm in panels A and B; 500 μm in panel C; 100 μm in panel D; and 50 μm in panels E and F.
Data were analyzed using unpaired t-tests (for two-group comparisons of subjects that were engaged in sexual behavior versus those that were not; see Results) and ANOVAs followed by pairwise comparisons with Fisher's PLSD (for comparisons of the six treatment groups, excluding the DS group). The data for the DS group were analyzed by correlating the number of directed songs with TH+Fos double-labeling. For all analyses, the dependent measure examined was the percent of TH-ir neurons that were double-labeled for Fos. Conservative corrections for multiple comparisons (e.g., Bonferroni) were not employed given the relatively low power of some analyses. Although this increases the possibility of false positives, the patterns of results were nonetheless consistent in cases where consistency would be expected (e.g., between CON and BCW control groups, and between COURT and MOUNT subjects).
Results
Table 1 presents absolute numbers of TH-ir and TH+Fos double-labeled neurons in the eight dopaminergic cell groups, and significant ANOVA results (based on percent of TH-ir neurons double-labeled with Fos) are shown in Figure 3. The absolute numbers of A15 TH-ir neurons shown in Table 1 differed significantly between groups (F5,22 = 4.193, P < 0.01), likely due to chance, but this should not influence the results reported below. Significant Fos responses were obtained for all dopaminergic cell groups except the A13 (F5,22 = 0.572, P > 0.10), caudal (hypothalamic) A14 (F5,22 = 0.604, P > 0.10) and A15 (F5,22 = 1.215, P > 0.10). The preoptic A14 neurons exhibited a trend toward group differences (F5,22 = 2.236, P = 0.08), with the highest level of TH+Fos colocalization being observed in the MOUNT group (Fig. 3H).
Table 1.
Numbers of TH-ir and TH-ir + Fos-ir cells (first and second line respectively for each cell group; all means ± SEM) in dopaminergic cell groups of male zebra finches exposed to various sociosexual stimuli. See Fig. 3 for group abbreviations and statistical analyses.
| Cell group
(#sections) |
CON | BCW | MALE | FIGHT | COURT | MOUNT |
|---|---|---|---|---|---|---|
| A8 (2) | 158.3 ± 35.8,
6.3 ± 0.9 |
129.8 ± 23.4,
4.5 ± 1.0 |
200.4 ± 14.5,
15.0 ± 2.2 |
131.2 ± 24.3,
18.8 ± 1.7 |
194.0 ± 26.5,
22.2 ± 1.9 |
139.6 ± 4.7,
12.8 ± 4.7 |
| A9 (3) | 385.0 ± 28.3,
0.0 ± 0.0 |
436.5 ± 24.7,
3.3 ± 2.0 |
455.0 ± 10.6,
1.0 ± 0.3 |
382.8 ± 40.0,
7.2 ± 1.2 |
437.4 ± 23.2,
4.0 ± 1.0 |
373.0 ±25.5,
4.2 ± 1.7 |
| A10 (5) | 387.8 ± 30.5,
3.0 ± 1.1 |
407.0 ± 42.9,
5.3 ± 1.5 |
399.4 ± 24.4,
7.2 ± 2.6 |
382.0 ± 47.5,
25.6 ± 6.0 |
467.0 ± 27.0,
15.0 ± 2.1 |
449.8 ± 58.4,
16.8 ± 3.3 |
| A11-CG (4) | 90.0 ± 15.1,
11.3 ± 3.3 |
94.7 ± 34.7,
7.0 ± 2.0 |
131.6 ± 11.5,
21.2 ± 2.4 |
106.2 ± 28.4,
28.6 ± 4.9 |
173.6 ± 19.1,
41.8 ± 11.1 |
137.8 ± 22.6,
29.6 ± 6.0 |
| A11-Hyp (2) | 88.3 ± 17.1,
7.0 ± 2.5 |
116.0 ± 24.0,
7.0 ± 3.1 |
111.8 ± 9.6,
6.6 ± 1.5 |
89.6 ± 14.0,
8.8 ± 2.3 |
119.4 ± 12.9,
16.0 ± 2.0 |
92.4 ± 9.8,
9.0 ± 3.3 |
| A12 (2) | 38.0 ± 13.4,
5.5 ± 2.0 |
49.5 ± 13.4,
9.5 ± 6.3 |
40.2 ± 7.5,
3.0 ± 0.6 |
65.4 ± 19.9,
6.8 ± 2.0 |
43.8 ± 9.9,
2.4 ± 1.2 |
23.4 ± 10.5,
0.4 ± 0.2 |
| A13 (1) | 30.8 ± 6.8,
1.5 ± 1.2 |
42.3 ± 10.15,
0.8 ± 0.5 |
29.2 ± 4.2,
0.2 ± 0.2 |
25.6 ± 1.0,
1.0 ± 0.8 |
35.6 ± 4.4,
1.8 ± 0.9 |
29.0 ± 4.5,
1.0 ± 0.5 |
| A14-Hyp (2) | 29.0 ± 6.9,
2.0 ± 0.7 |
43.8 ± 11.8,
2.8 ± 1.4 |
44.8 ± 7.5,
4.0 ± 1.0 |
36.4 ± 6.7,
2.0 ± 0.5 |
32.2 ± 4.1,
3.6 ± 1.4 |
35.0 ± 11.0,
3.0 ± 1.2 |
| A14-POA (2) | 23.8 ± 7.0,
0.1 ± 0.1 |
46.5 ± 8.0,
1.0 ± 0.4 |
52.4 ± 7.4,
0.8 ± 0.4 |
29.0 ± 4.6,
0.6 ± 0.2 |
37.8 ± 9.8,
1.3 ± 0.4 |
48.0 ± 7.6,
2.2 ± 0.6 |
| A15 (2) | 35.5 ± 7.6,
2.5 ± 1.0 |
73.0 ± 6.4,
4.5 ± 1.2 |
76.6 ± 5.6,
6.8 ± 4.0 |
46.6 ± 11.2,
2.8 ± 0.7 |
62.6 ± 5.5,
9.6 ± 4.0 |
51.6 ± 6.9,
2.0 ± 0.6 |
Fig. 3.
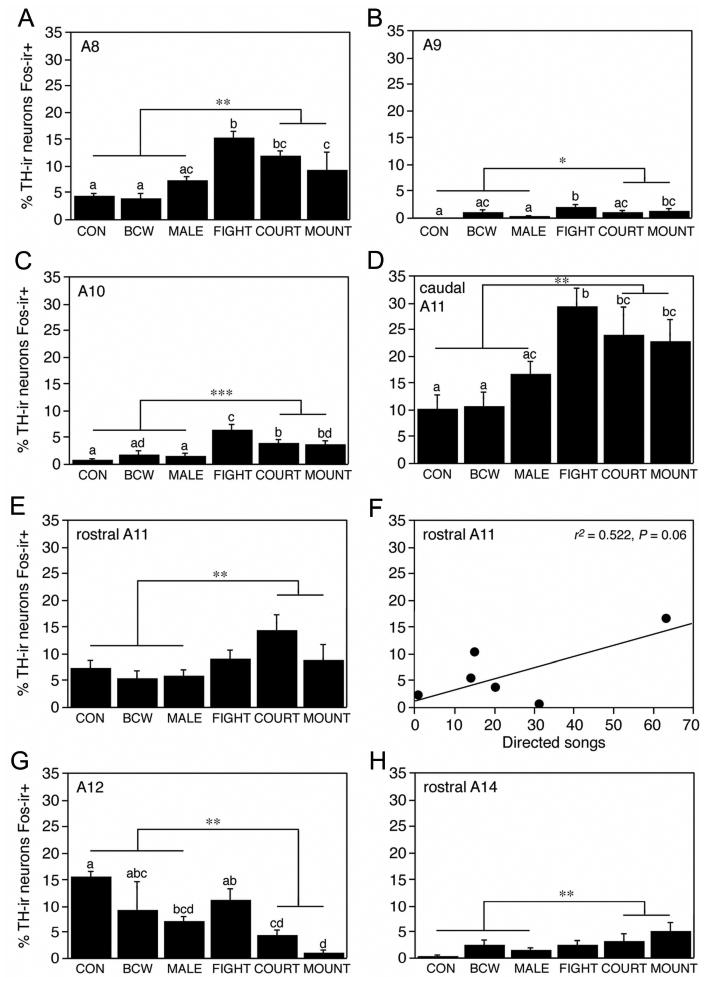
Percentage of TH-ir neurons that are Fos-ir+ (means ± SEM) following various sociosexual interactions in male zebra finches in the (A) A8 population of the rostral locus coeruleus, (B) A9 population of the substantia nigra, (C) A10 population of the ventral tegmental area, (D) A11 subpopulation of the central gray, (E-F) A11 subpopulation of the caudal hypothalamus, (G) A12 population of the tuberoinfundibular hypothalamus, and (H) rostral A14 subpopulation of the preoptic area. Different letters above the error bars denote significant group differences (Fisher's PLSD; P < 0.05) following significant one-way ANOVA. ANOVA results for the rostral A11 subpopulation (D) approach significance (ANOVA F5,22 = 2.531, P < 0.06). Asterisks denote significant pairwise comparisons between subjects that were engaged in sexual behavior (COURT and MOUNT) versus those that were not (CON, BCW, and MALE; *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t-tests). Groups are as follows: control (CON), heterospecific male stimulus (black-cheeked waxbill; BCW), male stimulus (MALE); mate competition (FIGHT), courtship with no mounting (COURT), and courtship with successful mounting (MOUNT). A separate group of subjects were allowed to court a female through a wire barrier, yielding the correlation between directed songs and double-labeling shown in panel F.
The remaining cell groups exhibited a diverse pattern of responses. Exposure to the heterospecific stimulus (BCW) did not produce a significant Fos response in any of the TH-ir cell groups, and exposure to a male stimulus (MALE) produced a significant effect on TH+Fos colocalization only in the A12 cell group (Fig. 3G). Overall, the Fos responses of the A12 neurons were distinct in that MALE, COURT and MOUNT subjects exhibited a decrease in TH+Fos colocalization relative to CON subjects (Fisher's PLSD; P < 0.05 following significant one-way ANOVA; F5,22 = 4.714, P < 0.005). This decrease was not observed for FIGHT subjects.
Significant increases in TH+Fos colocalization over CON males were observed for the FIGHT, COURT, and MOUNT subjects within the A8 (F5,22 = 6.263, P < 0.0001), A9 (F5,22 = 6.263, P < 0.0001), A10 (F5,22 = 6.263, P < 0.0001) and caudal A11 (F5,22 = 6.263, P < 0.0001) populations, as shown in Figure 3 (panels A-D, respectively). These three subject groups did not always exhibit comparable responses, however: FIGHT subjects exhibited significantly greater TH+Fos colocalization than did COURT and/or MOUNT subjects within the A8, A9 and A10 populations, but not the caudal A11 population.
A somewhat different pattern of results was obtained for the rostral A11 neurons of the caudal hypothalamus (Fig. 3E). Group differences for the rostral A11 approach significance (ANOVA F5,22 = 2.531, P < 0.06) and posthoc pairwise comparisons suggest that TH+Fos colocalization in the COURT group differs significantly (Fisher's PLSD P < 0.05) or near-significantly (P < 0.07) from all other groups. This pattern of trends is supported by a similarly near-significant correlation between directed song and TH+Fos colocalization in the subjects that were allowed to court females only through a wire barrier (F1,6 = 5.459, r2 = 0.522, P = 0.06; Fig. 3F).
Other correlations between TH+Fos colocalization and behavior (directed song or aggressive diplacements in the FIGHT group) did not reach or approach significance (P > 0.10; data not shown), with the exception of the correlation between aggressive displacements and double-labeling in the A8 population (F1,6 = 5.459, r2 = 0.725, P = 0.06).
One of the primary goals of the present study was to determine which dopaminergic cell groups are activated during sexual behavior. Thus, in order to maximize the statistical power of our analyses, we compared all subjects that expressed sexual behavior (subjects from the COURT and MOUNT groups) with those that did not (subjects from the CON, BCW and MALE groups) using unpaired t-tests. Subjects from the FIGHT condition were omitted (conservatively) to eliminate the contribution of aggressive behavior to the results. As shown in Figure 3, sexual behavior was accompanied by significant increases in TH+Fos colocalization within the A8, A9, A10, caudal A11 (CG), rostral A11 (hypothalamic) and rostral A14 (preoptic) cell groups (all P < 0.05). In contrast, sexual behavior was accompanied by a decrease in colocalization within the A12 cell group. With the exception of the findings for the rostral A14, these significant results were corroborated by ANOVA results, as presented above and in Figure 3.
Discussion
Although DA is known to influence a wide range of sociosexual behaviors, information on the involvement of specific DA neuronal populations remains limited. To date, the majority of relevant findings pertain to sexual behavior, and some cell groups have received much more attention than have others (e.g., the A10 neurons of the VTA are particularly well-studied). Thus, there remain many unanswered questions about 1) the range of sociosexual processes that the various DA populations may be involved in, and 2) whether some poorly studied DA cell groups are involved in sociosexual behaviors at all. The present experiments were designed to address these questions, and they yielded a variety of unexpected and novel results, in addition to confirming previous findings in mammals and/or birds.
Previous research has demonstrated that DA is released into the POA during copulation in male rodents (Hull et al., 1995) and this DA release is necessary for copulatory performance. DA also influences copulation in male birds (Castagna et al., 1997). Given that the strongest DA input to the POA arises in the A14 cell group of the medial hypothalamus, the prevailing hypothesis has been that the preoptic DA release observed during sexual behavior arises from A14 neurons. Weakly consistent with this view, we here observed a significant elevation in TH+Fos colocalization within the preoptic A14 neurons following sexual interactions. However, this effect was very modest, not specific to mounting, and was confined to the A14 subpopulation of the POA. More caudal A14 neurons of the hypothalamus showed no significant response to any sociosexual stimulus at all.
In addition to the A14 input, all midbrain DA populations are also known to project to the medial POA, at least in quail where this issue has been most thoroughly examined (Balthazart and Absil, 1997). These projections include approximately equivalent contributions from the A8 and A10 cell groups, a somewhat lighter projection from the A11 cell group, and a small projection from the A9 neurons. Our present findings demonstrate that sexual interactions induce a stronger Fos response in these other TH-ir populations than in the A14 neurons, and produce an exceptionally robust response in the caudal A11 neurons of the CG. The rostral A11 neurons of the caudal hypothalamus also exhibited a significant response to sexual interactions, and the pattern of results strongly suggests that these neurons may be involved in courtship singing.
To our knowledge, the hypothalamic A11 neurons have not been examined in previous functional investigations, but the CG A11 neurons are known to project to the telencephalic song circuit (Appletants et al., 2000; also see Appletants et al., 2001). The CG is postulated to serve as an interface between the POA and song circuitry (Riters and Alger, 2004; review: Goodson et al., 2005), and testosterone-induced singing in female canaries is also associated with an increased TH-ir innervation of the song system (Appletants et al., 2003).
Partially consistent with our present results, Charlier et al. (2005) report a significant elevation in TH+Fos colocalization in the midbrain A11 and A10 populations following sexual interactions in male quail. However, these authors do not report a significant Fos induction within the other cell groups that we here demonstrate to be responsive to sexual stimuli (A8, A9, rostral A11 and preoptic A14). These differences may reflect the different histochemical methods employed. Fos induction within A10 neurons has also been observed following copulation in rats (Balfour et al., 2004).
Although we found evidence for widespread activation of DA cell groups following sexual behavior, we found no evidence that any DA population is specifically activated in response to copulation. In fact, only in the A9 and A10 populations did the MOUNT subjects exhibit significantly greater TH+Fos colocalization than subjects that were exposed only to another male. However, we did obtain evidence that a subset of the A10 DA neurons of the VTA is selectively responsive to fighting. Thus, while a significant increase in TH-Fos colocalization within the A10 neurons was observed for COURT and MOUNT subjects (above control levels), FIGHT subjects exhibited an even greater response. This finding should be interpreted cautiously, however, because the fighting in our tests was induced by mate competition, and may therefore be partly confounded with sexual motivation. Further studies in a territorial species would help to resolve this issue (e.g., using a resident-intruder paradigm).
A variety of other evidence demonstrates a role for DA in aggression, including data from lizards (Matter et al., 1998) and mammals. In mammals, the A10 neurons in particular are known to influence agonistic behavior. Mesolimbic dopamine release in male rats increases substantially in relation to aggression and in anticipation of an aggressive interaction (Ferrari et al., 2003). In mice, TH mRNA and dopamine transporter mRNA both increase significantly within the VTA of males who repeatedly win aggressive encounters (Filipenko et al., 2001), and septal DA, which arises primarily in the VTA (Lindvall, 1975), influences aggression in rats (Clarke and File, 1982). Simulated territorial intrusions also increase the number of Fos-ir nuclei in the CG and VTA of male song sparrows (Melospiza melodia; Maney and Ball, 2003), suggesting a possible role for dopamine in the regulation of naturalistic territorial behavior.
The findings discussed above all point towards an increase in DA cellular activity following sociosexual interactions, and in studies with Fos, this is evidenced by an increase in TH+Fos colocalization (e.g., Balfour et al., 2004; Charlier et al., 2005; present study). The present finding that TH+Fos colocalization significantly decreases following sociosexual interactions in the tuberinfundibular A12 neurons is therefore particularly intriguing. Behavioral properties of the A12 cell group have not been studied in birds. However, findings in mammals suggest that these neurons are responsive to a variety of stressors and regulate prolactin secretion (e.g., Hollis et al., 2005). Footshock stress in ewes elicits dopamine release within the arcuate nucleus (which contains the A12 neurons), although long-term stress exposure leads to desensitization and a reduction from baseline levels (Tomaszewska and Przekop, 1999). TH+Fos double-labeling in the A12 neurons is increased in response to both physiological and immobilization stressors in mice (Pirnik and Kiss, 2005), and a subpopulation of these neurons also exhibit increased TH+Fos double-labeling following an immune challenge (Hollis et al., 2005). Although the responses of these neurons have not been examined in relation to social stressors, our present data are consistent with a role for these neurons in stress-related functions, given that the social isolation of the testing environment may have been stressful for a highly gregarious species such as the zebra finch. Thus, levels of TH+Fos double-labeling were relatively high in control subjects and decreased significantly following all interactions with other zebra finches except fighting (which may have been stressful). These combined observations demonstrate that the activity of the A12 neurons is highly sensitive to sociosexual stimuli, but the exact nature and function of these effects remains to be determined. Perhaps the most likely scenario is that these neurons provide a mechanism whereby sociosexual stimuli can influence prolactin secretion.
In summary, we have here confirmed the previously described response of A10 and CG A11 neurons to sexual interactions in males (Balfour et al., 2004; Charlier et al., 2005), but have also demonstrated that several other DA cell groups exhibit similar responses. These include the midbrain A8 and A9 cell groups, rostral A11 neurons of the hypothalamus, and preoptic A14 neurons. Previous hypotheses have focused largely on hypothalamic A14 neurons as being potentially important sources of the preoptic dopamine release that influences male sexual behavior (e.g., Dominguez and Hull, 2005), but we found that only the rostral (preoptic) subpopulation of A14 neurons showed a response to sexual interactions, not the hypothalamic subpopulation. This response was very weak (in terms of absolute numbers of double-labeled TH-ir neurons) compared to that observed in the hypothalamic A11 neurons and midbrain cell groups, although sexual interactions were accompanied by an approximately three-fold increase in TH+Fos colocalization within the rostral A14 over control levels, a percentage increase that is on par with the various midbrain groups. We additionally observed strong trends that suggest the hypothesis that hypothalamic A11 neurons may play a somewhat selective role in courtship behavior. Several of the midbrain cell groups may also contribute to aggression. In particular, a subset of A10 neurons appear to be selectively responsive to agonistic encounters, given that fighting in a mate competition paradigm induced a significant elevation in TH+Fos colocalization above the level observed in COURT and MOUNT subjects. Finally, we obtained a striking pattern of data for the tuberoinfundibular A12 neurons, demonstrating that sociosexual stimuli depress the Fos activity in this cell group.
Acknowledgments
This work was supported by NIH MH62656 to J.L.G. We thank Katrina Gonzaga, Mark Kurai, Hera Patail, and Yiwei Wang for assistance with tissue processing and/or data analysis.
Abbreviations
- AC
anterior commissure
- BSTm
medial bed nucleus of the stria terminalis
- Cb
cerebellum
- CG
midbrain central gray
- CO
optic chiasm
- CP
posterior commissure
- DA
dopamine
- DLM
medial dorsolateral nucleus of the anterior thalamus
- DLP
dorsolateral posterior nucleus
- DMP
posterior nucleus of the dorsomedial thalamus
- DSD
dorsal supraoptic decussation
- EM
nucleus ectomammillaris
- FA
frontal archo-pallial tract
- FPL
lateral forebrain bundle
- GLV
ventral lateral geniculate nucleus
- GP
globus pallidus
- Hb
habenula
- ICo
nucleus intercollicularis
- IM
nucleus isthmi, pars magnocellularis
- IP
interpeduncular nucleus
- IPC
nucleus isthmi, pars parvocellularis
- LA
lateral anterior nucleus
- LH
lateral hypothalamus
- LSt
lateral striatum
- MLd
nucleus mesencephalicus lateralis, pars dorsalis
- MPv
nucleus mesencephalicus profundus ventralis
- NIII
oculomotor nerve
- nIV
nucleus of the trochlear nerve
- nPC
nucleus of the pallial commissure
- OM
occipitomesencephalic tract
- OMd
oculomotor nucleus, dorsal part
- OMv
oculomotor nucleus, ventral part
- Ov
nucleus ovoidalis
- PBS
phosphate buffered saline
- POA
preoptic area
- Pt
pretectal nucleus
- PVN
paraventricular nucleus
- Rt
nucleus rotundus
- Ru
nucleus ruber
- SCE
stratum cellulare internum
- SCI
stratum cellulare internum
- Se
septum
- SLu
nucleus semilunaris
- SN
substantia nigra
- TeO
optic tectum
- TH
tyrosine hydroxylase
- ToS
torus semicircularis
- TrO
optic tract
- TSM
septomesencephalic tract
- Tu
tuberoinfundibular hypothalamus
- VLV
ventral nucleus of the lateral lemniscus
- VMH
ventromedial hypothalamus
- VTA
ventral tegmentum
- ZI
zona incerta
References
- Absil P, Das S, Balthazart J. Effects of apomorphine on sexual behavior in male quail. Pharmacol Biochem Behav. 1994;47:77–88. doi: 10.1016/0091-3057(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Robinson TM. Sex differences in aggressive behavior in zebra finches (Poephila guttata) J Comp Psychol. 1993;107:223–229. [Google Scholar]
- Adrio F, Anadon R, Rodriguez-Moldes I. Distribution of tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH) immunoreactivity in the central nervous system of two chondrostean fishes (Acipenser baeri and Huso huso) J Comp Neurol. 2002;448:280–297. doi: 10.1002/cne.10256. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis TJ, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailhache T, Balthazart J. The catecholaminergic system of the quail brain: immunocytochemical studies of dopamine β-hydroxylase and tyrosine hydroxylase. J Comp Neurol. 1993;329:230–256. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 Dopamine-receptor agonist and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese Quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Castagna C, Ball GF, Balthazart J. Effects of dopamine agonists on appetitive and consummatory male sexual behavior in Japanese quail. Pharmacol Biochem Behav. 1997;58:403–414. doi: 10.1016/s0091-3057(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early gene c-fos and Zenk (EGR-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviours. Pharmacol Biochem Behav. 1982;17:623–628. doi: 10.1016/0091-3057(82)90334-3. [DOI] [PubMed] [Google Scholar]
- Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J Neurosci. 2001;21:349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Filipenko ML, Alekseyenko OV, Beilina AG, Kamynina TP, Kudryavtseva NN. Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Brain Res Mol Brain Res. 2001;96:77–81. doi: 10.1016/s0169-328x(01)00270-4. [DOI] [PubMed] [Google Scholar]
- Giordano AL, Johnson AE, Rosenblatt JS. Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav. 1990;48:211–214. doi: 10.1016/0031-9384(90)90288-f. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJAJ. Distribution of tyrosine hydroxylase immunoreactivity in the brain of Typhlonectes compressicauda (Amphibia, Gymnophiona): Further assessment of primitive and derived traits of amphibian catecholamine systems. J Chem Neuroanat. 1994;8:19–32. doi: 10.1016/0891-0618(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: insights from studies on birds. Horm Behav. 2005;48:461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CF. Brief alteration in dopaminergic function during development causes deficits in adult reproductive behavior. J Neurobiol. 2004;61:301–308. doi: 10.1002/neu.20039. [DOI] [PubMed] [Google Scholar]
- Hollis JH, Lightman SL, Lowry CA. Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol. 2005;184:393–406. doi: 10.1677/joe.1.05839. [DOI] [PubMed] [Google Scholar]
- Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res. 1986;370:73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Messencephalic dopaminergic afferents to the lateral septal nucleus of the rat. Brain Res. 1975;87:89–95. doi: 10.1016/0006-8993(75)90785-4. [DOI] [PubMed] [Google Scholar]
- Maeda H, Sato T, Maki S. Effects of dopamine agonists on hypothalamic defensive attack in cats. Physiol Behav. 1985;35:89–92. doi: 10.1016/0031-9384(85)90176-3. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Matter JM, Ronan PJ, Summers CH. Central monoamines in free-ranging lizards: Differences associated with social roles and territoriality. Brain Behav Evol. 1998;51:23–32. doi: 10.1159/000006526. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mascia MS, Cortis L, Argiolas A. Extra-cellular dopamine increases in the paraventricular nucleus of male rats during sexual activity. Eur J Neurosci. 2003;17:1266–1272. doi: 10.1046/j.1460-9568.2003.02558.x. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: Immunocytochemical study of dopamine-β-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Pehek EA, Warner RK, Bazzett TJ, Bitran D, Band LC, Eaton RC, Hull EM. Microinjection of cis-flupenthixol, a dopamine antagonist, into the medial preoptic area impairs sexual behavior of male rats. Brain Res. 1988;443:70–76. doi: 10.1016/0006-8993(88)91599-5. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Kiss A. Fos expression variances in mouse hypothalamus upon physical and osmotic stimuli: co-staining with vasopressin, oxytocin, and tyrosine hydroxylase. Brain Res Bull. 2005;65:423–431. doi: 10.1016/j.brainresbull.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Pomerantz SM. Dopaminergic influences on male sexual behavior of rhesus monkeys: effects of dopamine agonists. Pharmacol Biochem Behav. 1992;41:511–517. doi: 10.1016/0091-3057(92)90366-n. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karle EJ, Anderson KD, Medina L. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJAJ, Reiner A, editors. Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge: Cambridge University Press; 1994. pp. 135–181. [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Sharf R, Lee DY, Ranaldi R. Microinjections of SCH 23390 in the ventral tegmental area reduce operant responding under a progressive ratio schedule of food reinforcement in rats. Brain Res. 2005;1033:179–185. doi: 10.1016/j.brainres.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Sweidan S, Edinger H, Siegel A. D2 dopamine receptor-mediated mechanisms in the medial preoptic-anterior hypothalamus regulate affective defense behavior in the cat. Brain Res. 1991;549:127–137. doi: 10.1016/0006-8993(91)90608-x. [DOI] [PubMed] [Google Scholar]
- Tillet Y. Catecholaminergic neuronal systems in the diencephalon of mammals. In: Smeets WJAJ, Reiner A, editors. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge: Cambridge University Press; 1994. pp. 207–236. [Google Scholar]
- Tomaszewska D, Przekop F. Catecholaminergic activity in the medial preoptic area and nucleus infundibularis-median eminence of anestrous ewes in normal physiological state and under stress condition. J Neural Transm. 1999;106:1031–1043. doi: 10.1007/s007020050221. [DOI] [PubMed] [Google Scholar]
- Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, Eaton RC, Hull EM. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Res. 1991;540:177–182. doi: 10.1016/0006-8993(91)90505-p. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE. Induction of social dominance by L-dopa treatment in Arctic charr. Neuroreport. 1992;3:243–246. doi: 10.1097/00001756-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards. Horm Behav. 2001;40:483–489. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]


