Abstract
Dehydroepiandrosterone (DHEA) is an endogenous adrenal steroid hormone with controversial actions in humans. We previously reported that DHEA has opposing actions in endothelial cells to stimulate phosphatidylinositol (PI) 3-kinase/Akt/endothelial nitric-oxide synthase leading to increased production of nitric oxide while simultaneously stimulating MAPK-dependent secretion of the vasoconstrictor ET-1. In the present study we hypothesized that DHEA may stimulate PI 3-kinase-dependent phosphorylation of FoxO1 in endothelial cells to help regulate endothelial function. In bovine or human aortic endothelial cells (BAEC and HAEC), treatment with DHEA (100 nm) acutely enhanced phosphorylation of FoxO1. DHEA-stimulated phosphorylation of FoxO1 was inhibited by pretreatment of cells with wortmannin (PI 3-kinase inhibitor) or H89 (protein kinase A (PKA) inhibitor) but not ICI182780 (estrogen receptor blocker), or PD98059 (MEK (MAPK/extracellular signal-regulated kinase kinase) inhibitor). Small interfering RNA knockdown of PKA inhibited DHEA-stimulated phosphorylation of FoxO1. DHEA promoted nuclear exclusion of FoxO1 that was blocked by pretreatment of cells with wortmannin, H89, or by small interfering RNA knockdown of PKA. DHEA treatment of endothelial cells increased PKA activity and intracellular cAMP concentrations. Transfection of BAEC with a constitutively nuclear FoxO1 mutant transactivated a co-transfected ET-1 promoter luciferase reporter. Treatment of BAEC with DHEA inhibited transactivation of the ET-1 promoter reporter in cells overexpressing FoxO1. ET-1 promoter activity and secretion in response to DHEA treatment was augmented by PI 3-kinase blockade and inhibited by MAPK blockade. We conclude that DHEA stimulates phosphorylation of FoxO1 via PI 3-kinase- and PKA-dependent pathways in endothelial cells that negatively regulates ET-1 promoter activity and secretion. Balance between PI 3-kinase-dependent inhibition and MAPK-dependent stimulation of ET-1 secretion in response to DHEA may determine whether DHEA supplementation improves or worsens cardiovascular and metabolic function.
Dehydroepiandrosterone (DHEA),2 a precursor in the biosynthesis of testosterone and estrogen, is an abundant circulating adrenal steroid hormone whose levels decline with increasing age (1, 2). Epidemiological studies correlate decreased circulating levels of DHEA with increased cardiovascular risk (3–6). Some human clinical investigations and animal studies show beneficial actions of DHEA administration to improve cardiovascular function (7–9). However, other carefully performed human studies are unable to document beneficial metabolic or cardiovascular effects of DHEA supplementation (10–13). Thus, putative health benefits of DHEA supplementation remain controversial. This is due in part to a poor understanding of the molecular mechanisms of action for DHEA. Some biological actions attributed to DHEA may be secondary to effects mediated indirectly by sex hormone derivatives of DHEA (14, 15) or regulation of expression of sex hormone receptors (16). However, an increasing body of evidence suggests that DHEA rapidly activates intracellular signaling pathways to mediate biological actions that are independent of secondary effects of sex hormones (17–20).
We recently reported that DHEA has acute non-genomic actions in primary vascular endothelial cells to stimulate phosphorylation of Akt and endothelial nitric-oxide synthase via PI 3-kinase-dependent pathways resulting in increased production of the vasodilator nitric oxide (NO) (19). In addition, we demonstrated that DHEA has opposing vascular actions to stimulate secretion of the vasoconstrictor ET-1 in a MAPK-dependent manner (19). Akt is known to phosphorylate the fork-head transcription factor FoxO1 leading to its nuclear exclusion (21, 22). FoxO1 helps to regulate cellular proliferation, differentiation, apoptosis, and glucose homeostasis (23–25). FoxO1 also plays important roles in regulating vascular homeostasis. Mice lacking FoxO1 die in utero from improper development of the vasculature (26). Overexpression of FoxO1 in primary endothelial cells impairs cell migration and tube formation, whereas knockdown of this transcription factor using siRNA enhances angiogenic functions (27, 28). FoxO1 is also a transcriptional repressor of endothelial nitric-oxide synthase (27). Other genes regulated by FoxO1 in endothelium include p27 kip1 (29), angiopoietin-2 (27, 30), and hydroxymethylglutaryl-CoA reductase (31). Taken together these findings suggest that FoxO1 integrates various cell signals at the transcriptional level that are relevant to endothelial function. In the present study, to elucidate additional downstream targets for DHEA-activated Akt in endothelial cells, we investigated whether FoxO1 regulates vasoactive actions of DHEA.
MATERIALS AND METHODS
Cell Culture—Bovine aortic endothelial cells (BAEC) (Cell Applications, San Diego, CA) or human aortic endothelial cells (HAEC) (Lonza, Walkersville, MD) in primary culture were grown in endothelial growth medium EGM-MV (BAEC, Lonza) or EGM-2 (HAEC, Lonza) and used between passages 3 and 6 as previously described (32).
Immunoblotting—Endothelial cells were grown in 60-mm dishes and serum-starved overnight (BAEC) or for 6 h (HAEC) in endothelial basal medium (Lonza). Cells were treated with DHEA (100 nm) (Sigma) for various times as indicated in the figure legends. Some cells were pretreated with ICI182780 (Tocris, Ellisville, MO), wortmannin (Sigma), H89 (Sigma), PD98059 (Sigma), PP2 (Calbiochem), SB203580 (EMD), or pertussis toxin (Sigma) before treatment with DHEA as indicated in the figure legends. Whole cell lysates were made with lysis buffer (100 mm NaCl, 20 mm Hepes, pH 7.9, 1% Triton X-100, 1 mm Na3VO4, 4 mm sodium pyrophosphate, 10 mm EDTA, 10 mm NaF, 1 mm phenylmethylsulfonyl fluoride, and complete protease inhibitor mixture (Roche Diagnostics). Lysates were immunoblotted according to standard methods (32) using antibodies against FoxO1 (Santa Cruz Biotechnology), phospho-FoxO1-Thr24 (Cell Signaling Technology), PKA (PKA C-α isoform, Cell Signaling Technology), or β-actin (Sigma). Immunoblots were quantified by scanning densitometry (GE Healthcare).
siRNA—HAECs were transfected with siRNA specifically targeting the catalytic subunit of human PKA with four sequences (1) GAA CAC ACC CUG AAU GAA A; 2) GAA CAC AGC CCA CUU GGA U; 3) CAA GGA CAA CUC AAA CUU A; 4) GCU AAG GGC AAA UGA ACG A (catalog #M-004649-01; gene official symbol, human PRKACA; Dharmacon, Chicago, IL)) or control scrambled siRNA (catalog #D-001210-03-05, Dharmacon) using Lipofectamine/PLUS Reagent (Invitrogen) for 3 h. Two days after transfection cells were serum-starved for 6 h and then treated with vehicle or DHEA (100 nm) for 30 min. Cells were lysed for immunoblotting as described above or fixed for immunohistochemistry as described below.
Immunohistochemistry—BAECs or HAECs were grown to 90% confluence in Lab-Tek chamber slides and serum-starved overnight (BAEC) or for 6 h (HAEC) in endothelial basal medium. Cells were then treated with vehicle or DHEA (100 nm, 30 or 60 min). Some cells were pretreated with wortmannin (100 nm) or H89 (25 μm) for 1 h before treatment with vehicle or DHEA. In some experiments, HAECs transfected with siRNA targeting PKA or scrambled siRNA were serum-starved for 6 h followed by treatment with vehicle or DHEA. Cells were then fixed with 4% paraformaldehyde at room temperature for 15 min and blocked in 0.05% Triton X-100 with 10% horse serum at room temperature for 15 min. The fixed cells were washed 3 times with PBS and incubated overnight at 4 °C with primary polyclonal rabbit antibodies (1:200 dilution) against phospho-FoxO1 (Cell Signaling Technology) or FoxO1 (Santa Cruz Biotechnology). The following day cells were washed three times with PBS and incubated with secondary antibodies (Alexa Fluor 586-conjugated goat anti-rabbit IgG; Molecular Probes, Eugene, OR) for 1 h at room temperature. Nuclear staining was performed by incubating cells with 4,6-diamidino-2-phenylindole (Molecular Probes) for 10 min. For visualization cells were washed three times with PBS and treated with ProLong Gold anti-fade reagent (Invitrogen). Images were evaluated using an Olympus IX81 epifluorescent microscope with appropriate filters and an attached charge-coupled device camera (Retiga Exi, Burnaby, British Columbia, Canada) in conjunction with IP Labs software (Scanalytics, Fairfax, VA).
PKA Activity Assay—PKA kinase activity was measured using a nonradioactive ELISA-based protein kinase assay kit (EMD Chemicals, Gibbstown, NJ). Briefly, BAECs cultured in 60-mm dishes were serum-starved overnight and then treated for 1 h with vehicle, DHEA (100 nm), or 3-isobutyl-1-methylxanthine (IBMX, 100 μm) (Sigma). Cells were washed twice with ice-cold PBS, and cell lysates were prepared with sample preparation buffer (50 mm Tris-HCl, 10 mm benzamidine 5 mm EDTA, 10 mm EGTA, and 1 mm phenylmethylsulfonyl fluoride, pH 7.5) and sonicated on ice for 10 s (5 times). Samples were then centrifuged at 100,000 × g for 1 h at 4 °C. The supernatant (cytosolic fraction) was collected, and protein concentration was measured with BCA reagent (Pierce). 50 mm β-mercapto-ethanol was added to each sample, and samples were incubated on ice for 30 min before kinase assay. 12-μl samples were used in each well, and assays were performed following the manufacturer's protocol. In some assays, bisindolylmaleimide 1 (150 μm, EMD) was preincubated with the cytosolic sample for 1 min at room temperature to compete with ATP binding. All samples were assayed in duplicate. Optical density (OD) of each sample was determined using a microplate reader, Power Wave X (Bio-Tek Instruments, Winooski, VT), and KC4 software (Bio-Tek) at a wavelength of 492 nm. PKA activity was expressed by normalizing OD reading to μg of sample used in each well.
cAMP Assay—Intracellular cAMP concentrations were measured in BAECs using an ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Briefly, BAECs at 90% confluence in 60-mm dishes were serum-starved overnight. Cells were then treated with vehicle, DHEA (100 nm for 0, 5, 15, or 30 min) or IBMX (100 μm, 1 h). Cells were washed 3 times with ice-cold PBS and lysed in provided cell lysis buffer. Samples were subjected to 3 freeze/thaw cycles and centrifuged at 600 × g for 10 min to remove debris. 100 μl of cell lysate from each sample was subjected to cAMP ELISA assay. A standard curve was prepared using provided cAMP standard with calibration dilution buffer. All samples, controls, and standards were assayed in duplicate. OD for each sample was determined at wavelengths of 450 and 540 nm as described above under “PKA Activity Assay.” The final OD was obtained by subtracting readings at 540 nm from readings at 450 nm to correct for optical imperfections in the plate. cAMP concentration in each sample was calculated by comparison with the standard curves.
ET-1 Promoter Activity—The human full-length ET-1 promoter in pGL2-firefly luciferase reporter vector (ET-1-luc) was kindly provided by Dr. Cam Patterson (University of North Carolina, Chapel Hill, NC) and has been described previously (33). pcDNA3-FLAG expression vectors for FoxO1 were kindly provided by Dr. Eric Tang (University of Michigan Medical School, Ann Arbor, MI) and have been previously described (34, 35). These included constructs containing the full-length open reading frame of wild-type FoxO1 (FoxO1-WT), the constitutively nuclear mutant FoxO1-AAA (3 Akt phosphorylation sites replaced by alanine, T24A/S256A/S319A), and FoxO1-H215R (point mutant with disrupted DNA binding site).
BAECs were grown to 80% confluence in 24-well plates, and each well of cells was co-transfected with 0.2 μg of ET-1-luc DNA and 0.1 μg of FoxO1-WT, FoxO1-AAA, or FoxO1-H215R using Lipofectamine/PLUS reagent for 3 h according to the manufacturer's protocol. To normalize for background luciferase activity, cells were also co-transfected with 10 ng of renilla luciferase reporter (pRL-TK, Promega). In some experiments cells were transfected with only ET-1-luc and pRL-TK DNA without FoxO1 constructs. Transfected cells were cultured in complete medium for 24 h and then serum-starved overnight. Cells were next treated with vehicle, DHEA (100 nm), or insulin (100 nm, Humulin, Eli Lilly) for 8 h. Some cells were treated with wortmannin (100 nm) or PD98059 (12.5 μm) with or without DHEA or insulin for 8 h. Luciferase activity was determined using the Dual-luciferase Reporter Assay System (Promega) in conjunction with a Lumat LB9501 luminometer (Berthold Technologies, Oak Ridge, TN). ET-1 promoter luciferase activity was normalized to renilla luciferase activity (internal control).
ET-1 Assay—BAECs grown in 35-mm dishes were serum-starved overnight. The next day media were replaced with fresh endothelial basal medium before treatment. BAECs were pretreated with vehicle, wortmannin (100 nm), or PD98059 (12.5 μm) for 1 h followed by treatment with vehicle or DHEA (100 nm) for 30 min. Conditioned media were collected from each dish, and cell lysates were prepared to determine protein concentration. For each sample, measurement of ET-1 in 100 μl of diluted conditioned media (1:1 dilution with PBS) was performed using an ELISA kit (Assay Designs, Ann Arbor, MI) according to the manufacturer's protocol. All samples were assayed in duplicate. The OD of each well was determined as described above under “PKA Activity Assay” at 450 and 580 nm. The reading at 450 nm was corrected by subtracting readings at 580 nm to correct for optical imperfections in the plate. Results were determined by comparison to standard curves and normalized to total protein concentration of cells in each well.
Statistical Analysis—Data are expressed as mean ± S.E. of multiple independent experiments. Unpaired t tests were used to evaluate differences where appropriate. p values <0.05 were considered to indicate statistical significance.
RESULTS
DHEA Acutely Stimulates Phosphorylation of FoxO1 in Vascular Endothelial Cells via PI 3-Kinase- and PKA-dependent Signaling—We previously demonstrated that DHEA has acute non-genomic actions to stimulate phosphorylation of Akt in BAEC (19). The transcription factor FoxO1 is a downstream target phosphorylated by Akt (24). Therefore, in the present study we examined the ability of DHEA to acutely stimulate phosphorylation of FoxO1 in BAECs. Treatment of cells with DHEA (100 nm) significantly enhanced phosphorylation of FoxO1 at Thr24 in a time-dependent manner with a maximal ∼2-fold effect after 30 min (Fig. 1). DHEA, a precursor of estradiol, weakly binds to the estrogen receptor (36, 37), and previous studies have implicated pertussis toxin-sensitive pathways in non-genomic actions of DHEA in endothelial cells (38, 39). To help rule out the possibility that effects of DHEA to stimulate phosphorylation of FoxO1 are mediated by the estrogen receptor or pertussis-toxin sensitive pathways, we stimulated BAEC with DHEA without or with pretreatment with ICI182780 (estrogen receptor inhibitor) or PTX (pertussis toxin) (Fig. 2, A and B). DHEA (100 nm, 30 min) and β-estradiol (20 nm, 5 min) both stimulated phosphorylation of FoxO1 when compared with vehicle-treated control cells (Fig. 2, A and B, lanes 1–3). Pretreatment of cells with ICI182780 only blocked the effect of β-estradiol, but not DHEA, to stimulate phosphorylation of FoxO1 (Fig. 2, A and B, lanes 4 and 5). Moreover, PTX pretreatment for 2 h (Fig. 2, A and B, lanes 7 and 8) or 24 h (data not shown) was unable to significantly block DHEA-stimulated phosphorylation of FoxO1. These results suggest that DHEA-stimulated phosphorylation of FoxO1 in endothelial cells does not require activation of estrogen receptors or PTX-sensitive pathways.
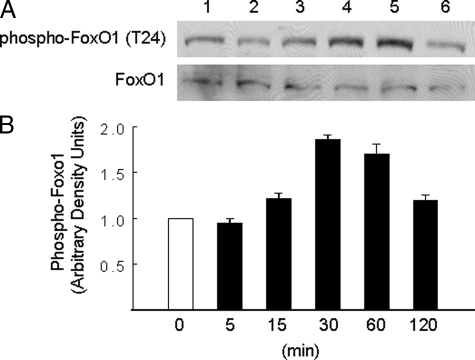
FIGURE 1.
DHEA acutely stimulated phosphorylation of FoxO1 in endothelial cells in a time-dependent manner. A, BAECs were serum-starved overnight and then treated with DHEA (100 nm) for the durations indicated in panel B. Whole cell lysates were immunoblotted with antibodies against phospho-FoxO1 or FoxO1. Representative immunoblots are shown for experiments that were repeated independently four times. B, immunoblots from four independent experiments were quantified by scanning densitometry, and the amounts of phospho-FoxO1 were normalized to total FoxO1 in each lane. Results in the bar graph are the mean ± S.E. The amount of phospho-FoxO1 in cell lysates was significantly increased over basal after treatment of cells with DHEA for 30 (p < 0.0001) or 60 min (p < 0.04).
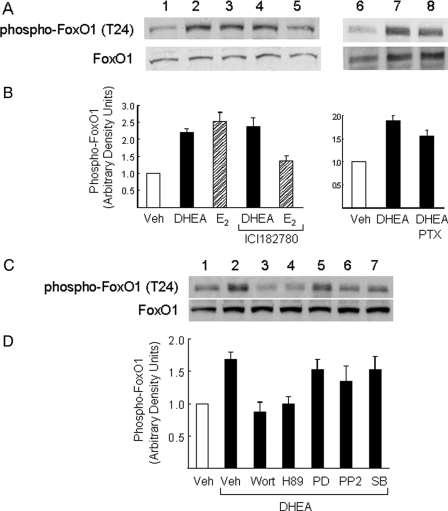
FIGURE 2.
Phosphorylation of FoxO1 in response to DHEA in endothelial cells was not mediated through estrogen receptor or Gi but requires activation of PI 3-kinase and PKA. A, BAECs were serum-starved overnight and then treated with vehicle (lane 1), DHEA (100 nm, 30 min; lanes 2, 4, 7, and 8), or β-estradiol (E2, 20 nm, 5 min; lanes 3 and 5). Some cells were pretreated with estrogen receptor antagonist ICI182780 (10 μm, 30 min; lanes 4 and 5) or PTX (100 ng/ml, 2 h; lane 8) before DHEA or E2 treatment. Whole cell lysates were immunoblotted with antibodies against phospho-FoxO or FoxO1. Representative immunoblots are shown for experiments that were repeated independently three times. B, immunoblots from three independent experiments were quantified by scanning densitometry, and the amounts of phospho-FoxO1 were normalized to total FoxO1 in each lane. Results are plotted as a bar graph (mean ± S.E.). The amount of phospho-FoxO1 in the cell lysates was significantly increased over basal after treatment of cells with DHEA or β-estradiol (p < 0.001 and 0.005, respectively). Pretreatment with ICI182780 or PTX did not significantly block this effect of DHEA (p > 0.6 and 0.5, respectively). PTX, pertussis toxin. C, BAECs were serum-starved overnight and then treated with vehicle (lane 1) or DHEA (100 nm, 30 min; lanes 2–7). Some cells were pretreated for 1 h with the PI 3-kinase inhibitor wortmannin (100 nm; lane 3), PKA inhibitor H89 (25 μm; lane 4), MEK inhibitor PD98059 (25 μm; lane 5), Src-family kinase inhibitor PP2 (1 μm; lane 6), or p38 MAPK inhibitor SB203580 (10 μm; lane 7) before DHEA treatment. D, immunoblots from four independent experiments were quantified by scanning densitometry, and the amounts of phospho-FoxO1 were normalized to total FoxO1 in each lane. Results in the bar graphs are the mean ± S.E. The amount of phospho-FoxO1 in cell lysates was significantly increased over basal after treatment of cells with DHEA. Pretreatment with wortmannin or H89 (but not other inhibitors) significantly blocked this effect of DHEA (p < 0.02 when compared with DHEA treatment alone). Veh, vehicle.
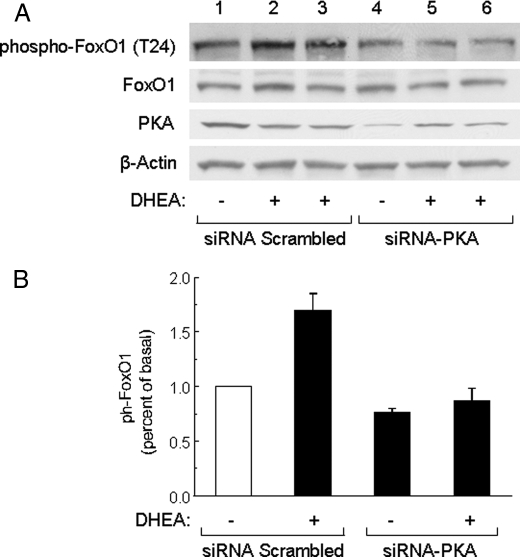
We next treated BAECs with a variety of chemical inhibitors including wortmannin, H89, PD98059, PP2, or SB203580 for 1 h before treatment with DHEA to determine which signaling pathways may be involved in DHEA-stimulated phosphorylation of FoxO1. Pretreatment with either wortmannin (PI 3-kinase inhibitor) or H89 (PKA inhibitor) completely blocked DHEA-stimulated phosphorylation of FoxO1 (Fig. 2, C and D, lanes 1–4). By contrast, pretreatment with PD98059 (MEK inhibitor), PP2 (Src inhibitor), or SB203580 (p38 MAPK inhibitor) did not significantly impair the ability of DHEA to stimulate phosphorylation of FoxO1 (Fig. 2, C and D, lanes 5–7). To further assess the role of PKA in DHEA-stimulated phosphorylation of FoxO1, we used siRNA oligonucleotides specifically targeting the human PKA catalytic subunit to reduce PKA expression in human endothelial cells. In preliminary experiments, a fluorescently tagged control oligonucleotide was used to determine the transfection efficiency of siRNA in HAEC under our conditions. We observed >90% transfection efficiency with our control oligonucleotides (data not shown). When compared with a control scrambled siRNA (Fig. 3A, third panel, lanes 1–3), siRNA targeting PKA specifically reduced PKA protein expression in transfected HAECs (Fig. 3A, third panel, lanes 4–6). Importantly, DHEA-stimulated phosphorylation of FoxO1 was substantially impaired in cells transfected with siRNA-PKA (Fig. 3, A, top panel, lanes 4–6, and B). Expression of FoxO1 protein was not affected by transfection with either the scrambled siRNA or siRNA targeting PKA (Fig. 3A, second panel). Taken together, our results suggest that DHEA-stimulated phosphorylation of FoxO1 in endothelial cells is mediated by both PI 3-kinase- and PKA-dependent signaling pathways.
FIGURE 3.
siRNA knockdown of PKA inhibited DHEA-stimulated phosphorylation of FoxO1. A, HAECs were transfected with scrambled control siRNA (lanes 1–3) or siRNA specifically targeting PKA (lanes 4–6). 48 h after transfection, cells were serum-starved for 6 h and treated with vehicle or DHEA (100 nm, 30 min) as indicated. Whole cell lysates were immunoblotted with antibodies against phospho-FoxO1, FoxO1, PKA, or β-actin. Representative immunoblots are shown for experiments that were repeated independently three times. B, immunoblots from three independent experiments were quantified by scanning densitometry, and the amounts of phospho-FoxO1 were normalized to total FoxO1. Results in the bar graph are the mean ± S.E.
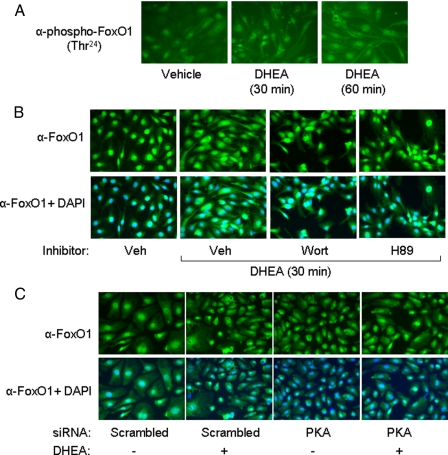
DHEA Treatment Promotes Nuclear Exclusion of FoxO1 in Endothelial Cells—One mechanism for regulation of FoxO1 function involves its nuclear exclusion in response to phosphorylation by Akt (24). Therefore, we assessed the effect of DHEA treatment on subcellular localization of FoxO1 using immunohistochemistry (Fig. 4). Treatment of BAECs with DHEA for 30 or 60 min significantly increased the amount of phosphorylated FoxO1 in the cytoplasm (Fig. 4A). Moreover, in HAECs, nuclear exclusion of FoxO1 caused by DHEA treatment for 30 min was significantly blocked by pretreatment of cells with either wortmannin or H89 (Fig. 4B). Because H89 may have other nonspecific actions in addition to inhibiting PKA, we also used siRNA to specifically knock down PKA. Similar to non-transfected HAECs (Fig. 4B), DHEA treatment promoted FoxO1 translocation from the nuclei to cytoplasm in HAECs transfected with scrambled control siRNA (Fig. 4C). However, in cells transfected with siRNA targeting PKA, DHEA-stimulated translocation of FoxO1 from the nucleus to the cytosol was substantially inhibited (Fig. 4C). These results support roles for both PI 3-kinase and PKA signaling in DHEA-stimulated translocation of FoxO1.
FIGURE 4.
DHEA stimulated phosphorylation of FoxO1 in intact endothelial cells and promoted nuclear exclusion of FoxO1 in a PI 3-kinase- and PKA-dependent manner. A, serum-starved BAECs were treated with vehicle or DHEA (100 nm) for 30 or 60 min. Cells were immunostained with anti-phospho-FoxO1 antibody as described under “Materials and Methods.” B, serum-starved HAECs were treated with vehicle or DHEA (100 nm, 30 min) as indicated. Some cells were pretreated for 1 h with vehicle, wortmannin (100 nm), or H89 (25 μm) before DHEA treatment. Cells were immunostained with FoxO1 antibody (top panels). Co-staining of the same cells with FoxO1 antibody and 4,6-diamidino-2-phenylindole (DAPI) is shown in the bottom panels. Veh, vehicle. C, HAECs were transfected with siRNA targeting PKA or scrambled control siRNA. Two days after transfection, cells were serum-starved for 6 h and treated with vehicle or DHEA (100 nm, 30 min) as indicated. Cells were immunostained with FoxO1 antibody (top panels). Co-staining of the same cells with 4,6-diamidino-2-phenylindole is shown in the bottom panels.
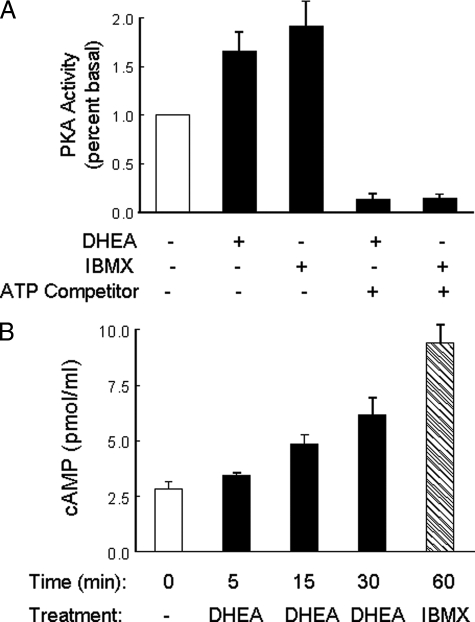
DHEA Treatment Increases PKA Activity and cAMP Concentration in Endothelial Cells—To evaluate whether DHEA activates PKA in endothelial cells, PKA activity was examined in cytosolic fractions isolated from BAECs treated with DHEA. Using an ELISA-based kinase assay with a PKA-specific peptide substrate, we found that DHEA treatment (100 nm, 1 h) induced an ∼70% increase in PKA activity above basal (Fig. 5A, p < 0.01) similar to that achieved with IBMX treatment (positive control). Effects of DHEA or IBMX on activation of PKA were inhibited by preincubation of samples with bisindolylmaleimide 1, an ATP binding competitor. DHEA-stimulated increases in PKA activity were not associated with changes in levels of expression of PKA protein (data not shown). Because cAMP is a primary intracellular activator of PKA, we also evaluated the ability of DHEA to increase cAMP levels in endothelial cells (Fig. 5B). When serum-starved BAEC were treated with DHEA (100 nm for 0, 5, 15, or 30 min), we observed a time-dependent increase in intracellular cAMP. Taken together, these data suggest that DHEA stimulates activation of PKA in endothelial cells through a mechanism that likely involves increased intracellular concentrations of cAMP.
FIGURE 5.
DHEA increased PKA activity and intracellular cAMP in endothelial cells. A, serum-starved BAECs were treated for 1 h with vehicle, DHEA (100 nm), or IBMX (100 μm). Cytosolic fractions were isolated and assayed for PKA activity using a nonradioactive kinase activity assay kit as described under “Materials and Methods.” Results of PKA activity are presented as percent basal (mean ± S.E. of five independent experiments performed in duplicate). In some assays an ATP binding competitor (bisindolylmaleimide 1, 150 μm) was preincubated with cytosolic fractions for 1 min at room temperature. Both IBMX and DHEA significantly increased PKA activity when compared with basal levels (IBMX versus basal, p < 0.005; DHEA versus basal, p < 0.01). These effects of DHEA and IBMX were completely blocked by bisindolylmaleimide 1. B, serum-starved BAECs were treated with DHEA (100 nm for 0, 5, 15, or 30 min) or IBMX (100 μm, 1 h). cAMP concentrations were measured in cell lysates using an ELISA-based kit as described under “Materials and Methods.” DHEA treatment significantly increased intracellular cAMP concentrations at 30 min when compared with 0 min (p < 0.01). Data shown are the mean ± S.E. from seven independent experiments performed in duplicate.
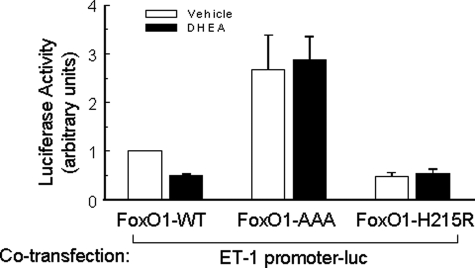
DHEA Inhibits ET-1 Promoter Activity Regulated by FoxO1—FoxO1 is an important transcription factor in endothelial cells that may help to regulate angiogenesis and endothelial function. Recently, we identified a novel FoxO1 binding site in the promoter of ET-1, a potent vasoconstrictor that contributes to endothelial dysfunction (40). To determine whether DHEA regulates ET-1 promoter activity through FoxO1, we assessed transactivation of an ET-1 promoter luciferase reporter in BAECs co-transfected with various FoxO1 expression vectors in the absence or presence of DHEA treatment for 8 h (Fig. 6). In cells co-transfected with a constitutively nuclear FoxO1 mutant (FoxO1-AAA) (without or with DHEA treatment), ET-1 promoter activity was increased to ∼2.5 times that observed in cells co-transfected with wild-type FoxO1 in the absence of DHEA treatment. In cells co-transfected with wild-type FoxO1, DHEA treatment significantly inhibited ET-1 promoter activity to a level similar to that observed in cells co-transfected with an inactive FoxO1 mutant with a disrupted DNA binding motif (FoxO1-H215R). Taken together, these results suggest that the ET-1 promoter is positively regulated by FoxO1, and phosphorylation of FoxO1 in response to DHEA treatment negatively regulates ET-1 promoter activity. Thus, one function for DHEA-stimulated phosphorylation of FoxO1 in endothelial cells may be to negatively regulate ET-1 promoter activity.
FIGURE 6.
DHEA inhibited the effect of FoxO1 to transactivate the ET-1 promoter. BAECs cultured in 24-well plates were co-transfected with an ET-1 promoter luciferase reporter construct and expression vectors for FoxO1-WT, FoxO1-AAA (constitutively nuclear mutant missing three Akt phosphorylation sites), or FoxO1-H215R (point mutant disrupting DNA binding site). One day after transfection, cells were serum-starved overnight and then treated with vehicle (open bars) or DHEA (100 nm, 8 h, closed bars) as indicated. A dual-luciferase reporter assay system was used to measure luciferase activity (mean ± S.E. of five independent experiments performed in triplicate), and data were normalized to the vehicle-treated FoxO1-WT group. DHEA treatment significantly blunted the effect of FoxO1-WT to transactivate the ET-1 promoter (p < 0.05).
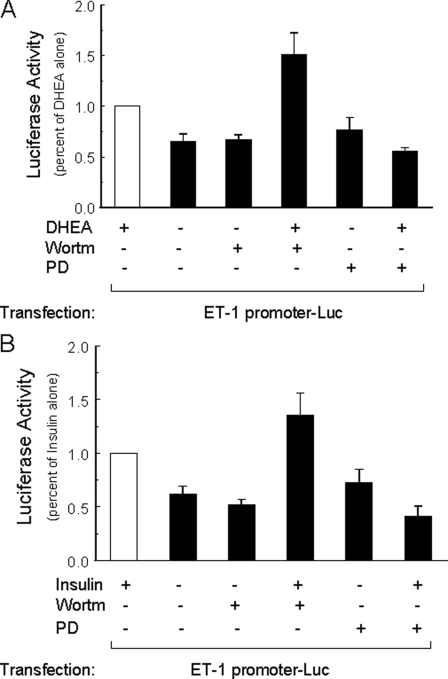
To further evaluate the effect of DHEA to regulate ET-1 promoter activity, we next assessed transactivation of the ET-1 promoter luciferase reporter in BAECs treated for 8 h with vehicle, DHEA, or insulin in the absence or presence of wortmannin or PD98059 (Fig. 7). When compared with vehicle-treated cells, ET-1 promoter activity was significantly increased in cells treated with either DHEA (Fig. 7A) or insulin (Fig. 7B) alone. Treatment with wortmannin or PD98059 alone did not significantly change ET-1 promoter activity when compared with vehicle treatment (Fig. 7, A and B). Interestingly, co-treatment of cells with wortmannin significantly enhanced effects of both DHEA and insulin to stimulate ET-1 promoter activity when compared with DHEA or insulin treatment alone. Conversely, co-treatment of cells with PD98059 and either DHEA or insulin resulted in ET-1 promoter activity levels that were comparable with those in vehicle-treated cells (Fig. 7, A and B). These results suggest that both DHEA and insulin have opposing effects on ET-1 promoter activity, 1) to inhibit ET-1 promoter activity through PI 3-kinase-dependent pathways and 2) to stimulate ET-1 promoter activity through MAPK-dependent pathways.
FIGURE 7.
Activation of ET-1 promoter in response to DHEA or insulin treatment was augmented by PI 3-kinase blockade and inhibited by MAPK blockade. BAECs grown in 24-well plates were transfected with ET-1 promoter luciferase reporter and renilla luciferase (internal control). One day later cells were serum-starved overnight and then treated for 8 h with vehicle, DHEA (100 nm), or insulin (100 nm) without or with wortmannin (100 nm), PD98059 (12.5 μm). Luciferase activity in each group was normalized to that in the group treated with DHEA alone (panel A, mean ± S.E. of eight independent experiments in triplicate) or insulin alone (panel B, mean ± S.E. of eight independent experiments in triplicate). When compared with cells treated with vehicle alone, ET-1 promoter activity was significantly increased in cells treated with either DHEA (panel A, p < 0.001) or insulin (panel B, p < 0.001). When compared with cells treated with DHEA or insulin alone, ET-1 promoter activity was further increased in cells co-treated with wortmannin (panel A, p < 0.03; panel B, p = 0.05) and inhibited in cells co-treated with PD98059 (panel A and B, p < 0.0001).
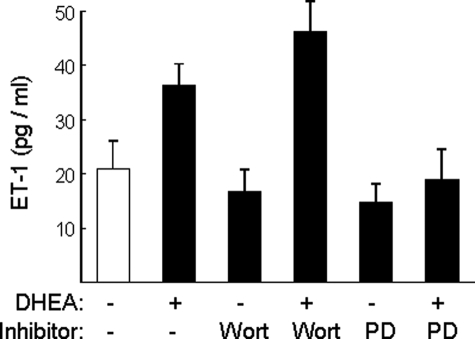
To determine whether the effect of DHEA on ET-1 promoter activity is mirrored in regulation of ET-1 secretion, we measured ET-1 in conditioned media collected from BAECs that were pretreated with vehicle, wortmannin, or PD98059 for 1 h followed by treatment with vehicle or DHEA for 30 min (Fig. 8). Consistent with our previously reported findings (19), DHEA treatment significantly increased ET-1 secretion from BAECs when compared with vehicle-treated control cells (Fig. 8, p < 0.03). Wortmannin or PD98059 alone did not significantly change ET-1 secretion when compared with vehicle treatment. However, pretreatment of cells with wortmannin further enhanced the ability of DHEA to stimulate ET-1 secretion (p = 0.05), whereas pretreatment of cells with PD98059 significantly reduced ET-1 secretion to the basal levels (p < 0.02, DHEA versus DHEA/PD98059; p > 0.4, DHEA/PD98059 versus vehicle). Thus, as with the effect of DHEA on ET-1 promoter activity, DHEA has opposing effects to inhibit ET-1 secretion through PI 3-kinase-dependent pathways and stimulate ET-1 secretion through MAPK-dependent pathways.
FIGURE 8.
DHEA-stimulated increase in ET-1 secretion is augmented by PI 3-kinase blockade and inhibited by MAPK blockade. BAECs grown in 35-mm dishes were serum-starved overnight and then pretreated with vehicle, wortmannin (100 nm), or PD-98059 (12.5μm) for 1 h followed by treatment of DHEA (100 nm) or vehicle for 30 min. ET-1 concentration was measured in conditioned media using ELISA (mean ± S.E. of six independent experiments performed in duplicate). When compared with vehicle treatment, DHEA treatment significantly increased ET-1 secretion (p < 0.03), whereas wortmannin or PD98059 alone did not significantly change ET-1 concentration in conditioned media. When compared with DHEA treatment alone, wortmannin pretreatment enhanced the effect of DHEA to increase ET-1 secretion (p = 0.05), whereas PD98059 blocked the ability of DHEA to stimulate ET-1 secretion (p < 0.02).
DISCUSSION
DHEA is an abundant adrenal steroid hormone whose molecular mechanisms of action are poorly understood. Circulating DHEA levels decrease with increasing age (4, 41). This is coupled with age-associated increases in insulin resistance, cardiovascular diseases, neurological disorders, and musculoskeletal frailty (42–44). Moreover, DHEA has biological actions in cell-based systems and animal models that mimic many of the actions of insulin and other hormones important for metabolic, cardiovascular, and neurological function (19, 45–49). Taken together, these observations have led to the hypothesis that DHEA supplementation may have beneficial health effects in humans to oppose age-related declines in metabolic, cardiovascular, neurological, and musculoskeletal function (50). DHEA is widely used as a popular nutritional supplement with putative anti-aging properties (51). However, clinical trials evaluating health benefits of DHEA supplementation in humans have yielded mixed results. Some studies report beneficial effects of DHEA to improve metabolic, cardiovascular, and neurological function (9, 48, 49, 52, 53). Nevertheless, other carefully performed, well powered clinical investigations using state-of-the-art methodology have found no benefit to DHEA supplementation in humans (10–13).
The underlying reasons for controversial and contradictory findings in well performed, adequately powered, clinical investigation of DHEA supplementation are not clear. One possibility is that DHEA supplementation may be beneficial only in certain physiological contexts, whereas it is ineffective or even harmful in other pathophysiological contexts. Our recent findings that DHEA regulates opposing vasoactive actions in vascular endothelium to stimulate production of the vasodilator NO by a PI 3-kinase-dependent mechanism while enhancing secretion of the vasoconstrictor ET-1 by MAPK-dependent pathways may be relevant for understanding the conflicting results of clinical trials with DHEA (19). That is, the net vasoactive action of DHEA supplementation (vasodilation or vasoconstriction) may be determined, in part, by the prevailing balance between distinct intracellular PI 3-kinase and MAPK signaling pathways present in a given physiological or pathophysiological context. For example, in SHR rats (a model of human metabolic syndrome with hypertension, insulin resistance, and overweight), hypertension, endothelial dysfunction, and insulin resistance are related to increased MAPK tone and decreased PI 3-kinase tone in the vascular endothelium (54). Interestingly, treatment of SHR rats with the insulin sensitizer rosiglitzone, the angiotensin converting enzyme (ACE) inhibitor enalapril, or the green tea polyphenol epigallocatechin gallate (EGCG) restores balance between MAPK and PI 3-kinase signaling in the endothelium resulting in decreased blood pressure, improved endothelial function, and enhanced insulin sensitivity (55, 56). In the present study we have discovered an additional mechanism whereby DHEA may favor vasodilator actions through PI 3-kinase and PKA-dependent inhibition of ET-1 synthesis and secretion. Our results suggest that opposing vascular actions of DHEA that are dependent on complex pathway-selective regulation of intracellular signal transduction pathways may help to determine whether DHEA supplementation has beneficial or detrimental effects on metabolic and cardiovascular health in humans. The concentrations of DHEA we use in the present study (100 nm) are higher than physiological circulating concentrations in humans (10–20 nm) (57, 58). However, this is not uncommon in biochemical studies of hormone actions where in vitro model systems are not as sensitive as in vivo conditions.
DHEA-stimulated Phosphorylation of FoxO1—In the present study we found that treatment of vascular endothelial cells with DHEA caused an acute time-dependent increase in phosphorylation of FoxO1 at the Akt phosphorylation site Thr24. This is consistent with our previous finding that DHEA acutely stimulates phosphorylation and activation of Akt in vascular endothelial cells (19) and the fact that FoxO1 is a substrate for Akt in other cell types (24). The phosphorylation of FoxO1 in response to DHEA treatment that we observed was not mediated by activation of the estrogen receptor or pertussis toxin-sensitive pathways. Using relatively specific chemical inhibitors we also ruled out roles for MAPK, Src-family kinases, and p38 MAPK. However, experiments using wortmannin and H89 suggested that activation of PI 3-kinase and PKA are required for DHEA-stimulated phosphorylation of FoxO1. Because Akt is downstream from PI 3-kinase, our results with wortmannin are not surprising. To further support the role of PKA, we demonstrated that specific and substantial reduction of PKA expression using siRNA blocked DHEA-stimulated phosphorylation of FoxO1.
Using immunohistochemistry, we demonstrated that DHEA treatment of endothelial cells resulted in acute phosphorylation of FoxO1 in intact cells that led to translocation of FoxO1 from the nucleus to the cytosol. Similar to our immunoblotting results, these effects were blocked in intact cells by pretreatment with wortmannin or H89 and in cells transfected with siRNA specifically targeting PKA. Our results in intact cells are consistent with previous studies showing that stimulation of cells with growth factors including insulin results in activation of Akt and other kinases that then phosphorylate specific serine/threonine motifs in FoxO1 leading to its exclusion from the nucleus and subsequent proteasomal degradation (34, 59–61).
DHEA Increased PKA Activity and Intracellular cAMP—In the present study we found that DHEA stimulated increased intracellular cAMP concentrations and elevated PKA activity without altering PKA expression in endothelial cells. These results suggest that PKA-dependent phosphorylation of FoxO1 is mediated by activation of PKA in response to DHEA treatment that increases intracellular cAMP levels in addition to DHEA-stimulated activation of PI 3-kinase signaling.
Effects of DHEA to Regulate ET-1 Synthesis and Secretion through FoxO1—In a related study we previously identified a novel FoxO1 binding site on the human ET-1 promoter (40). Genetic deletion of the gene for the FoxO1 transcription factor results in embryonic lethality due to a vascular phenotype (26, 62). Moreover, FoxO1 is the predominant FoxO isoform expressed in vascular endothelial cells (27). Therefore, it seems likely that DHEA-stimulated phosphorylation of FoxO1 in endothelial cells may play an important role in regulation of endothelial function. Indeed, we observed that overexpression of a constitutively nuclear FoxO1 mutant (missing Akt phosphorylation sites) strongly transactivated a human ET-1 promoter reporter construct. Furthermore, consistent with our findings that DHEA-stimulated phosphorylation of FoxO1 leads to its nuclear exclusion (Fig. 4) and that the ET-1 promoter is a transcriptional target for FoxO1 (40), DHEA treatment of endothelial cells overexpressing wild-type FoxO1 significantly diminished transactivation of a co-transfected ET-1 promoter reporter. Finally, expression of an inactive DNA binding domain mutant of FoxO1 also diminished transactivation of the ET-1 promoter reporter. Taken together, our results suggest that DHEA negatively regulates ET-1 promoter activity through phosphorylation of FoxO1 by a PI 3-kinase/Akt-dependent mechanism.
ET-1 is a potent vasoconstrictor (63) that plays important roles in vascular pathophysiology in part by mediating changes in endothelial permeability (64–67). Therefore, our results demonstrating DHEA-stimulated phosphorylation of FoxO1 negatively regulates ET-1 promoter activity are consistent with studies in Akt-1 knock-out mice that have a marked cardiovascular phenotype with increased vascular permeability, enhanced angiogenesis, and impaired vessel maturation (68). Our results are also concordant with previous studies demonstrating that DHEA treatment improves endothelial function and insulin sensitivity by a sex hormone receptor-independent mechanism (20, 69) and that DHEA protects endothelial cells against apoptosis through activation of PI 3-kinase/Akt signaling (39).
In a previous study we found that DHEA treatment of endothelial cells leads to an increase in ET-1 secretion that requires activation of MAPK (19). Consistent with this earlier finding, in the present study we observed that ET-1 promoter activity was also enhanced in response to insulin treatment of cells and that this effect was inhibited by pretreatment of cells with PD98059. Similarly, we observed that both ET-1 promoter activity and ET-1 secretion were enhanced in response to DHEA treatment and that these effects were inhibited by pretreatment of cells with PD98059. Importantly, pretreatment of cells with wortmannin significantly enhanced the ability of DHEA to stimulate ET-1 promoter activity and ET-1 secretion. Thus, in endothelial cells DHEA has opposing effects of stimulating ET-1 synthesis and secretion via MAPK-dependent pathways while inhibiting ET-1 synthesis and secretion via PI 3-kinase-dependent pathways that stimulate phosphorylation of FoxO1.
Our present results suggest a second mechanism for DHEA-stimulated activation of PI 3-kinase to favor vasorelaxant actions in the vasculature. That is, in addition to PI 3-kinase/Akt/endothelial nitric-oxide synthase activation leading to increased production of the vasodilator NO (19), PI 3-kinase/Akt/FoxO1 signaling may lead to a relative decrease in synthesis and secretion of the vasoconstrictor ET-1. Therefore, it seems likely that the balance between PI 3-kinase (vasodilator) and MAPK (vasoconstrictor) signaling in the cell in response to DHEA (or insulin) determines the net vasoactive action of DHEA (or insulin) (Fig. 9). The unequivocal identification and characterization of a specific cell-surface receptor for DHEA remains elusive despite intensive investigations for more than 50 years. The scope of our present study does not include identification of a DHEA receptor. However, we have elucidated a number of novel findings related to DHEA action in endothelial cells including a PKA-dependent pathway that is required in addition to PI 3-kinase pathways to regulate FoxO1 function to influence ET-1 promoter activity and secretion.
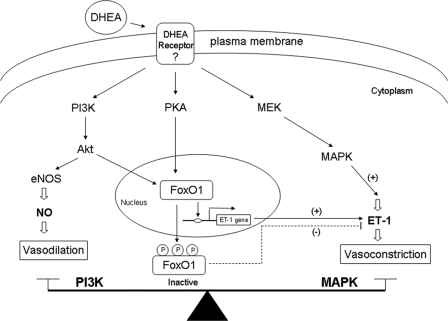
FIGURE 9.
Schematic model of signaling pathways that acutely regulate vascular actions of DHEA. As described previously (19), DHEA acutely activates PI 3-kinase/Akt/endothelial nitric-oxide synthase (eNOS) to stimulate production of the vasodilator NO. In addition, DHEA activates MAPK signaling to stimulate secretion of the vasoconstrictor ET-1. In the present study we demonstrated that DHEA acutely stimulates phosphorylation of FoxO1 using signaling pathways involving PKA and PI 3-kinase. Phosphorylated FoxO1 is excluded from the nucleus and translocated to the cytosol where it is unable to bind and activate the ET-1 promoter. Thus, a complex signaling network regulates opposing vascular actions of DHEA, and the net vasoactive action of DHEA is determined in part by the balance between PI 3-kinase- and MAPK-dependent signaling.
Our results may help to explain some of the contradictory findings among human therapeutic intervention studies with DHEA. That is, beneficial cardiovascular and metabolic effects of DHEA treatment may result if study subjects have PI 3-kinase tone outweighing MAPK tone in intracellular signaling pathways. Conversely, DHEA may have neutral or even detrimental cardiovascular and metabolic actions under pathological conditions of insulin resistance and endothelial dysfunction where MAPK tone predominates over PI 3-kinase tone in the vasculature (54–56, 70, 71). Thus, our findings that opposing vasoactive actions of DHEA are regulated by distinct MAPK- and PI 3-kinase-dependent signal transduction pathways may have important implications for the optimal design of future clinical trials of DHEA efficacy in humans. For example, in insulin-resistant states including aging, obesity, hypertension, and diabetes where PI 3-kinase signaling may be diminished and MAPK signaling may be enhanced (54–56, 70, 72), it may be helpful to evaluate combination therapy consisting of DHEA plus compounds that increase PI 3-kinase signaling in metabolic and vascular tissues (e.g. thiazoladinediones or ACE inhibitors).
In summary, we found that DHEA stimulates phosphorylation of FoxO1 via PI 3-kinase- and PKA-dependent signaling pathways in primary vascular endothelial cells that leads to decreased synthesis and secretion of ET-1. This represents an additional PI 3-kinase-dependent mechanism for DHEA to improve endothelial function (in addition to DHEA-stimulated production of NO). This complexity in the molecular mechanisms of vascular actions of DHEA may help to explain contradictory results from human intervention studies and may importantly inform the optimal design of future therapeutic trials.
This work was supported, in part, by the National Institutes of Health (Intramural Research Program, NCCAM) and by a Mentor-based award from the American Diabetes Association (to M. J. Q.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: DHEA, dehydroepiandrosterone; PI, phosphatidylinositol; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal-regulated kinase kinase; siRNA, small interfering RNA; BAEC, bovine aortic endothelial cells; HAEC, human aortic endothelial cells; PKA, protein kinase A; PBS, phosphate-buffered saline; ELISA, enzyme-linked immunosorbent assay; IBMX, 3-isobutyl-1-methylxanthine; OD, optical density.
References
- 1.Lasley, B. L., Santoro, N., Randolf, J. F., Gold, E. B., Crawford, S., Weiss, G., McConnell, D. S., and Sowers, M. F. (2002) J. Clin. Endocrinol. Metab. 87 3760-3767 [DOI] [PubMed] [Google Scholar]
- 2.Belanger, A., Candas, B., Dupont, A., Cusan, L., Diamond, P., Gomez, J. L., and Labrie, F. (1994) J. Clin. Endocrinol. Metab. 79 1086-1090 [DOI] [PubMed] [Google Scholar]
- 3.Slowinska-Srzednicka, J., Zgliczynski, S., Soszynski, P., Makowska, A., Zgliczynski, W., Srzednicki, M., Bednarska, M., Chotkowska, E., Woroszylska, M., Ruzyllo, W., and Sadowski, Z. (1991) J. Intern. Med. 230 551-553 [PubMed] [Google Scholar]
- 4.Barrett-Connor, E., and Goodman-Gruen, D. (1995) Ann. N. Y. Acad. Sci. 774 259-270 [DOI] [PubMed] [Google Scholar]
- 5.Johannes, C. B., Stellato, R. K., Feldman, H. A., Longcope, C., and McKinlay, J. B. (1999) J. Clin. Epidemiol. 52 95-103 [DOI] [PubMed] [Google Scholar]
- 6.Mazat, L., Lafont, S., Berr, C., Debuire, B., Tessier, J. F., Dartigues, J. F., and Baulieu, E. E. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 8145-8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon, G. B., Bush, D. E., and Weisman, H. F. (1988) J. Clin. Investig. 82 712-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams, J. R. (2000) Lipids 35 325-331 [DOI] [PubMed] [Google Scholar]
- 9.Kawano, H., Yasue, H., Kitagawa, A., Hirai, N., Yoshida, T., Soejima, H., Miyamoto, S., Nakano, M., and Ogawa, H. (2003) J. Clin. Endocrinol. Metab. 88 3190-3195 [DOI] [PubMed] [Google Scholar]
- 10.Nair, K. S., Rizza, R. A., O'Brien, P., Dhatariya, K., Short, K. R., Nehra, A., Vittone, J. L., Klee, G. G., Basu, A., Basu, R., Cobelli, C., Toffolo, G., Dalla Man, C., Tindall, D. J., Melton, L. J., III, Smith, G. E., Khosla, S., and Jensen, M. D. (2006) N. Engl. J. Med. 355 1647-1659 [DOI] [PubMed] [Google Scholar]
- 11.Percheron, G., Hogrel, J. Y., Denot-Ledunois, S., Fayet, G., Forette, F., Baulieu, E. E., Fardeau, M., and Marini, J. F. (2003) Arch. Intern. Med. 163 720-727 [DOI] [PubMed] [Google Scholar]
- 12.Welle, S., Jozefowicz, R., and Statt, M. (1990) J. Clin. Endocrinol. Metab. 71 1259-1264 [DOI] [PubMed] [Google Scholar]
- 13.Usiskin, K. S., Butterworth, S., Clore, J. N., Arad, Y., Ginsberg, H. N., Blackard, W. G., and Nestler, J. E. (1990) Int. J. Obes. 14 457-463 [PubMed] [Google Scholar]
- 14.Leiter, E. H., Beamer, W. G., Coleman, D. L., and Longcope, C. (1987) Metabolism 36 863-869 [DOI] [PubMed] [Google Scholar]
- 15.Stomati, M., Monteleone, P., Casarosa, E., Quirici, B., Puccetti, S., Bernardi, F., Genazzani, A. D., Rovati, L., Luisi, M., and Genazzani, A. R. (2000) Gynecol. Endocrinol. 14 342-363 [DOI] [PubMed] [Google Scholar]
- 16.Elbeltagy, K., Honda, K. I., Ozaki, K., Misugi, T., Tokuyama, O., Kimura, M., Kira, Y., and Ishiko, O. (2007) Fertil. Steril. 88 (Suppl. 4), 1135-1142 [DOI] [PubMed] [Google Scholar]
- 17.Liu, D., and Dillon, J. S. (2002) J. Biol. Chem. 277 21379-21388 [DOI] [PubMed] [Google Scholar]
- 18.Simoncini, T., Mannella, P., Fornari, L., Varone, G., Caruso, A., and Genazzani, A. R. (2003) Endocrinology 144 3449-3455 [DOI] [PubMed] [Google Scholar]
- 19.Formoso, G., Chen, H., Kim, J. A., Montagnani, M., Consoli, A., and Quon, M. J. (2006) Mol. Endocrinol. 20 1153-1163 [DOI] [PubMed] [Google Scholar]
- 20.Williams, M. R., Dawood, T., Ling, S., Dai, A., Lew, R., Myles, K., Funder, J. W., Sudhir, K., and Komesaroff, P. A. (2004) J. Clin. Endocrinol. Metab. 89 4708-4715 [DOI] [PubMed] [Google Scholar]
- 21.Kops, G. J., and Burgering, B. M. (1999) J. Mol. Med. 77 656-665 [DOI] [PubMed] [Google Scholar]
- 22.Van Der Heide, L. P., Hoekman, M. F., and Smidt, M. P. (2004) Biochem. J. 380 297-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakae, J., Biggs, W. H., III, Kitamura, T., Cavenee, W. K., Wright, C. V., Arden, K. C., and Accili, D. (2002) Nat. Genet. 32 245-253 [DOI] [PubMed] [Google Scholar]
- 24.Accili, D., and Arden, K. C. (2004) Cell 117 421-426 [DOI] [PubMed] [Google Scholar]
- 25.Greer, E. L., and Brunet, A. (2005) Oncogene 24 7410-7425 [DOI] [PubMed] [Google Scholar]
- 26.Furuyama, T., Kitayama, K., Shimoda, Y., Ogawa, M., Sone, K., Yoshida-Araki, K., Hisatsune, H., Nishikawa, S., Nakayama, K., Nakayama, K., Ikeda, K., Motoyama, N., and Mori, N. (2004) J. Biol. Chem. 279 34741-34749 [DOI] [PubMed] [Google Scholar]
- 27.Potente, M., Urbich, C., Sasaki, K., Hofmann, W. K., Heeschen, C., Aicher, A., Kollipara, R., DePinho, R. A., Zeiher, A. M., and Dimmeler, S. (2005) J. Clin. Investig. 115 2382-2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejana, E., Taddei, A., and Randi, A. M. (2007) Biochim. Biophys. Acta 1775 298-312 [DOI] [PubMed] [Google Scholar]
- 29.Potente, M., Fisslthaler, B., Busse, R., and Fleming, I. (2003) J. Biol. Chem. 278 29619-29625 [DOI] [PubMed] [Google Scholar]
- 30.Daly, C., Pasnikowski, E., Burova, E., Wong, V., Aldrich, T. H., Griffiths, J., Ioffe, E., Daly, T. J., Fandl, J. P., Papadopoulos, N., McDonald, D. M., Thurston, G., Yancopoulos, G. D., and Rudge, J. S. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 15491-15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisslthaler, B., Fleming, I., Keseru, B., Walsh, K., and Busse, R. (2007) Circ. Res. 100 e12-e21 [DOI] [PubMed] [Google Scholar]
- 32.Chen, H., Montagnani, M., Funahashi, T., Shimomura, I., and Quon, M. J. (2003) J. Biol. Chem. 278 45021-45026 [DOI] [PubMed] [Google Scholar]
- 33.Aitsebaomo, J., Kingsley-Kallesen, M. L., Wu, Y., Quertermous, T., and Patterson, C. (2001) J. Biol. Chem. 276 39197-39205 [DOI] [PubMed] [Google Scholar]
- 34.Tang, E. D., Nunez, G., Barr, F. G., and Guan, K. L. (1999) J. Biol. Chem. 274 16741-16746 [DOI] [PubMed] [Google Scholar]
- 35.Armoni, M., Harel, C., Karni, S., Chen, H., Bar-Yoseph, F., Ver, M. R., Quon, M. J., and Karnieli, E. (2006) J. Biol. Chem. 281 19881-19891 [DOI] [PubMed] [Google Scholar]
- 36.Chen, F., Knecht, K., Birzin, E., Fisher, J., Wilkinson, H., Mojena, M., Moreno, C. T., Schmidt, A., Harada, S., Freedman, L. P., and Reszka, A. A. (2005) Endocrinology 146 4568-4576 [DOI] [PubMed] [Google Scholar]
- 37.Iruthayanathan, M., Zhou, Y. H., and Childs, G. V. (2005) Endocrinology 146 5176-5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, D., and Dillon, J. S. (2004) Steroids 69 279-289 [DOI] [PubMed] [Google Scholar]
- 39.Liu, D., Si, H., Reynolds, K. A., Zhen, W., Jia, Z., and Dillon, J. S. (2007) Endocrinology 148 3068-3076 [DOI] [PubMed] [Google Scholar]
- 40.Reiter, C., Kim, J., and Quon, M. J. (2007) Diabetes 56 (Suppl. 1), A14 [Google Scholar]
- 41.Labrie, F., Belanger, A., Cusan, L., Gomez, J. L., and Candas, B. (1997) J. Clin. Endocrinol. Metab. 82 2396-2402 [DOI] [PubMed] [Google Scholar]
- 42.Schriock, E. D., Buffington, C. K., Hubert, G. D., Kurtz, B. R., Kitabchi, A. E., Buster, J. E., and Givens, J. R. (1988) J. Clin. Endocrinol. Metab. 66 1329-1331 [DOI] [PubMed] [Google Scholar]
- 43.Barrett-Connor, E., Khaw, K. T., and Yen, S. S. (1986) N. Engl. J. Med. 315 1519-1524 [DOI] [PubMed] [Google Scholar]
- 44.Valenti, G., Denti, L., Maggio, M., Ceda, G., Volpato, S., Bandinelli, S., Ceresini, G., Cappola, A., Guralnik, J. M., and Ferrucci, L. (2004) J. Gerontol. A Biol. Sci. Med. Sci. 59 466-472 [DOI] [PubMed] [Google Scholar]
- 45.Perrini, S., Natalicchio, A., Laviola, L., Belsanti, G., Montrone, C., Cignarelli, A., Minielli, V., Grano, M., De Pergola, G., Giorgino, R., and Giorgino, F. (2004) Diabetes 53 41-52 [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka, T., Miura, A., Kajita, K., Matsumoto, M., Sugiyama, C., Matsubara, K., Ikeda, T., Mori, I., Morita, H., Uno, Y., Mune, T., Kanoh, Y., and Ishizawa, M. (2007) Acta Diabetol. 44 219-226 [DOI] [PubMed] [Google Scholar]
- 47.Nakazora, H., and Kurihara, T. (2005) Intern. Med. 44 1247-1251 [DOI] [PubMed] [Google Scholar]
- 48.Morales, A. J., Nolan, J. J., Nelson, J. C., and Yen, S. S. (1994) J. Clin. Endocrinol. Metab. 78 1360-1367 [DOI] [PubMed] [Google Scholar]
- 49.Yen, S. S. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 8167-8169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tchernof, A., and Labrie, F. (2004) Eur. J. Endocrinol. 151 1-14 [DOI] [PubMed] [Google Scholar]
- 51.Tamler, R., and Mechanick, J. I. (2007) Endocrinol. Metabol. Clin. North Am. 36 533-552 [DOI] [PubMed] [Google Scholar]
- 52.Villareal, D. T., Holloszy, J. O., and Kohrt, W. M. (2000) Clin. Endocrinol. (Oxf) 53 561-568 [DOI] [PubMed] [Google Scholar]
- 53.Barnhart, K. T., Freeman, E., Grisso, J. A., Rader, D. J., Sammel, M., Kapoor, S., and Nestler, J. E. (1999) J. Clin. Endocrinol. Metab. 84 3896-3902 [DOI] [PubMed] [Google Scholar]
- 54.Potenza, M. A., Marasciulo, F. L., Chieppa, D. M., Brigiani, G. S., Formoso, G., Quon, M. J., and Montagnani, M. (2005) Am. J. Physiol. Heart Circ. Physiol. 289 813-822 [DOI] [PubMed] [Google Scholar]
- 55.Potenza, M. A., Marasciulo, F. L., Tarquinio, M., Quon, M. J., and Montagnani, M. (2006) Diabetes 55 3594-3603 [DOI] [PubMed] [Google Scholar]
- 56.Potenza, M. A., Marasciulo, F. L., Tarquinio, M., Tiravanti, E., Colantuono, G., Federici, A., Kim, J. A., Quon, M. J., and Montagnani, M. (2007) Am. J. Physiol. Endocrinol. Metab. 292 1378-1387 [DOI] [PubMed] [Google Scholar]
- 57.de Peretti, E., and Forest, M. G. (1978) J. Clin. Endocrinol. Metab. 47 572-577 [DOI] [PubMed] [Google Scholar]
- 58.Kalimi, M., Shafagoj, Y., Loria, R., Padgett, D., and Regelson, W. (1994) Mol. Cell. Biochem. 131 99-104 [DOI] [PubMed] [Google Scholar]
- 59.Biggs, W. H., III, Meisenhelder, J., Hunter, T., Cavenee, W. K., and Arden, K. C. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 7421-7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang, H., Regan, K. M., Wang, F., Wang, D., Smith, D. I., van Deursen, J. M., and Tindall, D. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1649-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K., and Fukamizu, A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 11285-11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosaka, T., Biggs, W. H., III, Tieu, D., Boyer, A. D., Varki, N. M., Cavenee, W. K., and Arden, K. C. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2975-2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K., and Masaki, T. (1988) Nature 332 411-415 [DOI] [PubMed] [Google Scholar]
- 64.Giachini, F. R., Callera, G. E., Carneiro, F. S., Tostes, R. C., and Webb, R. C. (2008) Expert Opin. Ther. Targets 12 327-339 [DOI] [PubMed] [Google Scholar]
- 65.Iglarz, M., and Clozel, M. (2007) J. Cardiovasc. Pharmacol. 50 621-628 [DOI] [PubMed] [Google Scholar]
- 66.Porter, L. P., McNamee, J. E., and Wolf, M. B. (2000) Microcirculation 7 347-356 [PubMed] [Google Scholar]
- 67.Narushima, I., Kita, T., Kubo, K., Yonetani, Y., Momochi, C., Yoshikawa, I., Shimada, K., and Nakashima, T. (1999) Naunyn-Schmiedebergs Arch. Pharmacol. 360 639-645 [DOI] [PubMed] [Google Scholar]
- 68.Chen, J., Somanath, P. R., Razorenova, O., Chen, W. S., Hay, N., Bornstein, P., and Byzova, T. V. (2005) Nat. Med. 11 1188-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aoki, K., Saito, T., Satoh, S., Mukasa, K., Kaneshiro, M., Kawasaki, S., Okamura, A., and Sekihara, H. (1999) Diabetes 48 1579-1585 [DOI] [PubMed] [Google Scholar]
- 70.Muniyappa, R., Montagnani, M., Koh, K. K., and Quon, M. J. (2007) Endocr. Rev. 28 463-491 [DOI] [PubMed] [Google Scholar]
- 71.Kim, J. A., Montagnani, M., Koh, K. K., and Quon, M. J. (2006) Circulation 113 1888-1904 [DOI] [PubMed] [Google Scholar]
- 72.Cusi, K., Maezono, K., Osman, A., Pendergrass, M., Patti, M. E., Pratipanawatr, T., DeFronzo, R. A., Kahn, C. R., and Mandarino, L. J. (2000) J. Clin. Investig. 105 311-320 [DOI] [PMC free article] [PubMed] [Google Scholar]