Abstract
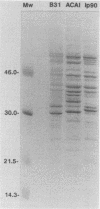
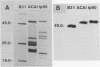
Biochemical and immunochemical studies of the outer membrane proteins of Borrelia burgdorferi have shown that the OspA and OspB proteins from strains of different geographic origins may differ considerably in their reactivities with monoclonal antibodies and in their apparent molecular weights. To further characterize this variation in Osp proteins between strains, the osp operons and deduced translation products from two strains, one from Sweden (ACAI) and one from eastern Russia (Ip90), were studied. Polyacrylamide gel electrophoresis and Western blot (immunoblot) analyses confirmed differences between ACAI, Ip90, and the North American strain B31 in their Osp proteins. The sequences of the ospA and ospB genes of ACAI and Ip90 were compared with that of the previously studied osp operon of B31 (S. Bergström, V. G. Bundoc, and A. G. Barbour, Mol. Microbiol. 3:479-486, 1989). The osp genes of ACAI and Ip90, like the corresponding genes of B31, were found on plasmids with apparent sizes of about 50 kb and are cotranscribed as a single unit. Pairwise comparisons of the nucleotide sequences revealed that the ospA genes of ACAI and Ip90 were 85 and 86% identical, respectively, to the ospA gene of strain B31 and 86% identical to each other. The ospB sequences of these two strains were 79% identical to the ospB gene of B31 and 81% identical to each other. There was significantly greater similarity between the ospA genes of the three different strains than there was between the ospA and ospB genes within each strain. These studies suggest that the duplication of osp genes in B. burgdorferi occurred before the geographical dispersion of strains represented by ACAI, Ip90, and B31.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam T., Gassmann G. S., Rasiah C., Göbel U. B. Phenotypic and genotypic analysis of Borrelia burgdorferi isolates from various sources. Infect Immun. 1991 Aug;59(8):2579–2585. doi: 10.1128/iai.59.8.2579-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64(6):506–512. [PubMed] [Google Scholar]
- Barbour A. G., Burman N., Carter C. J., Kitten T., Bergström S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991 Feb;5(2):489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Carter C. J., Burman N., Freitag C. S., Garon C. F., Bergström S. Tandem insertion sequence-like elements define the expression site for variable antigen genes of Borrelia hermsii. Infect Immun. 1991 Jan;59(1):390–397. doi: 10.1128/iai.59.1.390-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Immunochemical analysis of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Schrumpf M. E. Polymorphisms of major surface proteins of Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):83–91. doi: 10.1016/s0176-6724(86)80107-9. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman N., Bergström S., Restrepo B. I., Barbour A. G. The variable antigens Vmp7 and Vmp21 of the relapsing fever bacterium Borrelia hermsii are structurally analogous to the VSG proteins of the African trypanosome. Mol Microbiol. 1990 Oct;4(10):1715–1726. doi: 10.1111/j.1365-2958.1990.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Hoheisel J., Pohl F. M. Simplified preparation of unidirectional deletion clones. Nucleic Acids Res. 1986 Apr 25;14(8):3605–3605. doi: 10.1093/nar/14.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., Mayer L. W., Barbour A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985 Feb 8;227(4687):645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M., Duray P. H. Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:258–263. doi: 10.1111/j.1749-6632.1988.tb31859.x. [DOI] [PubMed] [Google Scholar]
- Kriuchechnikov V. N., Korenberg E. I., Shcherbakov S. V., Kovalevskii Iu V., Levin M. L. Identifikatsiia borrelii, izolirovannykh v SSSR ot kleshchei Ixodes persulcatus Schulze. Zh Mikrobiol Epidemiol Immunobiol. 1988 Dec;(12):41–44. [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L., Young K. K., Barbour A. G. Detection of Borrelia burgdorferi DNA by the polymerase chain reaction. Mol Cell Probes. 1990 Feb;4(1):73–79. doi: 10.1016/0890-8508(90)90041-w. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Postic D., Edlinger C., Richaud C., Grimont F., Dufresne Y., Perolat P., Baranton G., Grimont P. A. Two genomic species in Borrelia burgdorferi. Res Microbiol. 1990 May;141(4):465–475. doi: 10.1016/0923-2508(90)90072-x. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Hogan D., Schwan T. G. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J Clin Microbiol. 1991 Mar;29(3):524–532. doi: 10.1128/jcm.29.3.524-532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S. E., Burke J. F. Rapid isolation of miniprep DNA for double strand sequencing. Nucleic Acids Res. 1990 Aug 25;18(16):4948–4948. doi: 10.1093/nar/18.16.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Wallich R., Schaible U. E., Simon M. M., Heiberger A., Kramer M. D. Cloning and sequencing of the gene encoding the outer surface protein A (OspA) of a European Borrelia burgdorferi isolate. Nucleic Acids Res. 1989 Nov 11;17(21):8864–8864. doi: 10.1093/nar/17.21.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Anderson J. F., Baranton G., Barbour A. G., Hovind-Hougen K., Johnson R. C., Preac-Mursic V. Taxonomy of Borrelia spp. Scand J Infect Dis Suppl. 1991;77:108–129. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]