Abstract
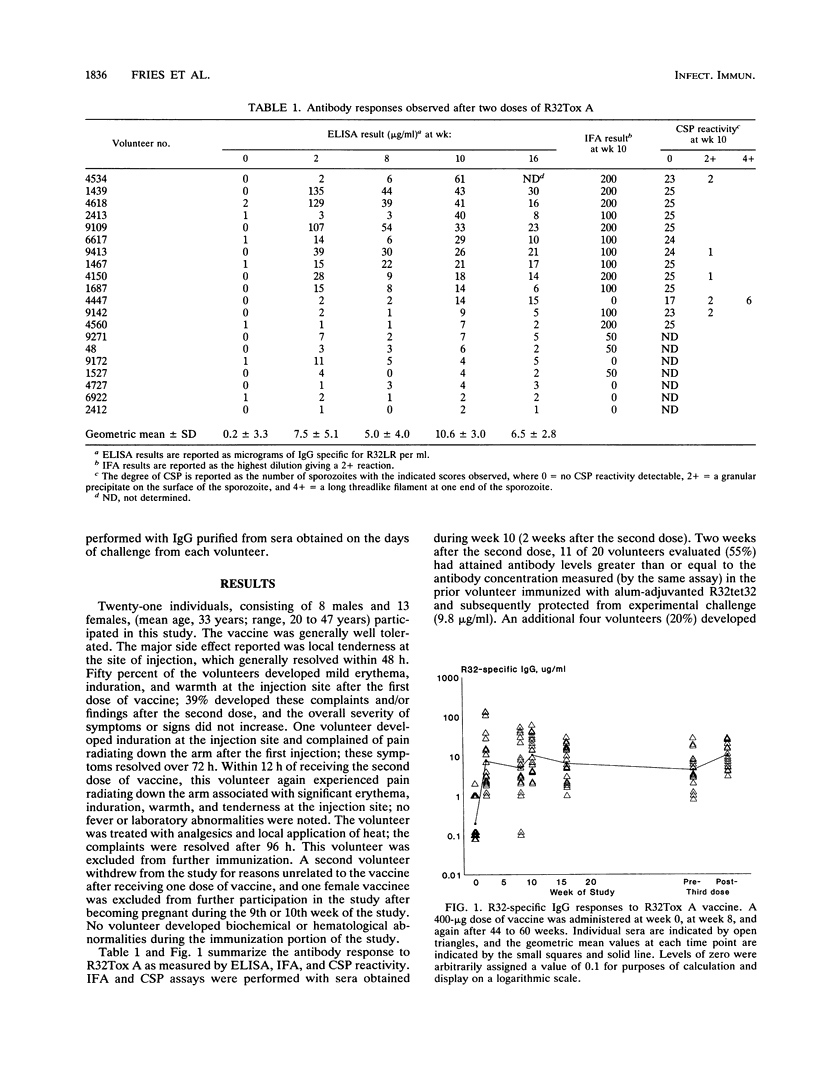
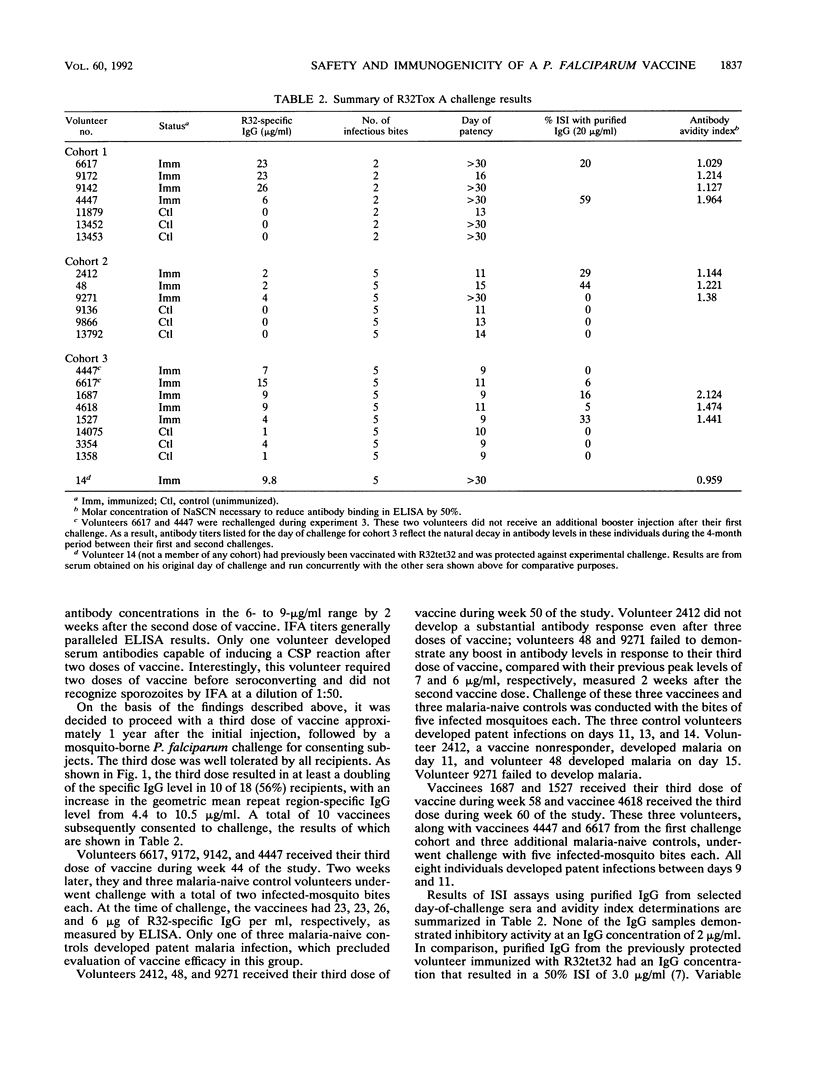
Twenty-one malaria-naive volunteers were immunized with a vaccine consisting of a 22-kDa recombinant peptide (R32LR), derived from the repeat region of Plasmodium falciparum circumsporozoite (CS) protein, covalently coupled to detoxified Pseudomonas aeruginosa toxin A. Nineteen volunteers received a second dose of vaccine at 8 weeks, and eighteen received a third dose at 8 to 12 months. The vaccine was well tolerated, with only one volunteer developing local discomfort and induration at the site of injection which limited function for 48 h. The geometric mean anti-CS immunoglobulin G antibody concentration 2 weeks after the second dose of vaccine was 10.6 micrograms/ml (standard deviation = 3.0 micrograms/ml). Eleven volunteers (52%) developed anti-CS antibody levels of greater than 9.8 micrograms/ml, the level measured in the one volunteer protected against P. falciparum challenge after immunization with the alum-adjuvanted recombinant protein R32tet32 in a prior study. Three separate experimental challenges were conducted with 10 volunteers 2 to 4 weeks after the third dose of vaccine. The four best responders, on the basis of antibody levels (6 to 26 micrograms/ml), were challenged with two infected-mosquito bites, but only one of four immunized volunteers and one of three malaria-naive controls became parasitemic. In a second challenge study using five infected-mosquito bites as the challenge dose, three of three malaria-naive control volunteers and two of three immunized volunteers developed malaria. The third vaccine was apparently completely protected. In the third and last challenge, three of three controls and five of five vaccinees became infected. Sera obtained on the days of challenge inhibited sporozoite invasion of hepatocytes variably in vitro (range, 45 to 90% inhibition), but the degree of inhibition did not correlate with protection. Although antibody against the CS repeat region may protect some individuals against experimental challenge, this protection cannot be predicted from antibody levels by current in vitro assays. The functionality and fine specificity of anti-CS antibody are probably critical determinants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Williams J. L., Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg. 1984;78(3):339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Gordon D. M., Richards R. L., Egan J. E., Hollingdale M. R., Gross M., Silverman C., Alving C. R. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. M., Cosgriff T. M., Schneider I., Wasserman G. F., Majarian W. R., Hollingdale M. R., Chulay J. D. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. Am J Trop Med Hyg. 1990 Jun;42(6):527–531. doi: 10.4269/ajtmh.1990.42.527. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Appiah A., Leland P., do Rosario V. E., Mazier D., Pied S., Herrington D. A., Chulay J. D., Ballou W. R., Derks T. Activity of human volunteer sera to candidate Plasmodium falciparum circumsporozoite protein vaccines in the inhibition of sporozoite invasion assay of human hepatoma cells and hepatocytes. Trans R Soc Trop Med Hyg. 1990 May-Jun;84(3):325–329. doi: 10.1016/0035-9203(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Que J. U., Cryz S. J., Jr, Ballou R., Fürer E., Gross M., Young J., Wasserman G. F., Loomis L. A., Sadoff J. C. Effect of carrier selection on immunogenicity of protein conjugate vaccines against Plasmodium falciparum circumsporozoites. Infect Immun. 1988 Oct;56(10):2645–2649. doi: 10.1128/iai.56.10.2645-2649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman L. S., Jones T. R., Long G. W., Paparello S., Schneider I., Paul C. F., Beaudoin R. L., Hoffman S. L. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am J Trop Med Hyg. 1990 Nov;43(5):441–445. doi: 10.4269/ajtmh.1990.43.441. [DOI] [PubMed] [Google Scholar]
- Vanderberg J., Nussenzweig R., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil Med. 1969 Sep;134(10):1183–1190. [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


