Abstract
Glaucoma is defined as a chronic and progressive optic nerve neuropathy, characterized by apoptosis of retinal ganglion cells (RGC) that leads to irreversible blindness. Ocular hypertension is a major risk factor, but in glaucoma RGC death can persist after ocular hypertension is normalized. To understand the mechanism underlying chronic RGC death we identified and characterized a gene product, α2-macroglobulin (α2M), whose expression is up-regulated early in ocular hypertension and remains up-regulated long after ocular hypertension is normalized. In ocular hypertension retinal glia up-regulate α2M, which binds to low-density lipoprotein receptor-related protein-1 receptors in RGCs, and is neurotoxic in a paracrine fashion. Neutralization of α2M delayed RGC loss during ocular hypertension; whereas delivery of α2M to normal eyes caused progressive apoptosis of RGC mimicking glaucoma without ocular hypertension. This work adds to our understanding of the pathology and molecular mechanisms of glaucoma, and illustrates emerging paradigms for studying chronic neurodegeneration in glaucoma and perhaps other disorders.
Vision impairment due to glaucoma affects 50 million people worldwide. In open angle glaucoma, visual field loss is caused by retinal ganglion cell (RGC)3 apoptosis, concomitant with elevated intraocular pressure (IOP). Current treatments are limited to reduction of high IOP. Unfortunately, whereas these treatments are often successful at normalizing IOP, progressive RGC death and visual field loss often continue (1–3). In addition ∼20% of patients are affected by normal tension glaucoma, a distinct optic nerve neuropathy in the absence of high IOP.
Proposed mechanisms of RGC apoptosis in glaucoma include: mechanical compression of the optic nerve head preventing axonal transport of neurotrophins required for RGC survival (also known as “physiologic axotomy”) (4), excitotoxic damage by hyperactive NMDA receptors, elevated glutamate, Ca2+ fluxes, and nitric oxide (5, 6); ischemic and other retinal injury leading to activation of microglia (7), β-amyloid toxicity (8), and inflammatory damage through tumor necrosis factor-α (TNFα) (9, 10). However, none of these mechanisms explain two key issues. First, all the cells in the inner retina are exposed to these deleterious effects, thus it is puzzling that RGCs are preferentially susceptible to apoptosis. Second, normalization of pressure often does not result in the complete arrest of RGC death, which continues chronically.
To address these questions, we hypothesized that a short span of ocular hypertension can trigger long-lived changes in retinal gene expression, changes that are deleterious to RGCs. Five unique criteria were set to identify intraocular pressure regulated early gene (IPREG) candidates. Altered gene expression should: (i) occur specifically in the retina and be caused specifically by ocular hypertension; (ii) occur relatively early following ocular hypertension and prior to RGC damage; (iii) be long-lived; (iv) independent of the continuous presence of high IOP; and (v) be linked to RGC death.
We identified potential IPREGs using gene arrays, and here we show work that validates one IPREG that meets all these criteria, α2-macroglobulin (α2M). α2M is an acute phase soluble protein whose known intrinsic and receptor-mediated activities can be linked to the above mechanisms postulated for glaucoma.
In this report we show that soluble α2M is up-regulated relatively early in two rat models of glaucoma, before significant RGC death, but it is not up-regulated after optic nerve axotomy. Up-regulated synthesis of α2M occurs predominantly in retinal glia, and is long-lived independently of continuous high IOP. α2M activity was linked to RGC death. Adding exogenous α2M to normal eyes caused apoptotic RGC death comparable with glaucoma but with normal IOP; whereas neutralization of α2M in glaucomatous eyes delayed RGC death, especially in combination with treatments that normalize high IOP.
Our data demonstrate that α2M is a strong mediator of RGC damage in glaucoma and is a potential therapeutic target for this incurable disease. This finding will contribute to our understanding of the complex molecular mechanisms underlying neurodegeneration in glaucoma.
MATERIALS AND METHODS
Optic Nerve Axotomy
Female Wistar rats between 250 and 300 g were anesthetized with a mixture of xylazine, acepromazine, and ketamine. The eye bulb was accessed by opening the dorsal orbita and partially removing the tear glands and orbital fat. The optic nerve (ON) was visualized by separating the superior rectus muscle followed by an incision of the eye retractor muscle. A longitudinal incision of the meninges was made 5 mm behind the bulbar exit of the ON, avoiding blood vessels. Sectioning of the ON was made 5 mm posterior of its exit from the eyeball.
Models for Inducing High IOP
Episcleral Cauterization Model—Cauterization was performed under anesthesia in female Wistar rats, 8 weeks of age, as described (11). Cauterization of three episcleral vessels in the right eye were done with a 30-min cautery tip (12). The left eye in each animal was used as normal IOP control after sham surgery (conjuctival incisions with no cauterization). Planar ophthalmoscopy was used to confirm normal perfusion of the retina at elevated IOP. Cauterization causes a chronic and stable increase of ∼1.7-fold in IOP without causing ischemia (1).
Hypertonic Model—Unilateral and chronic elevation of IOP was induced as described (13) by injection of a hypertonic saline solution into a single episcleral vein. A plastic ring was applied to the ocular equator to confine the injection to the limbal plexus. A microneedle was used to inject 50 μl of sterile 1.85 m NaCl solution through one episcleral vein. Following injection, the plastic ring was removed and the eyes were examined to assess the extent to which the saline solution traversed the limbal vasculature. Polysporin ophthalmic ointment (TMIMC Pfizer Canada Inc., ON, Canada) was applied to the operated eye and the animal was allowed to recover from the surgery. Animals were kept in a room with constant low fluorescent light (40–100 lux) to stabilize circadian IOP variations.
IOP Measurements
IOP was gauged using a Tonopen XL tonometer in awake animals under light anesthesia (intramuscular injection of ketamine, 4 mg/kg; xylazine, 0.32 mg/kg; and acepromazine, 0.4 mg/kg). The accuracy of the readings of the Tonopen compared with other instruments, even under anesthesia, has been established (14). The mean normal IOP of rats under light anesthesia was 12 mm Hg (range 10–14 mm Hg), and in cauterized eyes it is elevated to a stable average 21 mm Hg (range 18–24 mm Hg) for longer than 4 months (11, 12).
Pharmacological reduction of high IOP. A selective β-blocker (betaxolol 0.5%, Alcon) was applied daily as eye drops. Topical betaxolol administration results in full normalization of IOP after ∼3 days, and thereafter IOP continued to remain normalized, although betaxolol was applied. Betaxolol had no significant effect in the IOP of normal eyes.
Kinetics Analyses
Assessment of gene or protein expression, RT-PCR, Northern blots, Westerns blots, and quantification of retrograde-labeled RGCs were done on freshly isolated retinas from control (sham-operated, or axotomized) or from cauterized eyes at the indicated days after surgery.
RNA Preparation
Total RNA was isolated from retinal tissue using TRIzol (Invitrogen). RNA was purified using the RNeasy (Qiagen). The integrity and size distribution of RNA samples were assessed on RNA 6000 Nano LabChip (Agilent) using the 2100 bioanalyzer (Agilent).
RT-PCR
Retinas from control, high IOP, or axotomized animals were dissected on the indicated days. Total retinal RNA was extracted (TRIzol), DNA was digested (DNase, amplification grade, Invitrogen), and samples were re-purified after a second TRIzol extraction. For RT-PCR analysis single retinas were used (n = 3–5). One μg of total retinal RNA and specific primers were used to generate complementary cDNAs by semi-quantitative PCR analysis (11). Linear amplification of candidate genes was obtained after a total of 30 cycles, whereas β-actin (used as internal control) was in the linear range after 18 cycles. Agarose gels resolving the PCR products were scanned using a STORM 840 imaging system and quantitative analysis were performed using ImageQuant analysis software, in three independent RT-PCR experiments using three independently prepared RNA samples. Readings were averaged ± S.E., and data for each gene product in each group (normal IOP and high IOP) were normalized against β-actin as internal control. We verified that retinal β-actin mRNA levels did not vary in high IOP (data not shown).
In situ mRNA Hybridization Probes
For α2M RT-PCR probes we used (forward) GTGCTGCTCATGAAACCTGA (backward) CTTCGCCTAGTCTCTGTGGG primers that yield a PCR fragment of 390 base pairs. For amphiphysin RT-PCR probes we used (forward) TCGATGTGGAAAGCACTGAG (backward) CTTCTGAGTCTGAGGGCACC primers that yield a PCR fragment of 432 base pairs. The digoxigenin (DIG) PCR probe synthesis kit (Roche) was used with DIG-dUTP to generate DNA labeled with DIG, and labeled probes were purified. The DIG label increased the Mr of the products by 100–200 units indicating efficient labeling.
In situ Hybridization
Retinal cryosections (10 μm) were prepared from normal or day 21 glaucoma retinas. Sections on coverslips were air dried (5 min), and 80 ng of DIG-labeled probe in 20 μl of probe mixture and a coverslip were placed over each cryosection. Then slides were placed on a heat plate at 95 °C for 5 min to denature DNA, followed by cooling the slides for 5 min on ice. Slides were further incubated at 42 °C overnight in a humidified chamber. After removing coverslips, slides were washed twice 5 min in 2× SSC at 20 °C, and once for 10 min in 0.1× SSC at 42 °C. Negative controls used the same probes without DIG label. Detection was performed with the DIG Nucleic Acid Detection kit (Roche) following their instructions using nitro blue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate. To show the morphology after pictures from the in situ hybridization were taken, coverslips were removed and the retinal sections were washed gently in PBS, and then stained for 10–30 min in neutral red (5 mg/ml in water, pH 6.8, Roche), followed by washing in PBS, mounting, and new pictures were taken.
Western Blot Analyses
Single rat retinas were homogenized and lysed (150 mm NaCl, 50 mm Tris, pH 8.0, 2% Nonidet P-40, phenylmethylsulfonyl fluoride, leupeptin, and aprotinin) for 45 min. After centrifugation to remove nuclei and debris, soluble protein concentrations were determined (Bio-Rad). Fifteen μg of retinal proteins/lane were fractionated on a 12% SDS-PAGE, and transferred to a nitrocellulose membrane. The α2M protein was detected using goat polyclonal antibodies against α2M (Sigma and Calbiochem) and horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were revealed with enhanced chemiluminiscence (PerkinElmer Life Sciences). Pure α2M protein (Sigma) was loaded to quantify signals.
Intraocular Injections of Drugs
Solutions of activated α2M (Sigma), anti-α2M rabbit antibody (Calbiochem), control vehicle PBS, or control rabbit antibody (Sigma) were injected at the days 14 and 21 of glaucoma. The intraocular injections were in 2-μl volumes containing a total of 1 μg of α2M or 2 μg of antibody. Ocular pressure was not affected by intraocular injections, the high IOP eyes maintained high IOP and the normal IOP eyes maintained normal IOP (data not shown). For injections, a conjunctival incision was performed in the superior temporal quadrant of the eye. A puncture was made on the eye wall with a 30-gauge needle to allow the entrance of a cannula in the orbit. The tip of the needle was inserted at a 45° angle through the sclera into the vitreous body. This route of administration avoided injury to eye structures. A glass cannula (10 μm thickness) prepared with a microlectrode puller (Narishige) was connected through plastic tubing to a Hamilton syringe to dispense solutions. All animal procedures were approved by the McGill Animal Welfare Committee.
α2M Preparations
Commercial α2M (Sigma) required activation to become competent. α2M (10 mg/ml PBS) was activated with methylamine (100 mm) for 2 h at room temperature in the dark. The mixture was then dialyzed versus PBS. The Mr of activated α2M was smaller in Western blots (data not shown), indicating the expected processing.
Retrograde RGC Labeling
RGCs were labeled with 3% 1,1-dioctadecyl-3,3,3, 3-tetramethylindocarbocyanine perchlorate or with 3% Fluorogold (11). Briefly, rats were anesthetized and their heads were mounted in a stereotactic apparatus. Superior colliculi were exposed and the dye was injected in each hemisphere 5.8 mm behind Bregma, 1.0 mm lateral, and at depths of 5 and 3.5 mm.
Immunohistochemistry and Confocal Microscopy
Rats were perfused intracardially as described above, the eyes were enucleated and the anterior structures and the lens were removed. The remaining eyecups were immersed in 4% paraformaldehyde for 2 h, then transferred to 30% sucrose at 4 °C, embedded in OCT (Tissue-Tek, Miles Laboratories, IN), and frozen. Radial cryosections (10–14 μm) were placed onto gelatin-coated slides, blocked using 3% bovine serum albumin in PBS with 1% Triton for 30 min at room temperature, and exposed to the primary antibody for 2 h: anti-α2M antibody (rabbit, 1:200, Calbiochem; or goat, 1:100, Sigma) and/or anti-low density lipoprotein receptor-related protein (LRP)-1 receptor (1:200, Santa Cruz). Double staining was done with antibodies to the glial marker glial fibrillary acidic protein (mouse, 1:400 Chemicon), or to the neuronal marker Tubulin isoform β-III (mouse, 1:2000, Chemicon). Secondary antibodies were fluorescein isothiocyanate-conjugated anti-mouse, Cy3-conjugated anti-rabbit, or Alexa Fluor 488 anti-goat (used at 1:250, 1:1000, or 1;500 dilutions) for 1 h at room temperature. Confocal images were obtained using a Zeiss confocal microscope (LSM510). No bleed-through between the UV filter (FluoroGold) and the red filter (α2M, LRP-1) was observed in retinal sections labeled independently with each of these markers.
Flat-mounted Retinas, and Image Analyses
Seven days after retrograde labeling, rats were perfused by transcardial administration of phosphate buffer (PB), followed by 4% paraformaldehyde in PB, and the eyes were enucleated. After post-fixing for 1 h retinas were flat-mounted on glass slides (vitreous side up), air-dried, and cover-slipped with mounting medium (Molecular Probes), and studied by fluorescence microscopy (Zeiss) (11). For each retina, three digital images from each quadrant (superior, temporal, inferior, and nasal) were taken at ×20 magnification, for a total of 12 images per retina taken in a blinded fashion. All images were taken between ∼1 and ∼3 mm from the optic disc. Experimental groups included normal IOP, cauterized, cauterized + betaxolol, each ± injections. Experiments were reproduced independently for the indicated number of times. Each ×20 magnification field exposes an area of 0.2285 mm2 and in each independent experiment images spanning 10.97 to 21.93 mm2 per group were analyzed.
RGC Counting
RGCs were recognized in flat-mounted retinas by the presence of retrogradely transported dye. Microglia and macrophages, which may have incorporated 1,1-dioctadecyl-3,3,3, 3-tetramethylindocarbocyanine perchlorate or FluoroGold after phagocytosis of dying RGCs, were excluded from analysis based on their morphology and by immunostaining with antibodies against Isolectin-B4 and ED-1 (data not shown). RGCs counted in all 4 quadrants (12 images per retina) were averaged as the number of RGCs/mm2. Samples and images were coded and RGC counting was usually done by two experimenters, one of which was blinded to the code. Experiments were reproduced independently, and in particular the experiment where α2M was neutralized with antibodies was repeated 4 times independently.
Standardization of RGC Survival
Standardization of RGC loss in the test eyes were done versus normal IOP control eyes (100% RGC counts). Percent RGC loss were calculated using the formula (100 -(RGCTEST/RGCCONTROL) × 100). The RGCCONTROL counts correspond to the normal contralateral eye, because intra-rat variability (variability from right eye, OD versus left eye, OS, within a single rat) is lower than inter-rat variability (variability from rat to rat).
TUNEL Analyses
Control or experimental retinas were prepared for terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (UTP)-biotin nick end-labeling (TUNEL) staining and analysis, as described (16, 17). Three groups were studied: control contralateral normal eyes, cauterized eyes with high IOP for 2, 3, or 4 weeks, and eyes injected once with α2M and sacrificed 2, 3, or 4 weeks later. Retinas were dissected and fixed in 4% paraformaldehyde for 1 h, then washed three times in PBS (pH 7.4) and flat-mounted on coverslips with the RGC layer facing up. The retinas were treated for 10 min at room temperature with a solution of PBS + 1% Tween 20 containing 5 μg/ml Proteinase K, and were post-fixed (5 min at room temperature with 1% paraformaldehyde), followed by washing and blocking for 15 min. Whole retinas mounted on slides were incubated in TUNEL reaction mixture solution (Roche) 90 min at room temperature in humidified chambers. Slides were rinsed three times in PBS for 5 min each. Pictures were taken independently by 2 investigators (one in a blind fashion) from the flat-mounted retinas, under a microscope (×40 magnification). TUNEL+ cells were counted from the pictures, by 3 investigators (two in a blind fashion). Counts obtained independently were analyzed and were comparable, thus only “data sets” from one investigator are shown.
Data Analysis
Statistical analyses was performed using two-tailed paired t test and one-way analysis of variance with Tukey's post test with the commercial software GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA) with *, p < 0.05, ** p < 0.01, *** p < 0.001. All probability values were two-tailed; a level of 5% was considered significant. All data for % RGC loss is reported with S.E., and all the TUNEL data are reported with S.D.
RESULTS
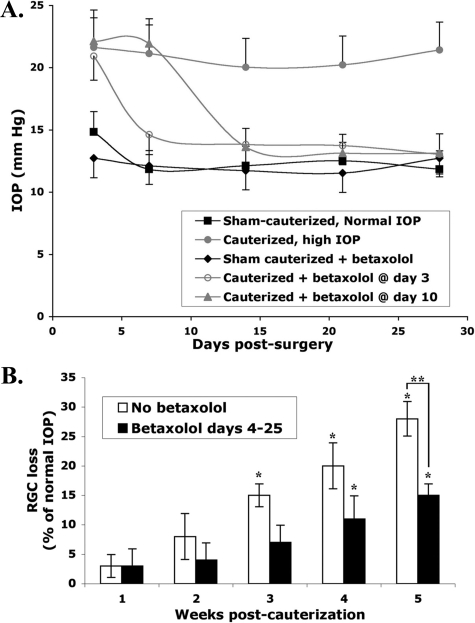
Rat Model of Glaucoma Mimicking IOP and RGC Death in Glaucoma—High IOP was induced in rat eyes by cauterizing three episcleral vessels of one eye to reduce aqueous humor outflow, and the contralateral eyes were used as controls. The IOP of cauterized eyes (∼21 mm Hg) was significantly higher than non-cauterized control eyes (∼12.6 mm Hg) for >2 months. Daily topical treatment with betaxolol lowered aqueous humor production and reduced high IOP. Daily betaxolol application started at day 3 post-cauterization fully normalized high IOP from day 7 onward (e.g. ∼3 days of daily betaxolol treatment are required for normalization of IOP). There were no significant differences in the IOP of cauterized eyes treated with betaxolol versus control non-cauterized contralateral eyes treated with or without betaxolol (Fig. 1A).
FIGURE 1.
Rat eyes with high IOP and normalized IOP progress toward glaucomatous RGC loss. A, mean IOP values ± S.D., n = 4–6 eyes/group. At day 0 eyes were surgically cauterized or were left normal. At days 3 or 10 post-cautery the indicated groups were treated with β-blocker (betaxolol 0.5%, Alcon Labs), the other groups were untreated. Daily treatment with β-blocker continued until day 28. Within 3 days of β-blocker treatment, cauterized eyes experienced a significant reduction of high IOP and their IOPs were not different from non-cauterized eyes as long as betaxolol was applied. B, progressive loss of RGCs triggered by short-term ocular hypertension. Mean RGC loss ± S.E., n = 6 retinas/group/experiment, from 3 independent experiments. Normalization of IOP with betaxolol (from day ∼7 onwards) reduces the rate of RGC loss, but does not prevent it. When betaxolol was used successful normalization of IOP were verified, but is not shown here for clarity. Normal non-cauterized eyes were obtained from 3 rats. In the test groups 6 rats for each time point had both eyes cauterized to elevate pressure, and one eye was treated with betaxolol to lower pressure. *, significant RGC loss compared versus normal non-cauterized retinas (p ≤ 0.01). **, significantly higher RGC loss in cauterized eyes compared versus cauterized eyes treated with betaxolol (p ≤ 0.01).
Chronic high IOP causes progressive and cumulative RGC loss (11). Using retrograde tracers that label RGC soma, we confirmed that at weeks 3, 4, and 5 post-cauterization there was a significant RGC loss of ∼15, ∼20, and ∼27% versus normal IOP contralateral eyes (Fig. 1B). Betaxolol normalization of IOP from day 7 onwards reduced the rate of RGC loss, but did not prevent it. At 5 weeks post-cauterization eyes treated with betaxolol had reduced RGC death versus cauterized eyes not treated with betaxolol. However, at weeks 4 and 5 post-cauterization there was a significant RGC loss of ∼11 and ∼15% versus contralateral eyes with normal IOP even though the IOPs measured in betaxolol-treated cauterized eyes were normal (Fig. 1B).
Thus, a lesser but still significant rate of chronic RGC loss was triggered by ∼1 week exposure to high IOP, independent of continuous high IOP. These animal data replicate the RGC loss or nerve fiber layer loss reported in patients medicated to lower IOP, affecting the visual field of 25% of subjects at 3 years and >70% at 10 years of treatment (2, 3).
RGC Death Induced by Optic Nerve Axotomy—To evaluate chronic RGC damage in glaucoma, we compared it versus an acute form of damage, optic nerve axotomy. In optic nerve axotomy minimal but detectable RGC death is observed after 4 days and ∼60% RGC death is found 10 days post-injury (18). Henceforth we compare day 28 of glaucoma versus day 4 after optic nerve axotomy, and day 42 of glaucoma versus day 10 after optic nerve axotomy because these time points in the in vivo models afford comparable RGC loss (19).
α2M Is an IPREG—Retinas were dissected out to ensure that only retinal mRNAs were prepared for gene expression studies. Retinal mRNA expression was compared in normal IOP control eyes versus eyes with ocular hypertension for 28 days. This was done to identify genes regulated during ocular hypertension.
A second comparison was done versus eyes whose ocular hypertension was normalized pharmacologically from day 3 onwards (in this group high IOP returns to normal levels by day 7). This was done to identify genes whose expression is altered early during ocular hypertension but that remain altered long after IOP is normalized.
A third comparison was done versus eyes whose optic nerves were severed. This was done to exclude genes regulated during irreversible RGC death.
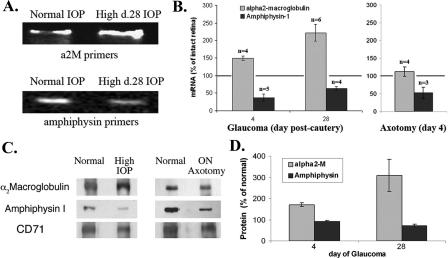
We identified 14 genes as potential IPREGs. A report on the array and the bioinformatics is in preparation.4 For the present report, we focused on validating α2M in vivo. We selected Amphiphysin-1 as a control because in the same gene array work it is not an IPREG. Amphiphysin-1 expression was significantly reduced in glaucoma but it was also reduced in optic nerve axotomy; indicating that it decreases during RGC stress or death rather than selectively with high IOP.
Quantitative RT-PCR studies using retinal mRNA showed up-regulation of α2M in glaucoma but not in optic nerve axotomy. In retinas subjected to 28 days of high IOP (n = 6) α2M mRNA was elevated an average 222 ± 24% (range 2–3-fold) versus control contralateral retinas with normal IOP (Fig. 2A). Even in retinas subjected to only 4 days of high IOP (n = 4), α2M mRNA was elevated on average 150 ± 7% (range 1.3 to 1.7-fold) versus control contralateral retinas with normal IOP (quantification in Fig. 2B). These data indicate that changes in retinal expression of α2M mRNA requires a short-term exposure to ocular hypertension. The kinetics of these changes precede detectable RGC death (as shown in Fig. 1B).
FIGURE 2.
Characterization of selected IPREGs. A and B, up-regulated α2-macroglobulin retinal mRNA in glaucoma. Retinas were dissected from eyes treated as indicated, and mRNAs were purified and studied by semi-quantitative RT-PCR. Amphiphysin is neuronal specific and is used as control. A, gels show representative data, with equal amounts of mRNA and equal gel loading for each primer set. B, quantification of RT-PCR data for α2M and Amphiphysin-1 mRNA (as in panel A), averaged ± S.E. (from the indicated n independent experiments, standardized to β-actin for loading). Note that in ON axotomy there is no change in α2M expression, but Amphiphysin-1 expression is reduced. C and D, retinal protein expression in normal versus glaucoma versus axotomized eyes. Retinas were dissected from eyes treated as indicated, and whole protein detergent extracts were studied by Western blotting with specific antibodies. CD71 is used as internal control (100%) because its expression does not change in any condition. C, representative data are shown. D, quantification of Western blot data for α2M and Amphiphysin-1 protein (as in panel C), averaged ± S.E. (n = 3 for day 4 and n = 9 for day 28 for α2M, and n = 4 for Amphiphysin).
In contrast, there were no changes in α2M mRNA after optic nerve axotomy (Fig. 2B), suggesting that changes to α2M mRNA expression is not related to RGC death and perhaps is induced selectively by ocular hypertension. Control studies showed that Amphiphysin-1 mRNA expression is rapidly reduced in glaucoma as well as in axotomy (Fig. 2B).
RT-PCR of retinas subjected to just 4–7 days of high IOP, followed by 21 days of normal IOP as a result of betaxolol treatment, showed that α2M mRNA expression remained elevated even though the measured IOPs were normal. These data indicate that the changes in α2M mRNA expression are long-lived, and independent of continuing high IOP for at least 21 days. In contrast, Amphiphysin-1 mRNA down-regulation can be reversed if the stress of ocular hypertension is resolved early on (data not shown).
α2M Is Specifically Regulated by Ocular Hypertension—To determine whether ocular hypertension, as opposed to RGC death, regulates gene expression we compared the glaucoma model versus optic nerve axotomy. Semi-quantitative Western blotting of retinal proteins were performed with samples from normal, day 28 high IOP, and day 4 ON axotomy retinas. Retinal α2M protein was significantly up-regulated in high IOP, but was not altered after optic nerve axotomy (Fig. 2C). In controls, there were comparable changes for Amphiphysin-1 (decreased ∼30%) both in the glaucoma and the optic nerve axotomy models. Thus, up-regulation of retinal α2M protein is not due to RGC death (i.e. it is not seen in optic nerve axotomy) and could be induced selectively by high IOP. In contrast, the reduction in retinal Amphiphysin-1 seems to be a marker of RGC stress or death.
Quantification of multiple experiments from glaucoma retinas at days 4 or 28, standardized to β-actin or to CD71 protein levels, showed up-regulation of α2M protein. After 4 days high IOP α2M protein was elevated an average 171 ± 9% (range 1.4 to 2.2-fold) versus control contralateral retinas with normal IOP. After 28 days high IOP α2M protein was elevated an average 309 ± 75% (range 2.3 to 4.6-fold) versus control contralateral retinas with normal IOP (Fig. 2D). Together, these quantitative data are consistent in showing up-regulation of α2M mRNA expression (Fig. 2B), and protein (Fig. 2D) in glaucoma but not in optic nerve axotomy.
Parallel studies of Amphiphysin-1 protein expression showed that this protein is down-regulated late in glaucoma. In retinas subjected to 4 days of high IOP, Amphiphysin-1 protein remained at 90 ± 5% (range 0.65 to 1-fold) versus control contralateral retinas with normal IOP. This was unexpected because Amphiphysin-1 mRNA is reduced at this time (Fig. 2B).
A difference between mRNA and protein levels may reflect a slow turnover of Amphiphysin-1 protein, because in retinas subjected to 28 days of high IOP Amphiphysin-1 protein was reduced to 70 ± 8% (range 0.50–0.80-fold) versus control contralateral retinas with normal IOP (Fig. 2D).
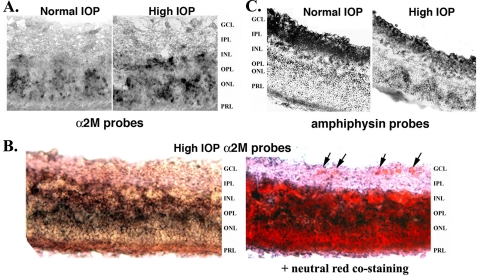
Localization of α2-Macroglobulin mRNA in Retina—In situ mRNA hybridization studies in retinas from normal IOP and from day 21 glaucoma showed α2M mRNA in the inner nuclear and the outer nuclear layers but not in the RGC layer (Fig. 3A).
FIGURE 3.
α2-Macroglobulin is preferentially expressed in glia. Retinas were dissected from normal or day 21 glaucoma eyes, and sections were prepared for in situ mRNA hybridization with DIG-labeled probes specific for α2M or Amphiphysin-1. A, normal versus glaucoma retinas labeled with α2M probes. Note the increase in α2M labeling intensity in the glaucoma retina. B, glaucoma retinas labeled with α2M probes, followed by staining with neutral red to show cell bodies (e.g. arrows in the retinal ganglion cell layer, GCL). C, normal versus glaucoma retinas labeled with Amphiphysin-1 probes. Note the reduction in Amphiphysin-1 labeling intensity in the glaucoma retina. GCL, retinal ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor layer.
Interestingly, most of the de novo up-regulation of α2M mRNA taking place in glaucoma occurs at the inner nuclear/inner plexiform layer (Fig. 3A). Hence, it seems that this anatomical region may mount a selective reaction to ocular hypertension.
To ascertain that RGCs do not express detectable α2M mRNA, sections of glaucoma retinas labeled with α2M probes were further stained with neutral red to better compare the retinal layers and to show cell bodies (e.g. arrows pointing to RGCs in the retinal ganglion cell layer, Fig. 3B). As control, in situ mRNA hybridization of serial sections with Amphiphysin-1 probes confirmed that mRNA expression takes place in the RGC layer, and that Amphiphysin-1 expression is reduced in glaucoma (Fig. 3C).
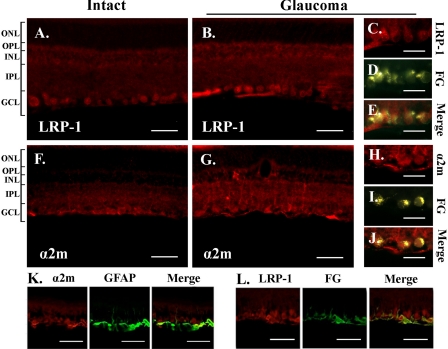
Immunohistochemical Localization of α2-Macroglobulin and Its Receptor in Retina—Because α2M is a soluble protein, we studied its localization and also that of its cellular receptor LRP-1 (CD91/LRP-1). Immunolocalization was tested in two different animal models of glaucoma to validate the findings. Cryosections were prepared from normal and glaucoma eyes and they were immunostained as shown in Fig. 4. Comparable data were obtained in both models, the hypertonic saline injection model (Fig. 4, A–J) and the cautery model (Fig. 4, K and L).
FIGURE 4.
Expression of LRP and up-regulation of α2-macroglobulin in glaucoma. Low magnification pictures of intact retina and glaucomatous retina immunostained with antibodies LRP-1 (A and B) and α2M (F and G). RGCs were first retrogradely labeled with fluorogold, intact or glaucoma retinas were dissected, and were immunostained with antibodies to LRP-1 (C–E) α2M (H and J). Retinas were dissected from glaucomatous eyes (high IOP day 28) and sections were immunostained with antibodies to glial fibrillary acidic protein (GFAP) and LRP-1 (K) or α2M (L). Data were acquired by fluorescence microscopy. RPE, retinal pigment epithelium; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars, 50 A, B, F, G, K, and L) and 25 μm (C–E and H–J).
In normal retinas, LRP-1 immunoreactivity was detected almost exclusively in the RGC layer (Fig. 4A). In glaucoma, LRP-1 was also detected in the RGC layer (Fig. 4B), and under higher magnification it co-localizes with the retrograde tracer Fluorogold that selectively labels RGCs (Fig. 4, C–E; also see Fig. 4L). In normal retinas α2M immunoreactivity was detected in the RGC layer, in putative Müller cells, and weakly in the inner plexiform layer (Fig. 4F). In glaucoma, α2M immunoreactivity was found in the RGC layer (Fig. 4G), and under higher magnification it co-localizes with the retrograde tracer Fluorogold that selectively labels RGCs (Fig. 4, H–J).
In glaucoma, α2M immunoreactivity was also increased in Müller cells end feet and retinal astrocytes where it co-localized with glial fibrillary acidic protein (Fig. 4K). Also, there was strong up-regulation of α2M protein in the inner nuclear layer and inner plexiform layer (Fig. 4G), and this was expected from the data obtained in in situ mRNA hybridization. In other experiments, co-localization of RGCs immunostained with the RGC-specific marker Tubulin βIII and either α2M immunoreactivity or LRP-1 immunoreactivity was also demonstrated (data not shown).
Because RGCs do not make α2M mRNA (Fig. 3) but α2M protein is detected on the RGC surface (Fig. 4), the data suggest that the α2M protein acts in a paracrine manner by binding to neuronal LRP-1 receptors. This mechanism requires α2M protein to be processed and secreted by glia and α2M protein to be bioavailable to RGCs.
α2-Macroglobulin Induces Glaucoma-like RGC Death, But with Normal Tension—To further evaluate the potential role and mechanism of α2M in RGC death, activated α2M protein was microinjected in normal eyes to determine whether glaucoma-like RGC death ensued. In this paradigm, a total of 1 μg of α2M were injected intraocularly in normal eyes. This quantity was selected based on α2M quantified from glaucomatous rat eyes, but the 1 μg was delivered as a bolus (2 injections) rather than allowing the build-up over time that would be expected in glaucoma. The normal contralateral eye injected with PBS vehicle were used as control in each rat. Intraocular injections did not alter normal IOP (data not shown).
Retrogradely-labeled surviving RGCs were counted at days 21 and 28 post-injections. In 4 independent experiments, activated α2M caused the progressive loss of RGCs: 10 ± 6% at day 21 (n = 8 rats) and a significant 19 ± 8% at day 28 (n = 5 rats; paired two-tailed t test p < 0.03).
In untreated glaucomatous eyes with high IOP we find similar RGC losses after 2 and 4 weeks (Fig. 1B). These data suggest that α2M induces progressive RGC death with a rate comparable with glaucoma, but with normal IOP.
Because ocular hypertension causes apoptotic RGC death by apoptosis (16, 17), TUNEL assays were used to compare the mechanism of RGC losses in glaucoma versus intraocular injection of activated α2M. Eyes were injected intraocularly at day 0 with 1 μg of activated α2M or with vehicle PBS, or were subjected to cauterization. TUNEL assays were done 14 or 28 days later using retinal cryosections. There were more TUNEL positive nuclei in the RGC layer of sections from eyes injected with α2M and from glaucomatous eyes compared versus control eyes (Table 1). Confirmable TUNEL assays were done on flat-mounted whole retinas. The RGC layer of retinas of eyes collected 21 days after injection of α2M had ∼3-fold more TUNEL positive nuclei versus control eyes injected with PBS (44 ± 15 versus 15 ± 7, n = 6). Together, these data suggest that α2M induces progressive RGC loss by apoptosis in a manner comparable with glaucoma, but with normal IOP.
TABLE 1.
α2-Macroglobulin induces apoptotic RGC death in the absence of high IOP Retinas were collected at days 14 or 28 post-challenge (cautery or α2M injection) and processed for TUNEL staining. Each data point represents the average TUNEL-positive counts ± S.D. (8 pictures at ×40 magnification per each retina) from the indicated n number of retinas.
| Treatment at day 0 | n | TUNEL+ cells |
|---|---|---|
| α2M (day 14 post-injection) | 4 | 38 ± 11 |
| α2M (day 28 post-injection) | 4 | 18 ± 6 |
| Glaucoma (day 14 post-cautery) | 2 | 26 ± 11 |
| Glaucoma (day 28 post-cautery) | 2 | 22 ± 14 |
| Untreated normal control | 4 | 3 ± 3 |
Neuroprotection I: Neutralization of α2-Macroglobulin Delays RGC Death in Glaucoma—To assess the role of α2M in glaucomatous RGC death, neutralizing antibodies to α2M were injected intraocularly in glaucomatous eyes, testing whether RGC death could be delayed. High IOP was induced by cauterization to trigger α2M overexpression and RGC damage. α2M neutralizing antibodies, control PBS, or control irrelevant antibodies were injected intraocularly at days 7 and 14 post-cauterization. Then, RGC counting was done at day 28 post-cauterization. High IOP was maintained throughout the experiment, and intraocular injections did not alter IOP.
In 2 independent experiments using eyes subjected to 28 days of high IOP, there were more fluorogold-labeled RGCs in the rats administered α2M neutralizing antibodies. RGC counting showed that intraocular injection of α2M neutralizing antibodies (n = 10) prevented the death of 71 ± 29% (p < 0.05) of the RGCs that are lost at day 28 glaucoma, compared with contralateral eyes treated with vehicle PBS (n = 4) or control irrelevant antibody (n = 6).
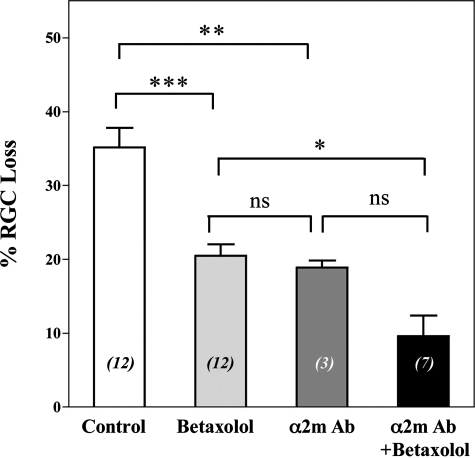
Neuroprotection II: Neutralization of α2-Macroglobulin Is Additive with Pressure Normalization—Because in glaucoma the standard therapy is the use of drugs that reduce ocular hypertension, a combination of α2M neutralizing therapy and pressure-reducing betaxolol was tested (Fig. 5). Given that each pharmacological treatment has a different mechanism of action, we anticipated additive or synergistic effects. In these experiments the end point was extended to day 42 glaucoma, to allow more extensive damage and RGC loss.
FIGURE 5.
Neutralization of α2-macroglobulin delays RGC death. % RGC loss calculated versus the corresponding normal IOP contralateral eyes, untreated. RGC labeling was done by retrograde labeling from the superior colliculi. End point for all animals was at day 42 of glaucoma. Each data point represents the average ± S.E. Statistical analyses with one-way analysis of variance, *, p < 0.05; **, p < 0.01; ***, p < 0.001, ns, not significant. Control indicates untreated glaucoma at day 42 (maximal damage). The numbers in italics between parentheses indicate the n. Betaxolol was given as topical eye drops daily, and anti-α2-macroglobulin neutralizing antibodies were given as intraocular injections at days 14 and 21 of glaucoma. The ocular pressure is not affected by intraocular injections (glaucoma remains glaucoma, normal remains normal).
Neutralizing antibodies were given at days 14 and 21 post-cauterization, as single therapy or in combination with daily deses betaxolol from day 14 until end point day 42. Surviving RGCs were counted at day 42. The corresponding contralateral untreated eyes with normal IOP were used as control 100% RGCs for each rat. In this experimental paradigm, the minimal damage expected is 8 ± 5% RGC loss, which is the damage that already occurred in eyes at day 14 high IOP (when therapy is initiated) and which is likely irreversible.
After 42 days of untreated high IOP, there is a significant loss of 35 ± 3% RGCs versus normal IOP. Normalization of IOP with daily application of betaxolol significantly reduced the loss of RGCs to 21 ± 2% (p < 0.001 versus untreated). Intraocular injection of anti-α2M antibody in glaucoma significantly reduced RGC loss to 19 ± 1% (p < 0.01 versus untreated). Treatment with daily betaxolol or 2 injections of anti-α2M antibody had comparable efficacy.
A combined treatment with anti-α2M antibody and with daily betaxolol was markedly neuroprotective, reducing RGC loss to 10 ± 3% (p < 0.05 versus betaxolol monotherapy). This % RGC loss is not different from the damage seen at day 14 high IOP (8 ± 5% RGC loss).
DISCUSSION
The in vivo evidence presented shows that short-term ocular hypertension regulates a key retinal gene product in two rat models of glaucoma. Changes in α2M are long-lasting and independent of continuous ocular hypertension; and this protein is critical in the cascade of events leading to RGC death.
Previous gene array studies reported differential gene expression in models of glaucoma (20–26). In other work, rotamase inhibitors have been used successfully in conjunction with pressure lowering drugs, validating FK506-binding proteins (or perhaps mammalian target of rapamycin downstream pathways) as targets (27).
However, none of these studies meet all five criteria for an IPREG candidate. We proposed that altered gene expression should: (i) occur specifically in retina and be caused specifically by ocular hypertension; (ii) occur relatively early following ocular hypertension and prior to RGC damage; (iii) be long-lived; (iv) independent of the continuous presence of high IOP; and (v) be linked to RGC death.
Using gene arrays we identified potential IPREGs that meet these criteria. The full gene array and bioinformatics work will be presented elsewhere. In the present report, we show in vivo data validating α2M as a mediator of RGC death in glaucoma and as a potential therapeutic target.
α2M Neurotoxic Mechanisms—Soluble α2M is an acute phase soluble protein with intrinsic and receptor-mediated activities. The activity of IPREG α2M can be linked to many of the postulated mechanisms of RGC death in glaucoma including immune bystander effects, neurotrophin deprivation, amyloid neurotoxicity, and glutamatergic stress.
Immune Bystander Effects—α2M is normally detectable in circulation and in tissues, but it is up-regulated by the TNFα and interleukin-6 as an acute phase response protein (28). In turn, α2M then binds interleukin-6 and TNFα and inhibits their clearance (29), thus extending pro-inflammatory effects (30, 31). α2M can thus be a downstream mediator of TNFα damage seen in glaucoma. Recently, TNFα was shown to cause RGC death through the induction of a putative mediator by glia (10). We propose that α2M fits the criteria for such a mediator made by glia. The notion of α2M acting as a mediator rather than as a toxic agent would be consistent with the fact that RGC death takes place with slower kinetics that does α2M up-regulation.
Neurotrophin Deprivation—Activated α2M binds to all neurotrophins (e.g. nerve growth factor) (32, 33) and inhibits their function (34–36). Thus α2M overexpression in glaucoma leads to decreased growth factor bioavailability, and potentially to RGC death because neurotrophins are required for maintenance of adult neurons.
Although nerve growth factor is up-regulated in glaucoma (11) it is insufficient to protect RGCs in glaucoma, and even pharmacological delivery of exogenous neurotrophins are not able to protect RGCs (19). The long-lived up-regulation of α2M in glaucoma may explain lack of efficacy by endogenous and exogenous neurotrophic growth factors. This notion is supported by previous work using a small molecule agonist of the TrkA nerve growth factor receptor (37), which is not neutralized by α2M. Unlike nerve growth factor, the small molecule agonist is effective in glaucoma and protects RGCs from death (19). Together these results support the view that that the neurotrophic deficit is not at the level of the TrkA receptor, but likely due in part by neutralization of neurotrophins by α2M.
Amyloid Neurotoxicity—α2M also binds to a cognate receptor LRP-1 (CD91/low-density lipoprotein receptor-related protein 1). α2M–LRP-1 interactions or up-regulation have the potential to cause neuronal death (38–43) in part due to Ca2+ fluxes through LRP-1 regulation of NMDA receptors (44–46).
LRP-1 is also implicated in clearance of amyloid protein. LRP-1-mediated amyloid clearance can be antagonized α2M (47), leading to elevated amyloid. Indeed, in glaucoma the neurotoxic role of elevated β-amyloid has been reported (8) and we postulated that elevated β-amyloid can be partially explained by up-regulated α2M.
It is noteworthy that α2M is up-regulated in AD (48), a disease where β-amyloid toxicity can be exacerbated by α2M (41, 42). Moreover, LRP–α2M interactions are directly deleterious to neurons, including RGCs (38–43). Parallels between AD and glaucoma have been made (1–3), and the molecular evidence discussed here supports this suspicion. On the other hand, under certain conditions and at low concentrations α2M can be neuroprotective (49) and can block apoE4 toxicity (50).
Glutamatergic Stress—α2M regulates Ca2+i through NMDA receptors (NMDA-R), and modulation of glutamate neurotransmission in hippoccampal neurons (44–46). Thus, up-regulation of α2M in the eye can potentiate the normal excitatory activity of NMDA-R leading to RGC death, as postulated by the excitotoxic hypothesis in glaucoma (51).
α2M Up-regulation Is Detrimental for RGCs—Adding exogenous α2M to the normal eye causes glaucoma-like RGC death but with normal tension. Neurotoxicity by α2M is apoptotic based on TUNEL, as reported for glaucoma (16, 17).
Because up-regulated α2M remains present long after ocular hypertension is normalized (1, 3, 52, 53), our findings might explain the continuous glaucomatous process. α2M may be involved in the condition known as “normal-tension glaucoma,” or may be useful to develop an animal model of this condition.
It is likely that α2M is not directly cytotoxic, but rather it induces pro-death signals in RGCs. This statement is based on three observations.
First, there is α2M mRNA and protein in normal retinas, obviously without toxicity. Indeed, low levels of α2M are reportedly neuroprotective (49) and can block apoE4 toxicity (50).
Second, in glaucoma α2M mRNA and protein are up-regulated very rapidly but significant RGC death is not detected until ∼14 days later. It is possible that α2M up-regulation leads to the build-up of β-amyloid, which is then directly or indirectly neurotoxic (8, 54).
Third, axonal loss has been reported to precede RGC death in a model of glaucoma (55) as well as in aging and Alzheimer disease (56, 57). Hence, it is possible that α2M up-regulation causes a slow process of axonal retraction and synaptic loss in RGCs that prevents efficient retrograde transport of the fluorogold label. This could result in delayed RGC loss, detected by reduced fluorogold labeling. We cannot absolutely rule out that decreased labeling of surviving RGCs might take place, but it is known that axonopathy or reduced axonal transport would lead to the eventual death of the RGCs nonetheless. Thus, we interpret a decrease in retrograde-labeled RGCs as the actual loss of these cells.
Neutralization of α2M Is Protective for RGCs—Neutralization of α2M in glaucoma is protective for RGCs even in the continuous presence of high IOP. This finding makes α2M a key factor in glaucoma. Importantly, neutralization of α2M in combination with pressure normalization is highly protective for RGCs. Additive effects were expected because pressure normalization is protective by itself because it removes the original stress of high IOP but only partially reduces α2M production.
Understandably, therapy initiated concomitant with damage has been deemed invalid (58). Our experimental paradigm treated pre-existing α2M up-regulation, and ongoing disease and RGC damage for at least 14 days. This is a challenging model designed to emulate progression to glaucoma. Our observations indicate that in addition to the standard approach of lowering high IOP, additional avenues may be required that include targeting the pro-death activity of up-regulated α2M (this paper), amyloid (8), and/or direct neuroprotection (19, 59–61).
Our work contributes to understanding the molecular mechanisms underlying the still obscure etiology of glaucoma. Further discovery and pharmacological manipulation of other IPREGs may result in the validation of novel mechanisms of neuronal death. These can be targeted for glaucoma therapy and potentially in other neurodegenerative conditions.
Acknowledgments
We thank Dr. Pietro Di Camilli for the Amphiphysin-1 probes. We gratefully acknowledge the assistance of H. Conway, Tra-Truong, Dr. P. Dergham, and Dr. H. Qin.
This work was supported, in whole or in part, by National Institutes of Health Grant CA82642. This work was also supported by Canadian Institutes of Health Research Grants MT13265 and MOP57690. Patents have been licensed to Mimetogen Pharmaceuticals Inc., and H. U. S. discloses interest in the company. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: RGC, retinal ganglion cell; IOP, intraocular pressure; TNFα, tumor necrosis factor-α; IPREG, intraocular pressure-regulated early gene; α2M, α2-macroglobulin; ON, optic nerve; RT, reverse transcriptase; PBS, phosphate-buffered saline; DIG, digoxigenin; TUNEL, terminal deoxynucleotidyl transferase-mediated UTP-biotin nick end-labeling; LRP-1, lipoprotein receptor-related protein-1; NMDA, N-methyl-d-asparatate.
Z. Shi, M. Rudzinski, K. Meerovitch, and H. U. Saragovi, unpublished data.
References
- 1.Rudzinski, M., and Saragovi, H. U. (2005) Curr. Med. Chem. 5 43-49 [Google Scholar]
- 2.Kass, M. A., Gordon, M. O., Hoff, M. R., Parkinson, J. M., Kolker, A. E., Hart, W. M., Jr., and Becker, B. (1989) Arch. Ophthalmol. 107 1590-1598 [DOI] [PubMed] [Google Scholar]
- 3.O'Brien, C., Schwartz, B., Takamoto, T., and Wu, D. C. (1991) Am. J. Ophthalmol. 111 491-500 [DOI] [PubMed] [Google Scholar]
- 4.Pease, M. E., McKinnon, S. J., Quigley, H. A., Kerrigan-Baumrind, L. A., and Zack, D. J. (2000) Investig. Ophthalmol. Vis. Sci. 41 764-774 [PubMed] [Google Scholar]
- 5.Vorwerk, C. K., Naskar, R., Schuettauf, F., Quinto, K., Zurakowski, D., Gochenauer, G., Robinson, M. B., Mackler, S. A., and Dreyer, E. B. (2000) Investig. Ophthalmol. Vis. Sci. 41 3615-3621 [PubMed] [Google Scholar]
- 6.Siu, A. W., Leung, M. C., To, C. H., Siu, F. K., Ji, J. Z., and So, K. F. (2002) Exp. Eye Res. 75 401-406 [PubMed] [Google Scholar]
- 7.Halpern, D. L., and Grosskreutz, C. L. (2002) Ophthalmol. Clin. North Am. 15 61-68 [DOI] [PubMed] [Google Scholar]
- 8.Guo, L., Salt, T. E., Luong, V., Wood, N., Cheung, W., Maass, A., Ferrari, G., Russo-Marie, F., Sillito, A. M., Cheetham, M. E., Moss, S. E., Fitzke, F. W., and Cordeiro, M. F. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 13444-13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tezel, G., Li, L. Y., Patil, R. V., and Wax, M. B. (2001) Investig. Ophthalmol. Vis. Sci. 42 1787-1794 [PubMed] [Google Scholar]
- 10.Nakazawa, T., Nakazawa, C., Matsubara, A., Noda, K., Hisatomi, T., She, H., Michaud, N., Hafezi-Moghadam, A., Miller, J. W., and Benowitz, L. I. (2006) J. Neurosci. 26 12633-12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudzinski, M., Wong, T. P., and Saragovi, H. U. (2004) J. Neurobiol. 58 341-354 [DOI] [PubMed] [Google Scholar]
- 12.Laquis, S., Chaudhary, P., and Sharma, S. (1998) Brain Res. 784 100-104 [DOI] [PubMed] [Google Scholar]
- 13.Zhou, Y., Pernet, V., Hauswirth, W. W., and Di Polo, A. (2005) Mol. Ther. 12 402-412 [DOI] [PubMed] [Google Scholar]
- 14.Moore, C., Epley, D., Milne, S., and Morrison, J. (1995) Curr. Eye Res. 14 711-717 [DOI] [PubMed] [Google Scholar]
- 15.Deleted in proof
- 16.Kerrigan, L. A., Zack, D. J., Quigley, H. A., Smith, S. D., and Pease, M. E. (1997) Arch. Ophthalmol. 115 1031-1035 [DOI] [PubMed] [Google Scholar]
- 17.Ji, J., Chang, P., Pennesi, M. E., Yang, Z., Zhang, J., Li, D., Wu, S. M., and Gross, R. L. (2005) Vision Res. 45 169-179 [DOI] [PubMed] [Google Scholar]
- 18.Di Polo, A., Aigner, L. J., Dunn, R. J., Bray, G. M., and Aguayo, A. J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3978-3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi, Z., Birman, E., and Saragovi, H. U. (2007) Dev. Neurobiol. 67 884-894 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez, M. R., Agapova, O. A., Yang, P., Salvador-Silva, M., Ricard, C. S., and Aoi, S. (2002) Glia 38 45-64 [DOI] [PubMed] [Google Scholar]
- 21.Pang, I. H., Johnson, E. C., Jia, L., Cepurna, W. O., Shepard, A. R., Hellberg, M. R., Clark, A. F., and Morrison, J. C. (2005) Investig. Ophthalmol. Vis. Sci. 46 1313-1321 [DOI] [PubMed] [Google Scholar]
- 22.Miyahara, T., Kikuchi, T., Akimoto, M., Kurokawa, T., Shibuki, H., and Yoshimura, N. (2003) Investig. Ophthalmol. Vis. Sci. 44 4347-4356 [DOI] [PubMed] [Google Scholar]
- 23.Lo, W. R., Rowlette, L. L., Caballero, M., Yang, P., Hernandez, M. R., and Borras, T. (2003) Investig. Ophthalmol. Vis. Sci. 44 473-485 [DOI] [PubMed] [Google Scholar]
- 24.Zenkel, M., Poschl, E., von der Mark, K., Hofmann-Rummelt, C., Naumann, G. O., Kruse, F. E., and Schlotzer-Schrehardt, U. (2005) Investig. Ophthalmol. Vis. Sci. 46 3742-3752 [DOI] [PubMed] [Google Scholar]
- 25.Esson, D. W., Popp, M. P., Liu, L., Schultz, G. S., and Sherwood, M. B. (2004) Investig. Ophthalmol. Vis. Sci. 45 4450-4462 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed, F., Brown, K. M., Stephan, D. A., Morrison, J. C., Johnson, E. C., and Tomarev, S. I. (2004) Investig. Ophthalmol. Vis. Sci. 45 1247-1258 [DOI] [PubMed] [Google Scholar]
- 27.Huang, W., Fileta, J. B., Dobberfuhl, A., Filippopolous, T., Guo, Y., Kwon, G., and Grosskreutz, C. L. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 12242-12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegenka, U. M., Buschmann, J., Lutticken, C., Heinrich, P. C., and Horn, F. (1993) Mol. Cell. Biol. 13 276-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gourine, A. V., Gourine, V. N., Tesfaigzi, Y., Caluwaerts, N., Van Leuven, F., and Kluger, M. J. (2002) Am. J. Physiol. 283 R218-R226 [DOI] [PubMed] [Google Scholar]
- 30.James, K., van den Haan, J., Lens, S., and Farmer, K. (1992) Immunol. Lett. 32 49-57 [DOI] [PubMed] [Google Scholar]
- 31.Wu, S. M., Patel, D. D., and Pizzo, S. V. (1998) J. Immunol. 161 4356-4365 [PubMed] [Google Scholar]
- 32.Wolf, B. B., and Gonias, S. L. (1994) Biochemistry 33 11270-11277 [DOI] [PubMed] [Google Scholar]
- 33.Gonias, S. L., Carmichael, A., Mettenburg, J. M., Roadcap, D. W., Irvin, W. P., and Webb, D. J. (2000) J. Biol. Chem. 275 5826-5831 [DOI] [PubMed] [Google Scholar]
- 34.Skornicka, E. L., Shi, X., and Koo, P. H. (2002) J. Neurosci. Res. 67 346-353 [DOI] [PubMed] [Google Scholar]
- 35.Lee, P. G., and Koo, P. H. (1999) J. Neurosci. Res. 57 872-883 [PubMed] [Google Scholar]
- 36.Chiabrando, G. A., Sanchez, M. C., Skornicka, E. L., and Koo, P. H. (2002) J. Neurosci. Res. 70 57-64 [DOI] [PubMed] [Google Scholar]
- 37.Maliartchouk, S., Feng, Y., Ivanisevic, L., Debeir, T., Cuello, A. C., Burgess, K., and Saragovi, H. U. (2000) Mol. Pharmacol. 57 385-391 [PubMed] [Google Scholar]
- 38.Herz, J. (2003) J. Clin. Investig. 112 1483-1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birkenmeier, G., Osman, A. A., Kopperschlager, G., and Mothes, T. (1997) FEBS Lett. 416 193-196 [DOI] [PubMed] [Google Scholar]
- 40.Fabrizi, C., Businaro, R., Lauro, G. M., and Fumagalli, L. (2001) J. Neurochem. 78 406-412 [DOI] [PubMed] [Google Scholar]
- 41.Fabrizi, C., Businaro, R., Lauro, G. M., Starace, G., and Fumagalli, L. (1999) Exp. Neurol. 155 252-259 [DOI] [PubMed] [Google Scholar]
- 42.Kovacs, D. M. (2000) Exp. Gerontol. 35 473-479 [DOI] [PubMed] [Google Scholar]
- 43.Bu, G., Cam, J., and Zerbinatti, C. (2006) Ann. N. Y. Acad. Sci. 1086 35-53 [DOI] [PubMed] [Google Scholar]
- 44.Bacskai, B. J., Xia, M. Q., Strickland, D. K., Rebeck, G. W., and Hyman, B. T. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 11551-11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu, Z., Strickland, D. K., Hyman, B. T., and Rebeck, G. W. (2002) J. Biol. Chem. 277 14458-14466 [DOI] [PubMed] [Google Scholar]
- 46.Qiu, Z., Hyman, B. T., and Rebeck, G. W. (2004) J. Biol. Chem. 279 34948-34956 [DOI] [PubMed] [Google Scholar]
- 47.Waldron, E., Jaeger, S., and Pietrzik, C. U. (2006) Neurodegener. Dis. 3 233-238 [DOI] [PubMed] [Google Scholar]
- 48.Qiu, Z., Strickland, D. K., Hyman, B. T., and Rebeck, G. W. (2001) J. Neuropathol. Exp. Neurol. 60 430-440 [DOI] [PubMed] [Google Scholar]
- 49.Hayashi, H., Campenot, R. B., Vance, D. E., and Vance, J. E. (2007) J. Neurosci. 27 1933-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto, Y., Jiang, H., Niikura, T., Ito, Y., Hagiwara, A., Umezawa, K., Abe, Y., Murayama, Y., and Nishimoto, I. (2000) J. Neurosci. 20 8401-8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WoldeMussie, E., Yoles, E., Schwartz, M., Ruiz, G., and Wheeler, L. A. (2002) J. Glaucoma 11 474-480 [DOI] [PubMed] [Google Scholar]
- 52.Hart, W. M., Jr., and Becker, B. (1982) Ophthalmology 89 268-279 [DOI] [PubMed] [Google Scholar]
- 53.Kwon, Y. H., Kim, Y. I., Pereira, M. L., Montague, P. R., Zimmerman, M. B., and Alward, W. L. (2003) J. Glaucoma 12 409-416 [DOI] [PubMed] [Google Scholar]
- 54.McKinnon, S. J. (2003) Front. Biosci. 8 s1140-1156 [DOI] [PubMed] [Google Scholar]
- 55.Soto, I., Oglesby, E., Buckingham, B. P., Son, J. L., Roberson, E. D., Steele, M. R., Inman, D. M., Vetter, M. L., Horner, P. J., and Marsh-Armstrong, N. (2008) J. Neurosci. 28 548-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruno, M. A., Clarke, P. B., Seltzer, A., Quirion, R., Burgess, K., Cuello, A. C., and Saragovi, H. U. (2004) J. Neurosci. 24 8009-8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saragovi, H. U. (2005) J. Neurochem. 95 1472-1480 [DOI] [PubMed] [Google Scholar]
- 58.Levin, L. A. (2001) J. Glaucoma 10 Suppl. 1, S19-S21 [DOI] [PubMed] [Google Scholar]
- 59.Hartwick, A. T. (2001) Optomol. Vis. Sci. 78 85-94 [DOI] [PubMed] [Google Scholar]
- 60.Lipton, S. A. (2001) Prog. Brain Res. 131 712-718 [PubMed] [Google Scholar]
- 61.Farkas, R. H., and Grosskreutz, C. L. (2001) Int. Ophthalmol. Clin. 41 111-130 [DOI] [PubMed] [Google Scholar]