Abstract
Neuropeptides of the arginine vastocin (AVT) family, which include the mammalian peptides arginine vasopressin (AVP) and oxytocin (OXT), comprise neuroendocrine circuits that range from being evolutionarily conserved to evolutionarily diverse. For instance, the functions and anatomy of the AVT/AVP projections to the pituitary (which arise in the preoptic area and hypothalamus) are strongly conserved, whereas those of extrahypothalamic AVT/AVP circuits are species-specific and change rapidly over evolutionary time. AVT/AVP circuits arising in the medial bed nucleus of the stria terminalis (BSTm) exhibit species-specific evolution in relation to mating system in mammals (monogamous versus non-monogamous) and sociality in songbirds (gregarious versus relatively asocial). In estrildid songbirds, AVT neurons in the BSTm increase their Fos expression only in response to “positively-valenced” social stimuli (stimuli that normally elicit affiliation), whereas “negative” stimuli (which elicit aggression or aversion) produce no response or even suppress Fos expression. Relative to territorial species, gregarious species show 1) greater social induction of Fos within AVT neurons, 2) a higher baseline of Fos expression in AVT neurons, 3) more AVT neurons in the BSTm, and 4) a higher density of V1a-like binding sites in the lateral septum. Furthermore, septal AVT infusions inhibit resident-intruder aggression, but facilitate aggression that is motivated by mate competition (an affiliative context). The functional profile of the BSTm AVT neurons is therefore quite distinct from that of hypothalamic AVT/AVP neurons, particularly those of the paraventricular nucleus (PVN), which are classically stress-responsive. This is paradoxical, given that AVT/AVP projections from the PVN and BSTm likely overlap. Despite this overlap, each AVT/AVP cell group should produce a distinct pattern of modulation across brain regions. Relative weighting of hypothalamic and BSTm nonapeptide circuitries may therefore be an important determinant of approach-avoidance behavior, and may be a prime target of natural selection related to sociality.
Keywords: vasotocin, vasopressin, oxytocin, isotocin, evolution, social behavior
Nonapeptides and the Patterning of Behavior
Neuropeptides of the arginine vasotocin (AVT) family, the nine amino acid “nonapeptides,” are important generators of behavioral diversity, perhaps more so than any other neurochemical systems. This is due in large part to the dramatic plasticity of socially-relevant nonapeptide circuits. Plastic responses to steroid hormones and/or photoperiod cues are commonly observed for nonapeptide systems (Goodson and Bass, 2001, De Vries and Panzica, 2006) and these responses promote temporal patterns of behavior that are appropriate to the season, immediate social context, and physiological state of the animal. Nonapetide circuits are likewise plastic in the evolutionary sense. This is particularly observed with respect to receptor distributions, which can vary qualitatively across species (Insel et al., 1991, Insel and Shapiro, 1992, Insel et al., 1994, Bester-Meredith et al., 1999, Goodson et al., 2006), thereby setting up dramatic species differences in behavior.
On a much shorter timescale, the nonapeptides significantly influence context-dependent patterns of socially-elicited neural response. As addressed in the final section, different cell groups that produce a given nonapeptide may exhibit quite divergent (in some ways opposite) responses to social stimuli, and the projections of these cell groups may even converge onto some of the same targets. However, each population should produce a different pattern of modulation across behaviorally-relevant brain regions. Differential “weighting” of activity in basal forebrain networks is an important determinant of social behavior output (Newman, 1999, Goodson, 2005), hence the distinct patterns of release from the various nonapeptide cell groups could produce very different (even opposite) behavior patterns, both within and between species.
Deep History of the Nonapeptides
AVT is present in all non-mammalian vertebrates examined to date and is the ancestral nonapeptide form for all of the vertebrate nonapeptides (Acher and Chauvet, 1995, Acher et al., 1995). Given this basal position of AVT, the vertebrate nonapeptides are here collectively referred to as the “vasotocin family.” However, AVT is structurally similar to a variety of invertebrate nonapeptides (Fujino et al., 1999), including a form in the nerve net of the freshwater hydra (Hydra attenuata), suggesting that the AVT-like nonapeptides are much more ancient than AVT itself and have an evolutionary history that dates back to the Precambrian era, more than 600 million years ago (Acher and Chauvet, 1995, Acher et al., 1995). Furthermore, recent analyses show that AVT neurophysin neurons within neurosecretory brain regions of both annelids and fish express the same tissue-specific microRNA and combinations of transcription factors. These neurons appear to serve as sensory-neurosecretory linkages, and are likely among the most conserved elements of the neurosecretory brain (Tessmar-Raible et al., 2007).
The primary nonapeptides in vertebrates are shown in Figure 1. As shown, AVT is the sole nonapeptide form in agnathans (jawless vertebrates). The AVT gene was duplicated in early fishes, approximately 450 million years ago, forming a second oxytocin-like (OXT-like) peptide. The most common OXT-like form in fishes is isotocin (IT), which is present in all teleosts (bony fishes) thus far examined. Sometime prior to the water-to-land transition, IT was replaced in the common ancestor of tetrapods by mesotocin, which is retained in crossopterygian fish and all tetrapods except eutherian and protherian mammals, which express OXT. Despite these multiple modifications, AVT and OXT differ by only one amino acid. Evolution on the AVP side of the family has been even more conservative, with AVP replacing AVT in most mammals. AVT and AVP also differ at only one position (reviews: (Moore, 1992, Acher and Chauvet, 1995, Acher et al., 1995, Moore and Lowry, 1998).
Figure 1.
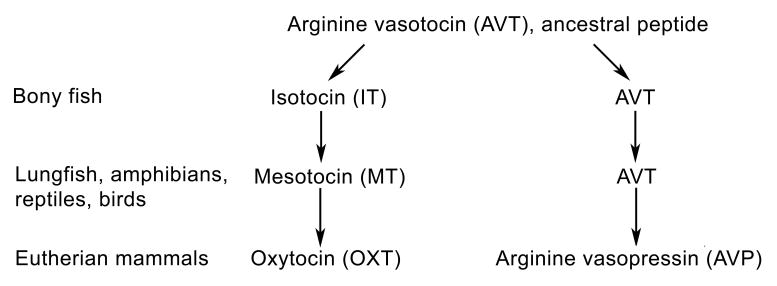
Evolution of the vasotocin nonapeptide family in vertebrates (after Acher and Chauvet, 1995). Only main features are shown. Secondary duplications and other peptide forms are present in some vertebrate groups (e.g., marsupials and cartilaginous fish; see the second text section).
Most vertebrates possess two nonapeptide forms, including an AVP-like form and an OXT-like form. However, secondary duplications are present in a variety of groups, particularly the cartilaginous fishes, and some groups therefore express three or even four nonapeptides. At least six different OXT-like peptides are expressed in cartilaginous fish (OXT, glumitocin, valitocin, aspargtocin, asvatocin and phasvotocin), with one or two forms being present in any given species. Secondary duplications are also common in marsupials, which express three different AVP-like forms (AVP, lysipressin and phenypressin) in addition to both mesotocin and OXT. Most eutherians express only AVP and OXT, although lysipressin replaces AVP in pigs (Moore, 1992, Acher and Chauvet, 1995, Acher et al., 1995).
Evolution of Central Nonapeptide Circuits: Anatomical and Behavioral Basics
Evolutionary conservation in the structure of the nonapeptides, as described above, is substantially mirrored by conservative evolution in the locations of main cell groups in the brain. In all vertebrates, the AVP-like and OXT-like peptides are produced by populations of magnocellular and parvocellular neurons in the preoptic area (POA) and anterior hypothalamus (AH). Magnocellular neuron populations are present in the magnocellular POA of fish and amphibians; these are homologous to magnocellular nonapeptide neurons in the supraoptic nucleus of the hypothalamus (SON) of reptiles, birds and mammals. Parvocellular neuron populations are found in the parvocelluar POA of fishes and posterior POA of amphibians, which are homologous to parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN) in amniotes (Moore and Lowry, 1998, Goodson and Bass, 2001). These cell groups project to the neurohypophysis as well as the adenohypophysis, allowing the nonapeptides to exert a wide range of peripheral effects. Of particular interest for the present review are actions that may be integrated with the central regulation of social behaviors. These include the regulation of milk ejection by OXT, and the regulation of adrenocorticotropic hormone secretion by AVT/AVP (Witt, 1995, Carter, 1998, Goodson and Bass, 2001, Keverne and Curley, 2004).
Teleost fish tend to produce AVT and IT only in the POA, although other small AVT populations have been identified (Batten et al., 1990, Holmqvist and Ekstrom, 1995, Goodson and Bass, 2000b, Goodson et al., 2003). In contrast, a dramatic expansion in the number of AVT cell groups accompanied the water-to-land transition, with up to 19 cell AVT cell groups occurring in a single tetrapod species (the roughskin newt, Taricha granulose; Lowry et al., 1997). Not surprisingly, AVT exerts a diversity of behavioral actions in newts (Thompson and Moore, 2000, Rose and Moore, 2002, Thompson and Moore, 2003).
Given that teleosts tend to produce AVT and IT exclusively (or near-exclusively) within the major magnocellular and parvocellular nuclei that are common to all vertebrates (Moore and Lowry, 1998, Goodson and Bass, 2001), the anatomical and behavioral “fundamentals” of nonapeptide systems are particularly tractable to examine in teleost fish. In vocalizing teleosts of the family Batrachoididae, AVT-immunoreactive (-ir) and IT-ir pathways target an interconnected set of midbrain and forebrain regions that comprise an evolutionarily conserved “social behavior network” (including amygdalar, septal, hypothalamic and tegmental regions) (Goodson and Bass, 2000b, Goodson and Bass, 2002, Goodson et al., 2003). Both AVT and IT modulate fictive social vocalizations in these fish (Goodson and Bass, 2000b, Goodson and Bass, 2000a) and neurophysiologically-guided tract tracings demonstrate that vocally-active brain areas receive direct projections from the parvocellular POA, but not the magnocellular POA (Goodson and Bass, 2002). Given that nonapeptide distributions in these vocal teleosts are highly similar to those in tetrapods, the tracing results suggest that the parvocellular neurons of the POA-AH give rise to the most ancient, socially-relevant nonapeptide circuits in the brain. In addition to social vocalization, AVT also modulates aggression in fish (Semsar et al., 2001, Lema and Nevitt, 2004, Santangelo and Bass, 2006), and similarly, the nonapeptides influence aggression and social communication across a wide range of other vertebrate taxa (Goodson and Bass, 2001, Thompson et al., 2006).
The expansion of AVT/AVP circuitries in tetrapods includes some features that are taxonomically widespread and some that are taxon-specific. For instance, of the 19 AVT populations in newts, several appear to be highly derived, but many are likely homologous to cell groups in other tetrapod species and thus represent the ancestral tetrapod state (Lowry et al., 1997, Moore and Lowry, 1998). Most notable in this regard are the AVT/AVP neurons of the suprachiasmatic nucleus, which are fundamental to the expression of many biological rhythms, and those of the medial bed nucleus of the stria terminalis (BSTm), which are implicated in a variety of social behaviors. The homology (across vertebrate classes) of AVT/AVP neurons in the BSTm is particularly well supported by the virtual ubiquity of sex steroid sensitivity in these cells and their projections to the lateral septum (LS). Sexual dimorphisms in cell number and fiber density (male > female) are also commonly observed in the AVT/AVP circuitry of the BSTm and LS (Moore and Lowry, 1998, De Vries and Panzica, 2006).
Standing in stark contrast to the conservation described above are the distributions of the nonapeptide receptors. Whereas receptor distributions for most neurochemicals are relatively conserved, those for OXT and AVT/AVP are highly variable and species-specific, with widespread and qualitative differences being observed even between species that are very closely related (Insel et al., 1991, Insel et al., 1994, Goodson et al., 2006). This observation clearly accounts for much of the functional diversity and evolutionary plasticity that is observed within the vasotocin family, as described below.
Evolutionary Plasticity of Nonapeptide Systems Generates Social Diversity
If we examine the spectrum of neurotransmitters, neuromodulators, and receptor types that are present in vertebrates, one thing becomes immediately clear – that the nonapeptides exhibit levels of plasticity, sexual dimorphism and species-specificity that are far beyond that of other neurochemical systems. Certainly there are highly conserved features, as described above (e.g., in peptide structure and major cell groups), but nonapeptide receptor distributions evolve at an astonishing rate and nonapeptide systems exhibit a myriad of hormone-dependent features. Thus, there is a general tendency for certain features of nonapeptide systems to be temporally plastic and evolutionarily labile. This means that nonapeptide circuits provide more “grist for the mill” of behavioral evolution than do other neurochemical systems, and we can therefore expect to find an extraordinary level of species-specificity in nonapeptide anatomy (particularly in receptor distributions) and related behavioral functions. At the same time, since the nonapeptides are arguably the “easiest” thing for natural selection to target in relation to behavior (given the available variation and plasticity), we can expect to find a great deal of mechanistic convergence in relation to specific kinds of derived behaviors.
Given the widespread presence of sex differences in AVT/AVP systems (De Vries and Panzica, 2006), it is perhaps not surprising that AVT is associated with highly derived forms of sexual plasticity (Foran and Bass, 1999). Teleost fish are the most remarkable in this respect and exhibit a dazzling array of sexual plasticity, including serial sex change, terminal sex change (both male-to-female and female-to-male) and the presence of multiple reproductive phenotypes (Bass and Grober, 2001). The nonapeptides are implicated in all of these forms of plasticity. For instance, rapid increases in AVT mRNA are correlated with dominance behavior during socially-mediated sex change in bluehead wrasse (Thalassoma bifasciatum) (Godwin et al., 2000), and endogenous AVT is necessary for the assumption of dominance behavior in this species (Semsar and Godwin, 2004).
Whereas sex-changing fish may transiently express both male-typical and female-typical traits, some sexually polymorphic species stably express phenotypes that display a mixture of sex-typical characters. Polymorphic species therefore offer the opportunity to examine the extent to which sex-typical peptidergic mechanisms can be dissociated from gonadal sex. The most direct evidence of such dissociation comes from studies of vocal-motor physiology in the sexually polymorphic plainfin midshipman fish (Portichthys notatus). Fictive social vocalizations in the midshipman can be electrically elicited from a number of brain areas, including the AH (Goodson and Bass, 2002), and delivery of nonapeptides and antagonists into the AH produces morph-specific effects. The ancestral male morph in the midshipman is the Type I male, which vocally courts females and defends a nest site. AVT inhibits vocal-motor bursting in Type I males, whereas an AVP V1 antagonist facilitates bursting. Administrations of IT and an OXT antagonist are without effect. Females exhibit a pattern of nonapeptide effects that is the reverse of Type I males, such that they sensitive to IT manipulations, but not AVT manipulations (Goodson and Bass, 2000a). The third reproductive phenotype, the Type II male morph (which sneak or satellite spawns), is an evolutionarily derived phenotype that expresses an interesting amalgamation of sexual characteristics. Their appearance and size are similar to females; they exhibit the simple vocal repertoire of females; and like females, they visit nests of Type I males only to spawn, leaving the Type I males to provide parental care (Bass, 1996). However, relative to body size, their testes are many times larger are those of the Type I males. Hence, in Type II males, the POA-AH regulate the pituitary-gonadal axis in a hypermasculinized manner. Despite this, hypothalamic delivery of nonapeptides and antagonists in Type II males produces a pattern of effects that is virtually identical to females, not Type I males (Goodson and Bass, 2000a).
The results in midshipman demonstrate that gonadal sex and sex-typical behavioral modulation can be uncoupled from each other, even within brain regions that regulate the pituitary-gonadal axis. The developmental mechanisms that differentiate morphs remain to be determined, and it is not yet clear whether midshipman possess sex chromosomes. However, findings in mice clearly show that both genomic and non-genomic factors can contribute to the sexual differentiation of AVP systems (De Vries et al., 2002). Such dual effects should offer natural selection greater flexibility in relation to AVT/AVP systems, and could represent a mechanism whereby sex-typical behavioral modulation can become uncoupled from gonadal sex.
Although the functional significance of morph-specific nonapeptide effects in the midshipman remains to be elucidated, it is notable that similar sex-specific effects of OXT and AVP are observed in monogamous prairie voles (Microtus ochrogaster): Endogenous OXT is required for pair bonding in female prairie voles, whereas endogenous AVP is required for pair bonding in males (Insel and Hulihan, 1995, Young and Wang, 2004). Whether these similarities in voles and midshipman reveal a conserved vertebrate trend or represent convergent evolution remains to be determined.
A more clear case of evolutionary convergence is exhibited between monogamous voles and other monogamous mammals. Monogamous and non-monogamous species of Peromyscus mice and Microtus voles exhibit widespread differences in the distributions of AVP V1a receptors and OXT receptors (Insel et al., 1991, Insel and Shapiro, 1992, Insel et al., 1994). In general, though, the pattern of species differences in the vole comparisons is different than the pattern of species differences in mice, suggesting that not all of the variation in receptor distribution is related to mating system. Even so, multiple studies of monogamous and non-mongamous species (including voles, mice and primates) point towards two brain areas as being relevant for monogamous pair bonding – the nucleus accumbens in the case of OXT receptors, and the ventral pallidum in the case of AVP V1a receptors (Wang et al., 1997, Young et al., 1999, Liu and Wang, 2003, Lim et al., 2004a). Convergent evolution is particularly clear in the case of V1a distributions, since in each comparison of monogamous and non-monogamous species, the monogamous species exhibits a higher density of V1a receptors in the ventral pallidum (Young and Wang, 2004). These species differences are both necessary and sufficient to account for species differences in behavior, as established through site-specific manipulations of V1a gene expression (Pitkow et al., 2001, Lim et al., 2004b, Lim and Young, 2004).
Vasotocin and the Evolution of Avian Sociality
In addition to differing in mating system, the monogamous and non-monogamous rodents discussed above diverge in other aspects of social organization. For example, the monogamous vole species often form small groups and exhibit biparental care (Getz et al., 2005), whereas the non-monogamous species typically do not. Given that the nonapeptides are relevant for the regulation of various affiliative and paternal behaviors in addition to pair bonding (Wang et al., 1994, Parker and Lee, 2001, Bales et al., 2004), the question arises as to whether peptidergic mechanisms evolve specifically in relation to a given aspect of social organization (such as mating system, parental care or sociality) or whether multiple dimensions of behavior are obligatorily linked together in relation to nonapeptide function. However, rodents do not offer good opportunities to address these questions, since the various aspects of behavior cannot be adequately uncoupled in comparative studies of different species (for discussion, see Goodson et al., 2006).
In contrast to rodents, however, some bird groups offer excellent opportunities to isolate various aspects of social organization as quasi-independent variables. Finches and waxbills of the family Estrildidae are particularly useful in this regard, since sociality (i.e., grouping behavior) can be isolated from other aspects of behavior and ecology. The estrildids are all monogamous (forming long-term or life-long pair bonds) and exhibit biparental care. Most of the species are moderately social – flocking during the non-breeding season and loosely distributing for breeding. However, some species have evolved to be gregarious year-round and a few species have evolved highly colonial breeding. At the other end of the continuum, a small number of estrildid species live year-round as relatively asocial male-female pairs and aggressively defend territories (Skead, 1975, Goodwin, 1982). Our studies have included five species of estrildids, including two territorial species that likely evolved territoriality independently; two highly gregarious species that independently evolved coloniality; and an intermediate, modestly gregarious species (Goodson et al., 2005, Goodson et al., 2006, Goodson and Wang, 2006).
Our primary research focus in these birds has been on the response characteristics of the AVT neurons in the BSTm. These neurons likely contribute to the regulation of multiple affiliative behaviors, including pair bonding in rodents (Young and Wang, 2004, De Vries and Panzica, 2006), but no research had previously determined how different classes of social stimuli elicit responses in these neurons. To address this issue, we used double-label immunocytochemistry to examine the induction of Fos (a marker of neuronal activity) in AVT-ir neurons following a control manipulation or exposure to a same-sex conspecific through a wire barrier (both sexes were examined, although no sex differences were observed). A significant interaction effect was obtained (species × condition), reflecting the fact that in the territorial species, exposure to a same-sex conspecific tended to decrease the colocalization of immunoreactive AVT and Fos, whereas colocalization tended to increase in the highly gregarious species. The modestly gregarious species exhibited virtually no change in the colocalization of AVT and Fos (Goodson and Wang, 2006). Importantly, the testing paradigm that we employed limits the overt expression of social behaviors, thus the species differences in Fos response should primarily reflect differences in perceptual or motivational processes, not differences in behavioral response.
The data just described suggest the hypothesis that BSTm AVT neurons may exhibit increases in Fos expression following exposure to “positive” stimuli that normally elicit affiliative responses, but not to “negative” stimuli that normally elicit aggression or aversion. Such a valence sensitivity could readily account for divergent responses to same-sex stimuli in territorial and gregarious species. Two additional findings firmly support our hypothesis. First, in the territorial violet-eared waxbill (Uraeginthus granatina), exposure to a same-sex conspecific produces a significant decrease in AVT-Fos colocalization, whereas exposure to a positive social stimulus, the subject's pair bond partner, produces a very robust increase (Fig. 2A). Similarly, in the highly gregarious zebra finch (Taeniopygia guttata), significant increases in AVT-Fos colocalization are observed in a mate competition paradigm if the subjects are allowed to court, but not if the subjects are aggressively subjugated (Fig. 2B; Goodson and Wang, 2006). Recent findings from our lab further demonstrate that the AVT-ir neurons of the BSTm are sensitive only to positive social stimuli, since positive non-social stimuli are without effect (J. L. Goodson, unpublished observations).
Figure 2.
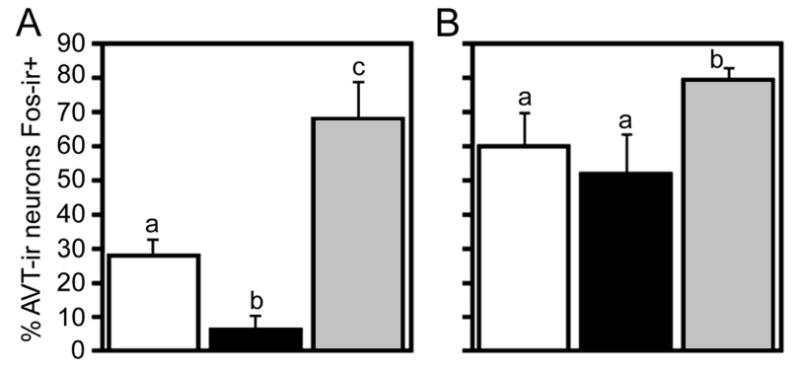
AVT neuronal responses to social stimuli (modified from Goodson and Wang, 2006). A. Percentage of AVT-ir neurons in the BSTm that express Fos-ir nuclei (means ± SEM) in the relatively asocial violet-eared waxbill following exposure to a control condition (open bars), a same-sex conspecific (black bars), or the subject's pair bond partner (grey bars). Different letters above the bars indicate significant group differences (Mann-Whitney tied P < 0.05 following significant Kruskal-Wallis, tied P = 0.003). Total n = 16. B. AVT+Fos colocalization in control (open bars), subjugated (black bars) and non-subjugated (grey bars) zebra finches exposed to mate competition (Kruskal-Wallis tied P = 0.03). No sex differences were observed and sexes are shown pooled. Total n = 15.
Consistent with these findings, we found that 1) the two highly gregarious, colonially-breeding species have significantly more AVT-ir neurons in the BSTm than do the other species, 2) the territorial species have lower baseline levels of AVT-Fos colocalization than do the gregarious species, and 3) the three gregarious species have significantly higher densities of V1a-like binding sites in the LS than do the territorial species (Fig. 3) (Goodson et al., 2006, Goodson and Wang, 2006). In rodents, septal V1a receptors promote active social interaction and facilitate social recognition (Landgraf et al., 2003). These are functions that should be in higher demand for gregarious species than for territorial species.
Figure 3.
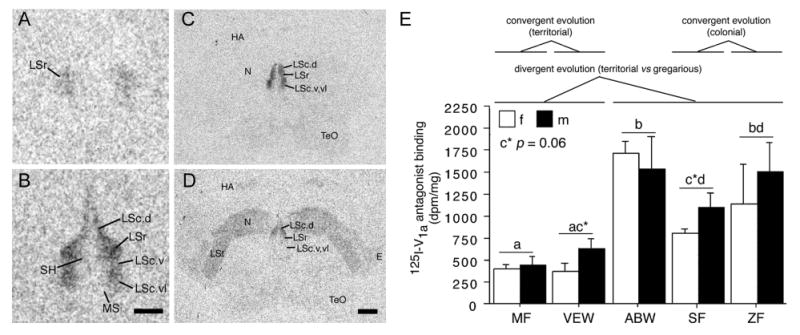
Species differences in linear 125I-V1a antagonist binding (modified from Goodson et al., 2006). A-B. Representative binding in the septum of the territorial violet-eared waxbill (VEW; A), and moderately gregarious Angolan blue waxbill (ABW; B). C-D. Representative sections for a male Angolan blue waxbill and male spice finch (colonial), respectively, showing species differences in binding for the nidopallium (N) and other areas of the forebrain. E. Linear 125I-V1a antagonist binding in the dorsal (pallial) portion of the lateral septum (LS), shown as decompositions per minute/mg (dpm/mg; means ± SEM). Different letters above the error bars denote significant species differences (Fisher's PLSD following significant ANOVA; p < 0.05). The scale bar in B corresponds to 500 μm in panels A-B; the scale bar in D corresponds to 1 mm in panels C-D. Abbreviations: E, entopallium; HA, apical part of the hyperpallium; LSc, caudal division of the lateral septum (dorsal, ventrolateral, and ventral zones denoted as LSc.d, LSc.vl, and LSc.v, respectively); LSr, rostral division of the lateral septum; LSt, lateral striatum; MS, medial septum; N, nidopallium; SH, septohippocampal septum; TeO, optic tectum.
Sociality and Septal Neuropeptides: What is Being Modulated?
Manipulations of septal AVT produce results that are consistent with the results described above, and support the idea that the AVT projection from the BSTm to the LS promotes affiliative behavior. For instance, intraseptal AVT infusions reduce resident-intruder aggression in two species of songbirds that independently evolved territoriality – the violet-eared waxbill (Goodson, 1998b) and the field sparrow (Spizella pusilla; Fig. 4A) (Goodson, 1998a). In the colonial zebra finch, AVT actually increases aggression during competition to court (Goodson and Adkins-Regan, 1999, Goodson et al., 2004), but this is aggression that is specifically linked to appetitive sexual behavior (i.e., an affiliative context) (Adkins-Regan and Robinson, 1993).
Figure 4.
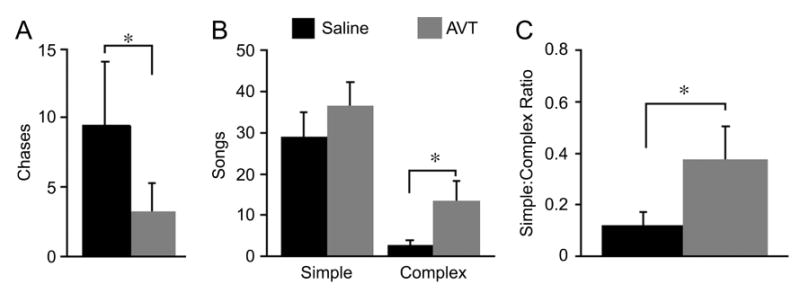
Effects of intraseptal AVT infusions in male field sparrows housed on semi-natural territories (field-based flight cages placed in natural habitat; modified from Goodson, 1998a). A. Chases during a 15-minute resident-intruder test following infusion of saline control or 100 ng AVT. B. The number of simple and complex songs given spontaneously during the dawn singing period; these song types are multipurpose and strictly agonistic, respectively (Nelson and Croner, 1991). C. The ratio of complex to simple songs, showing an increase in the agonistic content of song following intraseptal infusion of AVT. All data are shown as means ± SEM; *P < 0.05, Wilcoxon signed ranks; n = 7.
These context-dependent effects on aggression are consistent with the hypothesis that AVT is influencing a broader emotional state such as anxiety. For instance, heightened anxiety may reduce aggression in a territorial bird that is faced with an intruder, but increase aggression in a gregarious zebra finch that is competing for a mate. Similar context-dependent effects are observed in male field sparrows housed outdoors on semi-natural territories. As just mentioned, resident-intruder aggression in field sparrows is decreased by intraseptal AVT infusions. However, spontaneous use of an agonistic song type during the “dawn chorus” (a time of elevated singing) is increased (Fig. 4B-C) (Goodson, 1998a). Both the decrease in overt aggression (when faced with an actual intruder) and the increase in agonistic singing (when an animal is attempting to keep other animals away) could potentially result from an increase in anxiety.
In support of this idea, septal V1a receptors influence anxiety in rodents (Landgraf et al., 1995) and V1a-like receptors modulate neural responses of the LS to stress in song sparrows (Melospiza melodia) (Goodson and Evans, 2004). In general, manipulations of AVT/AVP that influence social behavior also tend to influence anxiety (Pitkow et al., 2001, Bielsky et al., 2004).
Neuromodulatory Patterning: Overlapping Circuits and Distinct Behavioral States
Different populations of AVT/AVP neurons can have different (even opposing) functional profiles and yet exhibit overlapping projections to the same brain areas. For instance, the AVT/AVP neurons of the BSTm appear to promote affiliative responses to social stimuli (Young and Wang, 2004, Goodson and Wang, 2006) and inhibit resident-intruder aggression (Goodson, 1998b, Goodson, 1998a), whereas AVT/AVP neurons in PVN are well-known to regulate behavioral and physiological responses to stress (Engelmann et al., 2004). Yet other AVP neurons of the AH promote offensive, resident-intruder aggression (Ferris et al., 1997, Delville et al., 2000, Gobrogge et al., 2007). These findings are hard to reconcile with each other, particularly when we consider that the projections of the different populations can be closely juxtaposed and that AVT/AVP often act in a paracrine fashion. That is, AVT/AVP signals from the diverse populations are clearly going to be mingled at their postsynaptic targets. The LS provides a concrete example: Although the BSTm provides the only direct AVP innervation of the LS (De Vries and Buijs, 1983, De Vries and Panzica, 2006), neurophysiological studies indicate that AVP projections from the PVN also influence LS activity (Disturnal et al., 1986, Disturnal et al., 1987). Dendritic release of the nonapeptides (Landgraf and Neumann, 200) provides still another means of mingling.
How can AVT/AVP influence behavior in a context-dependent manner if it is released in response to such disparate stimuli as sexual, stressful or agonistic events? Or, as in the case of AVT/AVP arising from the BSTm and AH populations, have directly opposite effects on the same kind of behavior? The answer to this paradox becomes clear if we examine the functional properties of basal forebrain (“limbic”) circuits that regulate behavior. The “social behavior network” is characterized by extensive interconnectivity and a relative lack of linearity (Newman, 1999, Goodson, 2005). Each “node” in this network is involved in the regulation of multiple forms of social behavior, including those that may seem to be incompatible with each other. However, it is not the amount of activity at any one node that determines the form of behavioral output. Rather, it is the distinct pattern of activation across the entire network that generates distinct behavioral states. Note that although there are two different models for these circuits (Newman, 1999, Swanson, 2000, Choi et al., 2005), both require that we consider the relative amount of activity across the network nodes – that is, the overall topography of the activation pattern.
By this conceptualization, it does not matter that the PVN and BSTm may each produce the same effect on a given postsynaptic target (e.g., the LS). What is important is that AVT/AVP projections from the PVN and BSTm will each elicit a different pattern of postsynaptic effects across the social behavior network, since the projections from these populations are not going to be entirely overlapping. One pattern may promote stress or aversion behavior, and the other may promote affiliation. Hypothetically then, evolutionary modifications to behavior will involve changing the relative strengths of functionally distinct nonapeptide systems (arising in the BSTm, PVN, AH, etc.), which would yield different patterns of postsynaptic modulation, and hence different patterns of behavior. Such changes to weighting could be achieved by various means, including evolutionary modifications to cell number; basal and socially-elicited levels of gene activity; and postsynaptic receptor densities. All of these things have occurred during social evolution in the estrildid finches and/or microtine voles, with good evidence for both evolutionary divergence and convergence in relation to behavior (Young and Wang, 2004, Goodson et al., 2006, Goodson and Wang, 2006). Although not yet examined in an evolutionary context, modifications to the various modes of intracerebral release and signaling could likewise produce dramatic variations in behavior. These modes include volume transmission of neuropeptides following dendritic release, and more targeted release from axon terminals (Landgraf and Neumann, 2004). Almost limitless combinations of these modes could be employed across behaviorally-relevant brain regions, yielding an endless variety of context-dependent and species-specific patterns of neuromodulation.
Summary
Nonapeptide circuits are an evolutionarily ancient component of the brain, and they exhibit numerous anatomical and functional features that are strongly conserved across the vertebrate classes. Nonetheless, certain features are evolutionarily plastic, particularly receptor distributions, which allows the nonapeptides to influence behavior in a species-specific manner. At least in some cases, evolution in nonapeptide circuits appears to take a predictable course, given that various anatomical and functional features have evolved both divergently and convergently in relation to mating system in mammals and sociality in birds. Evolutionary modifications to nonapeptide signaling may take a variety of forms, all of which likely produce species-specific patterns of neuromodulation across brain regions. Importantly, these evolutionary modifications may adjust the relative influence of the various nonapeptide cell groups, which can be functionally distinct and even functionally opposed.
Acknowledgments
Support provided by National Institutes of Health grant MH62656.
Abbreviations
- AH
anterior hypothalamus
- AVP
arginine vasopressin
- AVT
arginine vasotocin
- BSTm
medial bed nucleus of the stria terminalis
- IT
isotocin
- LS
lateral septum
- OXT
oxytocin
- POA
preoptic area
- PVN
paraventricular nucleus of the hypothalamus
References
- Acher R, Chauvet J. The neurohypophysial endocrine regulatory cascade: Precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–289. doi: 10.1006/frne.1995.1009. [DOI] [PubMed] [Google Scholar]
- Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv Exp Med Biol. 1995;395:615–627. [PubMed] [Google Scholar]
- Adkins-Regan E, Robinson TM. Sex differences in aggressive behavior in zebra finches (Poephila guttata) J Comp Psychol. 1993;107:223–229. [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363. [Google Scholar]
- Bass AH, Grober MS. Social and neural modulation of sexual plasticity in teleost fish. Brain Behav Evol. 2001;57:293–300. doi: 10.1159/000047247. [DOI] [PubMed] [Google Scholar]
- Batten TFC, Cambre ML, Moons L, Vandesande F. Comparative distribution of neuropeptide-immunoreactive systems in the brain of the green molly Poecilia latipinna. J Comp Neurol. 1990;302:893–919. doi: 10.1002/cne.903020416. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Disturnal JE, Veale WL, Pittman QJ. Modulation by arginine vasopressin of glutamate excitation in the ventral septal area of the rat brain. Can J Physiol Pharmacol. 1987;65:30–35. doi: 10.1139/y87-006. [DOI] [PubMed] [Google Scholar]
- Disturnal JE, Veale WL, Pittman QL. The ventral septal area: electrophysiological evidence for putative arginine vasopressin projections onto thermoresponsive neurons. Neuroscience. 1986;19:795–802. doi: 10.1016/0306-4522(86)90299-x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran CM, Bass AH. Preoptic GnRH and AVT: Axes for sexual plasticity in teleost fish. Gen Comp Endocrinol. 1999;116:141–152. doi: 10.1006/gcen.1999.7357. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Nagahama T, Oumi T, Ukena K, Morishita F, Furukawa Y, Matsushima O, Ando M, Takahama H, Satake H, Minakata H, Nomoto K. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J Exp Zool. 1999;284:401–406. doi: 10.1002/(sici)1097-010x(19990901)284:4<401::aid-jez6>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- Getz LL, McGuire B, Carter CS. Social organization and mating system of free-living prairie voles Microtus ochrogaster: a review. Acta Zoologica Sinica. 2005;51:178–186. [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Godwin J, Sawby R, Warner RR, Crews D, Grober MS. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav Evol. 2000;55:77–84. doi: 10.1159/000006643. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost, Porichthys notatus. J Comp Neurol. 2000b;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Estrildid finches of the world. Cornell University Press; Ithaca, NY: 1982. [Google Scholar]
- Holmqvist BI, Ekstrom P. Hypophysiotrophic systems in the brain of the Atlantic salmon: Neuronal innervation of the pituitary and the origin of pituitary dopamine and nonapeptides identified by means of combined carbocyanine tract tracing and immunocytochemistry. J Chem Neuroanat. 1995;8:125–145. doi: 10.1016/0891-0618(94)00041-q. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema SC, Nevitt GA. Exogenous vasotocin alters aggression during agonistic exchanges in male Amargosa River pupfish (Cyprinodon nevadensis amargosae) Horm Behav. 2004;46:628–637. doi: 10.1016/j.yhbeh.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004a;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004b;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Richardson CF, Zoeller TR, Miller LJ, Muske LE, Moore FL. Neuroanatomical distribution of vasotocin in a urodele amphibian (Taricha granulosa) revealed by immunohistochemical and in situ hybridization techniques. J Comp Neurol. 1997;385:43–70. doi: 10.1002/(sici)1096-9861(19970818)385:1<43::aid-cne3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Moore FL. Evolutionary precedents for behavioral actions of oxytocin and vasopressin. Ann N Y Acad Sci. 1992;652:156–165. doi: 10.1111/j.1749-6632.1992.tb34352.x. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Croner LJ. Song categories and their functions in the field sparrow (Spizella pusilla) Auk. 1991;108:42–52. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles) Horm Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Santangelo N, Bass AH. New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc Roy Soc B. 2006;273:3085–3092. doi: 10.1098/rspb.2006.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- Skead DM. Ecological studies of four Estrildines in the central Transvaal. Ostrich. 1975 11:1–55. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Moore FL. Vasotocin stimulates appetitive responses to the visual and pheromonal stimuli used by male roughskin newts during courtship. Horm Behav. 2000;38:75–85. doi: 10.1006/hbeh.2000.1610. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Moore FL. The effects of sex steroids and vasotocin on behavioral responses to visual and olfactory sexual stimuli in ovariectomized female roughskin newts. Horm Behav. 2003;44:311–318. doi: 10.1016/s0018-506x(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GD. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): Studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997;768:147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Witt DM. Oxytocin and rodent sociosexual responses: From behavior to gene expression. Neurosci Biobehav Rev. 1995;19:315–324. doi: 10.1016/0149-7634(95)00006-z. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


