Abstract
Small ubiquitin-related modifiers (SUMOs) regulate diverse cellular processes through their covalent attachment to target proteins. Vertebrates express three SUMO paralogs: SUMO-1, SUMO-2, and SUMO-3 (SUMO-2 and SUMO-3 are ∼96% identical and referred to as SUMO-2/3). SUMO-1 and SUMO-2/3 are conjugated, at least in part, to unique subsets of proteins and thus regulate distinct cellular pathways. However, how different proteins are selectively modified by SUMO-1 and SUMO-2/3 is unknown. We demonstrate that BLM, the RecQ DNA helicase mutated in Bloom syndrome, is preferentially modified by SUMO-2/3 both in vitro and in vivo. Our findings indicate that non-covalent interactions between SUMO and BLM are required for modification at non-consensus sites and that preferential SUMO-2/3 modification is determined by preferential SUMO-2/3 binding. We also present evidence that sumoylation of a C-terminal fragment of HIPK2 is dependent on SUMO binding, indicating that non-covalent interactions between SUMO and target proteins provide a general mechanism for SUMO substrate selection and possible paralog-selective modification.
Post-translational protein modifications play essential roles in regulating all aspects of cell function. Small ubiquitin-related modifiers (SUMOs)2 are unusual post-translational modifications, because they themselves are proteins of ∼100 amino acids (1, 2). Through covalent attachment to lysine residues in target proteins, sumoylation regulates a wide range of processes, including transcription activation, DNA synthesis and repair, nucleocytoplasmic transport, and chromosome segregation. The molecular mechanisms by which sumoylation affects individual proteins, and thus this diversity of processes, are in many cases target protein-specific. An emerging theme, however, is that sumoylation often promotes interactions between modified proteins and downstream factors containing SUMO-interacting motifs (SIMs) (1). To date, a single conserved SIM has been identified that consists of a hydrophobic core ((V/I)X(V/I)(V/I)) followed or preceded by a negatively charged cluster of amino acids (3-6). Although only a limited number of SIM-containing proteins has been functionally characterized to date, a large number of proteins contains this motif and is thus predicted to interact non-covalently with SUMO.
Invertebrate organisms express only a single SUMO, whereas vertebrates express three paralogs capable of covalent conjugation to other proteins: SUMO-1, SUMO-2, and SUMO-3. SUMO-2 and SUMO-3 are ∼96% identical to each other (and thus referred to collectively as SUMO-2/3); however, they are only ∼45% identical to SUMO-1. Increasingly, evidence suggests that SUMO-1 and SUMO-2/3 have distinct cellular functions. Proteomic studies have, for example, shown that SUMO-1 and SUMO-2/3 are conjugated to only partially overlapping subsets of proteins (7, 8). In addition, localization studies indicate that SUMO-1 and SUMO-2/3 are conjugated to unique subsets of proteins that localize to different subcellular domains (9, 10). SUMO-1 and SUMO-2/3 also have distinct dynamic properties within the nucleoplasm of cells and are regulated differentially in response to cell stress (11-13). Finally, genetic studies have linked SUMO1 haploinsufficiency to cleft lip and/or palate in humans and mice, indicating that SUMO-1 and SUMO-2/3 play non-redundant roles during development (14, 15).
Despite of the evidence that SUMO-1 and SUMO-2/3 are conjugated to only partially overlapping subsets of proteins and have unique properties and functions, it remains unclear how different proteins are selectively modified by one paralog relative to another. ATP-dependent activation of SUMO-1, SUMO-2, and SUMO-3 involves the same E1 activating enzyme, a heterodimer consisting of Aos1 and Uba2 (16, 17). Following activation, all three paralogs are subsequently transferred to a single E2 conjugating enzyme, Ubc9 (18-20). In addition, although multiple different SUMO E3 ligases have been identified, none characterized to date are known to confer paralog-selective modification. Thus, based on current understanding of the enzymes involved in target protein selection and sumoylation, mechanisms for paralog-selective modification remain unclear.
Bloom syndrome is an autosomal recessive human genetic disease caused by mutations in the gene encoding for BLM, a protein belonging to the RecQ family of DNA helicases (21). BLM has essential roles in DNA replication, repair, and homologous recombination and is thus intimately involved in the maintenance of genome integrity (22-24). Due to loss of functional BLM, individuals with Bloom syndrome are predisposed to a wide variety of cancers due to genome instability. We have previously reported that BLM is modified by SUMO and that sumoylation regulates its distribution between promyelocytic leukemia (PML) nuclear bodies, where it resides in undamaged cells, and sites of DNA repair, where it accumulates in response to DNA damage (25). These previous studies also suggested that BLM is preferentially modified by SUMO-2/3. Here, we have used BLM as a model substrate to investigate molecular mechanisms regulating paralog-selective sumoylation. Using in vitro and in vivo assays, we demonstrate that the sumoylation of BLM is dependent on non-covalent interactions between SUMO and SIMs present in BLM. Moreover, we also demonstrate that the preferential association of BLM with SUMO-2/3 determines its paralog-selective sumoylation. Based on additional analysis of the homeodomain interacting protein kinase 2 (HIPK2), we propose that non-covalent interactions between target proteins and the SUMO moiety of charged Ubc9 defines an alternative mechanism for substrate recognition and modification that is distinct from direct Ubc9 binding. Importantly, this mechanism provides a means for paralog-selective sumoylation.
EXPERIMENTAL PROCEDURES
Plasmid and Recombinant Proteins—BLM-(1-447) and full-length mouse RanGAP1 constructs for in vitro transcription and translation, as well as the green fluorescent protein-BLM (1-458/NLS) construct for in vivo expression, were described previously (25, 26). The FLAG-tagged BLM (1-458/NLS) mammalian expression vector was generated by subcloning BLM (1-458/NLS) into the pFLAG-3XFlag vector modified from pFLAG (Sigma-Aldrich) (9). HIPK2-(800-1049) was constructed by inserting amino acids 800-1049 of HIPK2 into pENTR vector (Invitrogen). BLM, HIPK2 SIM mutants and the SUMO-2(QFI) mutant protein (containing the following amino acid substitutions: Q35A, F36A, and I38A) expression vectors were generated by PCR-based QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA) using corresponding wild-type vectors as template. The SUMO-1/2 chimera construct was generated by replacing the coding region for amino acids 31-51 of SUMO-1 with the coding region for amino acids 26-46 of SUMO-2 using PCR-based mutagenesis followed by standard cloning procedures. All mutations and constructs were verified by DNA sequence analysis.
Expression and purification of BLM-(1-431) from bacteria was performed as described previously (27). Recombinant E1 (Aos1/Uba2) and E2 (Ubc9) enzymes were expressed and purified from Escherichia coli using standard procedures and essentially as described (28). GST, GST-tagged SUMO-1, SUMO-2, and SUMO mutant proteins were purified by affinity chromatography on glutathione-Sepharose 4B beads (GE Healthcare, Piscataway, NJ) and eluted with 20 mm reduced glutathione or by cleavage with thrombin using standard procedures.
In Vitro Sumoylation—BLM-(1-447) wild-type and SIM mutants, RanGAP1 and HIPK2 wild-type, and SIM mutant were produced by in vitro transcription and translation in rabbit reticulocyte lysate in the presence of [35S]methionine according to the manufacturer's instructions (Promega, Madison, WI). Unless otherwise noted, 2 μl of translation product was added to a 20-μl reaction containing 200 nm E1 enzyme, 600 nm Ubc9, 1.0 μm SUMO-1 or SUMO-2 or SUMO mutant proteins, 1 mm ATP, 20 units/ml creatine phosphokinase, 5 mm phosphocreatine, 0.6 mg/ml inorganic pyrophosphatase, 20 mm HEPES-KOH (pH 7.3), 110 mm potassium acetate, 2 mm magnesium acetate, and 1 mm dithiothreitol. For sumoylation of RanGAP1, reactions were modified to contain 15 nm E1, 45 nm E2, and 0.5 μm recombinant SUMO proteins. Unless otherwise noted, reactions were incubated at 37 °C for the indicated times and stopped by addition of SDS sample buffer and analyzed by SDS-PAGE and autoradiography. Purified recombinant BLM (amino acids 1-431) was SUMO-modified using similar reaction conditions, resolved by SDS-PAGE, and analyzed by immunoblotting with the BLM-specific antibody sc-13584 (Santa Cruz Biotechnology, Santa Cruz, CA). Competition experiments were performed with excess SUMO as indicated in the text and figure legends.
Quantifications of band intensities for modification assay results were performed using Fujifilm MultiGauge software (Fujifilm Corp.). The data are representative of results from multiple experiments. Data points, however, were derived from the accompanying gels only.
In Vitro Binding Assays—Recombinant GST, GST-tagged SUMO-1, SUMO-2, SUMO-2(QFI), or SUMO-1/2 chimera proteins were incubated in glutathione-coated 96-well plates (Pierce Biotechnology, Rockford, IL) overnight at 4 °C according to manufacturer's instructions. Equal protein loading and immobilization was verified by Coomassie Blue staining and/or anti-GST immunoblot analysis before and after binding. Following binding, wells were blocked with 2% bovine serum albumin in assay buffer (20 mm HEPES-KOH (pH 7.3), 110 mm potassium acetate, 2 mm magnesium acetate, 1 mm EGTA, 0.05% Tween 20) for 1 h at room temperature. In vitro translated proteins were diluted into 100 μl of assay buffer and incubated with the immobilized proteins for 1 h at room temperature. After five washes with assay buffer, proteins were eluted with SDS sample buffer and resolved by SDS-PAGE and autoradiography. Alternatively, bound radioactive counts were determined using a liquid scintillation counter. All binding assays were repeated in triplicate.
Transfections and Immunopurifications—293T cells were cultured using standard conditions and transfected with empty FLAG vector, or vectors coding for FLAG-tagged CENP-E tail domain (9), FLAG-tagged wild-type BLM (1-458/NLS) or FLAG-tagged SIM double mutant BLM (1-458/NLS) using Lipofectamine-Plus reagent according to the manufacturer's instruction (Invitrogen). 48 h after transfection, cells were lysed in a modified radioimmune precipitation assay buffer containing 50 mm Tris (pH 7.8), 150 mm NaCl, 2 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 20 mm N-ethylmaleimide, 1% SDS, and protease inhibitors (Roche Applied Science). Lysates were sonicated and clarified by centrifugation at 20,000 × g for 10 min at 4 °C. The supernatants were diluted with 1 × radioimmune precipitation assay buffer to adjust the final SDS concentration to 0.1%. Immunopurification and immunoblot analysis was performed as described (9).
Immunofluorescence Microscopy—HeLa cells were cultured using standard conditions and grown on glass coverslips. Cells were transfected with green fluorescent protein-tagged wild-type BLM (1-458/NLS) or green fluorescent protein-tagged SIM double mutant BLM (1-458/NLS) expression vectors. 24 h after transfection, cells were fixed with 2% formaldehyde in phosphate-buffered saline for 30 min, washed with phosphate-buffered saline, and permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 5 min. Immunofluorescence analysis was performed essentially as described (26) using anti-PML monoclonal antibody sc-966 (Santa Cruz Biotechnology) followed by Alexa-594-conjugated goat anti-mouse secondary antibody (Invitrogen). Coverslips were mounted and visualized using a Zeiss Axiovert fluorescence microscope (Carl Zeiss, Thornwood, NY).
RESULTS
BLM Is Preferentially Modified by SUMO-2 in Vitro—To explore mechanisms regulating its preferential modification by SUMO-2/3, we compared the ability of BLM to be modified by SUMO-1 and SUMO-2 using in vitro sumoylation assays. In the first set of assays, an N-terminal fragment of BLM that is both necessary and sufficient for sumoylation (amino acids 1-447) was expressed in rabbit reticulocyte lysate and incubated in modification reactions with either SUMO-1 or SUMO-2 (Fig. 1A). Consistent with previous observations, BLM-(1-447) was modified by both SUMO-1 and SUMO-2, but a clear preference for SUMO-2 modification was evident. Whereas ∼30% of the input BLM-(1-447) was modified by SUMO-1 after a 60-min incubation, ∼90% of the input was modified by SUMO-2/3 (Fig. 1B). Ladders of both SUMO-1 and SUMO-2 conjugates were detected at early time points, indicative of BLM modification at multiple different lysine residues and/or the attachment of polymeric chains. At later time points, BLM shifted to very high molecular mass conjugates in reactions containing SUMO-2, likely due to the elongation of polymeric chains. The preferential modification of BLM under these assay conditions was unique, because other SUMO substrates, including RanGAP1 (Fig. 1, C and D) and SP100 and PML (data not shown), were modified by SUMO-1 and SUMO-2 at similar rates under similar conditions.
FIGURE 1.
BLM is preferentially modified by SUMO-2 in vitro. A, an N-terminal fragment of BLM (amino acids 1-447) was expressed in vitro and incubated for the indicated times (minutes) in modification reactions containing either SUMO-1 or SUMO-2. Proteins were detected by autoradiography, and the percentage of BLM modified at each time point was determined by quantification of band intensities (B). C, RanGAP1 was expressed in vitro and incubated for the indicated times (minutes) in modification reactions containing either SUMO-1 or SUMO-2. Proteins were detected by autoradiography, and the percentage of RanGAP1 modified at each time point was determined by quantification of band intensities (D). E, recombinant BLM-(1-431) was incubated for the indicated times (minutes) in modification reactions containing either SUMO-1 or SUMO-2. Proteins were detected by immunoblotting with a BLM-specific antibody, and the percentage of BLM modified at each time point was determined by quantification of band intensities (F).
Because reticulocyte lysate contains factors that could influence BLM sumoylation, we next performed assays using only recombinant proteins purified from bacteria. BLM-(1-431) was expressed and purified from bacteria and incubated in reactions containing recombinant E1 activating and E2 conjugating enzymes and either SUMO-1 or SUMO-2 for varying lengths of time. Reactions were resolved by SDS-PAGE and analyzed by immunoblotting with an antibody specific for BLM (Fig. 1E). Surprisingly, preferential SUMO-2/3 modification of BLM-(1-431) was again observed using these assay conditions, with nearly all of the input protein being modified by SUMO-2 after a 2-h incubation compared with ∼30% of the input being modified by SUMO-1 at a similar time point (Fig. 1F). Notably, SUMO-1 and SUMO-2 were activated and formed thiol ester intermediates with the E2 conjugating enzyme with similar kinetics under these assay conditions (data not shown) (29). These findings indicate that the preferential SUMO-2 modification of BLM is independent of accessory factors and is thus a property intrinsic to BLM.
BLM Binds Preferentially to SUMO-2—Yeast two-hybrid assays suggested that BLM binds preferentially to SUMO-2 (25). To further investigate the SUMO-binding activity of BLM, in vitro binding assay were performed. BLM-(1-447) was expressed in vitro and incubated with GST, GST-tagged SUMO-1, or SUMO-2 (Fig. 2A). Consistent with the yeast two-hybrid results, we found that BLM-(1-447) interacted preferentially with SUMO-2 compared with SUMO-1, with between 5 to 7 times greater binding consistently observed in assays containing SUMO-2 (Fig. 2B). Based on these findings, we hypothesized that sumoylation of BLM may be determined by its ability to interact non-covalently with the SUMO moiety of charged Ubc9. Preferential modification of BLM by SUMO-2 would thus reflect its ability to more efficiently recruit SUMO-2 charged Ubc9 due to its greater affinity for SUMO-2 relative to SUMO-1. This hypothesis was also guided by knowledge that BLM is modified at multiple different lysine residues, none of which exist within the ψKXE consensus sequence recognized directly with Ubc9 (25, 30).
FIGURE 2.
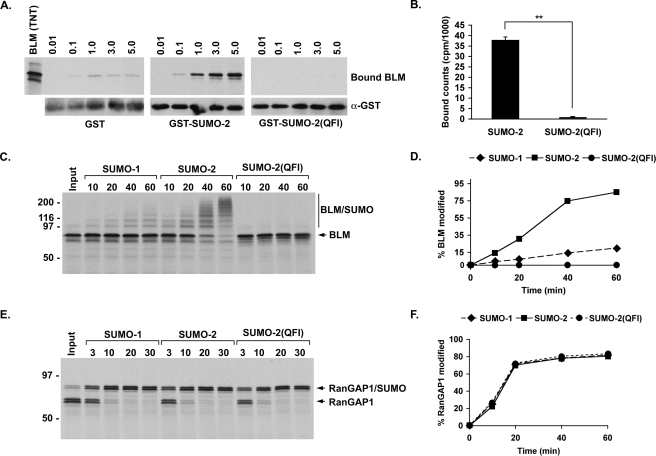
BLM binds non-covalently and preferentially to SUMO-2, and its sumoylation is inhibited by excess SUMO. A, increasing amounts of in vitro expressed BLM-(1-447) (microliters) was incubated with GST or GST-tagged SUMO-1 or SUMO-2. Bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE and autoradiography. 1 μl of BLM-(1-447) was loaded for reference (BLM (TNT)). All binding assays contained equivalent amounts of GST and GST-tagged SUMO proteins as determined by immunoblot analysis of eluted proteins with an anti-GST antibody. Quantification of BLM bound in assays containing 5 μl of TnT product was determined by liquid scintillation counting. Plotted values represent the mean ± S.D. from three independent experiments. *, p < 0.001 (paired t test) (B). C, in vitro expressed BLM-(1-447) was incubated for 60 min in modification reactions containing increasing concentrations of SUMO-2 (1, 1.5, 2, 4, 6, and 11 μm). Reactions were resolved by SDS-PAGE and analyzed by autoradiography. D, RanGAP1 was expressed in vitro and incubated for 30 min at room temperature using identical assay conditions and analysis as in C. E, the percentage of BLM or RanGAP1 modified at each concentration of SUMO-2 was determined by quantification of band intensities.
If non-covalent interactions between BLM and SUMO play a role in covalent modification, the presence of increasing concentrations of excess SUMO would be predicted to inhibit modification. To test this prediction, BLM-(1-447) was expressed in vitro and modified in reactions containing increasing concentrations of SUMO-2 (Fig. 2C). In the presence of 1 μm SUMO-2, BLM was efficiently modified to ∼90%. As the level of SUMO-2 was incrementally increased from 1 to 11 μm, however, a progressive decrease in BLM sumoylation was observed (Fig. 2E). Inhibition of SUMO-2 modification of BLM was also observed in reactions containing increasing concentrations of activation incompetent forms of either SUMO-1 or SUMO-2, with excess SUMO-2 inhibiting slightly more effectively (supplemental Fig. S1). Importantly, no difference in sumoylation of RanGAP1 was observed between reactions containing the lowest concentrations of SUMO-2 and those containing the highest concentrations (Fig. 2, D and E). Thus, BLM recognition and modification involves a mechanism sensitive to excess free SUMO and distinct from the mechanism mediating RanGAP1 recognition and modification.
We next identified residues in BLM essential for SUMO binding using a collection of mutants and in vitro SUMO binding assays. Using this approach, we identified a region of BLM between amino acids 212 and 237 that was essential for SUMO binding and that had also previously been found to be essential for sumoylation (25). Sequence analysis of this region revealed the presence of two hydrophobic patches preceded by stretches of serines and charged amino acids, sequences similar to the known consensus SIM (Fig. 3A) (3, 4, 6). To test whether the hydrophobic residues in each putative SIM are critical for interactions between BLM and SUMO, mutations were made in one or the other of the motifs, or in both motifs simultaneously. Using binding assays similar to those described above, we found that mutating individual motifs reduced both SUMO-1 and SUMO-2 binding activity to approximately one-third of that observed with the wild-type protein (Fig. 3, B and C). The single SIM mutant proteins, however, retained their preferential binding to SUMO-2 relative to SUMO-1. By simultaneously mutating hydrophobic residues in both SIMs, SUMO-1 and SUMO-2 binding was reduced to nearly undetectable levels (Fig. 3, B and C).
FIGURE 3.
BLM contains tandem SIMs essential for SUMO binding and modification. A, functional analysis identified an ∼30 amino acid N-terminal region of BLM essential for SUMO binding. As illustrated, this segment contains two putative SIMs consisting of hydrophobic patches flanked by serine, glutamic acid, and aspartic acid residues. Mutant BLM-(1-447) containing alanine substitutions in the first SIM (M1), the second SIM (M2), or both SIMs (DM) were produced as shown. B, wild-type BLM-(1-447), the two single SIM mutants (M1 and M2) and the double SIM mutant (DM) were expressed in vitro. Translation products (Input) were incubated with GST or GST-tagged SUMO-1 or SUMO-2. Bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE and autoradiography. All binding assays contained equivalent amounts of GST and GST-tagged SUMO proteins as determined by immunoblot analysis of eluted proteins with an anti-GST antibody. C, binding assays were performed as in B. Relative binding was determined by liquid scintillation detection of eluted proteins. Plotted values represent the mean ± S.D. from three independent experiments. **, p < 0.05 (paired t test). D, in vitro expressed wild-type BLM-(1-447) and SIM mutants were incubated in modification reactions containing either SUMO-1 or SUMO-2 for 60 min. Proteins were detected by autoradiography, and the percentage of wild-type and mutant protein modified by SUMO-1 or SUMO-2 was determined by quantification of band intensities (E).
Using these BLM-(1-447) SIM mutant proteins, we next performed in vitro sumoylation assays to test whether non-covalent SUMO binding by BLM was critical for its covalent modification (Fig. 3D). Wild-type and mutant BLM-(1-447) proteins were expressed in vitro and modified in reactions containing either SUMO-1 or SUMO-2 (Fig. 3D). Mirroring the decreases in SUMO-1 and SUMO-2 binding, the SUMO-1 and SUMO-2 modification efficiencies of the single SIM mutant proteins were both reduced by ∼30% compared with modification of wild-type BLM-(1-447) (Fig. 3E). As predicted from their preferential SUMO-2 binding, SUMO-2 was still preferentially conjugated to the single SIM mutants. Most significantly, SUMO-1 and SUMO-2 modification of the double SIM mutant protein was nearly undetectable under these same assay conditions (Fig. 3, D and E).
SIM-interacting Residues in SUMO-2 Mediate BLM Binding and Modification Preference—We next expressed a mutant form of SUMO-2 predicted to be unable to interact non-covalently with BLM. This mutant contained three amino acid substitutions at residues within the second β-strand of SUMO-2 known to be critical for SIM interactions (3, 31). In vitro binding assays revealed that this mutant protein, SUMO-2(QFI), is defective for non-covalent interactions with BLM-(1-447) (Fig. 4, A and B). We next assayed for the ability of this SUMO-2 mutant to be conjugated to BLM-(1-447) using in vitro sumoylation assays. Consistent with non-covalent SUMO binding being required for BLM sumoylation, SUMO-2(QFI) was not covalently conjugated to BLM-(1-447) using assay conditions where both SUMO-1 and SUMO-2 were (Fig. 4, C and D). Although not conjugated to BLM, SUMO-2(QFI) was activated and formed thiol ester intermediates with Ubc9 comparable to SUMO-1 and SUMO-2 (data not shown). Moreover, SUMO-2(QFI) was conjugated to RanGAP1 at rates similar to both SUMO-1 and wild-type SUMO-2, further indicating its functionality and the distinct mechanisms mediating BLM and RanGAP1 modification (Fig. 4, E and F).
FIGURE 4.
SIM interacting residues of SUMO-2 are essential for BLM binding and modification. A, increasing amounts of in vitro expressed BLM-(1-447) (microliters) was incubated with GST or GST-tagged SUMO-2 or SUMO-2(QFI). Bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE and autoradiography. All binding assays contained equivalent amounts of GST and GST-tagged SUMO proteins as determined by immunoblot analysis of eluted proteins with an anti-GST antibody. Quantification of BLM bound in assays containing 5 μl of TnT product was determined by liquid scintillation counting. Plotted values represent the mean ± S.D. from three independent experiments. **, p < 0.05 (paired t test) (B). C, BLM-(1-447) was expressed in vitro (Input) and incubated in modification reactions containing SUMO-1, SUMO-2, or SUMO-2(QFI) for the indicated times (minutes). Proteins were detected by autoradiography, and the percentage of BLM modified at each time point was determined by quantification of band intensities (D). E, RanGAP1 was expressed in vitro (Input) and incubated in modification reactions containing SUMO-1, SUMO-2, or SUMO-2(QFI) for the indicated times (minutes). Proteins were detected by autoradiography, and the percentage of RanGAP1 modified at each time point was determined by quantification of band intensities (F).
To address the issue of paralog selectivity, we produced a SUMO-1/2 chimera in which the SIM-binding region of SUMO-1 (consisting of α-helix 1 and β-strand 2) was replaced with the similar region from SUMO-2 (Fig. 5). Using in vitro binding assays, we found that BLM-(1-447) bound the SUMO-1/2 chimera with characteristics comparable to SUMO-2, indicating that the α-1 and β-2 regions are the principle determinants of interactions with BLM (Fig. 6, A and B). To test whether the enhanced interaction of the SUMO-1/2 chimera with BLM affected the rate of covalent conjugation, we next performed in vitro sumoylation assays using wild-type SUMO-1, SUMO-2, and the SUMO-1/2 chimera (Fig. 6C). As previously observed, SUMO-2 was conjugated more efficiently to BLM-(1-447) relative to SUMO-1, as indicated by the near complete modification of BLM by SUMO-2 at 60 min compared with only ∼25% modification by SUMO-1 at the same time point (Fig. 6D). Using identical assay conditions, the SUMO-1/2 chimera was conjugated to BLM at a rate comparable to that observed with SUMO-2, with BLM again being nearly completely modified at 60 min (Fig. 6, C and D). In contrast, rates of RanGAP1 modification were similar with SUMO-1, SUMO-2, and the SUMO-1/2 chimera (Fig. 6, E and F).
FIGURE 5.
Construction of a chimeric SUMO-1/2 protein. A, a crystal structure (Protein Data Bank 1Z 5S) illustrating interactions between the SIM of Nup358 and the first α-helix and second β-strand of SUMO-1 (46). B, as illustrated, a chimeric SUMO-1/2 protein was produced by replacing the first α-helix and second β-strand of SUMO-1 with the same region from SUMO-2.
FIGURE 6.
Paralog-selective sumoylation of BLM is defined by SUMO binding. A, increasing amounts of in vitro expressed BLM-(1-447) (microliters) was incubated with GST or GST-tagged SUMO-1, SUMO-2, or SUMO-1/2 chimera. Bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE and autoradiography. Binding assays contained equivalent amounts of GST and GST-tagged SUMO proteins as determined by immunoblot analysis of eluted proteins with an anti-GST antibody. Quantification of BLM bound in assays containing 5 μl of TnT product was determined by liquid scintillation counting. Plotted values represent the mean ± S.D. from three independent experiments. *, p < 0.001; **, p < 0.05 (paired t test) (B). C, in vitro expressed BLM-(1-447) (Input) was incubated in modification reactions containing SUMO-1, SUMO-2, or the SUMO-1/2 chimera for the indicated times (minutes). Proteins were detected by autoradiography and the percentage of BLM modified at each time point was determined by quantification of band intensities (D). E, in vitro expressed RanGAP1 (Input) in modification reactions containing SUMO-1, SUMO-2, or the SUMO-1/2 chimera for the indicated times (minutes). Proteins were detected by autoradiography, and the percentage of RanGAP1 modified at each time point was determined by quantification of band intensities (F).
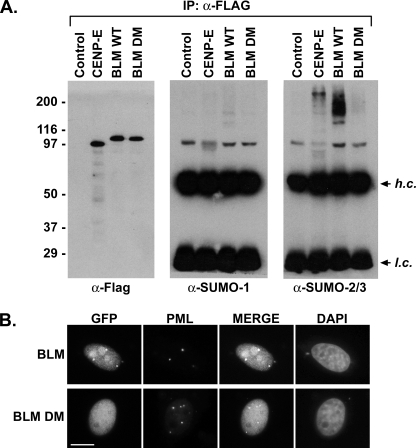
SIM-dependent Preferential SUMO-2/3 Modification of BLM in Vivo—To verify and characterize preferential sumoylation of BLM in vivo, cells were transfected with plasmids encoding for FLAG-tagged BLM-(1-458/NLS) or the double SIM mutant form of BLM-(1-458/NLS). Cells were also transfected with a plasmid coding for the C-terminal tail domain of CENP-E as a positive control (9). Immunopurifications with FLAG-specific antibodies were performed from cell lysates under protein denaturing conditions, and blots were probed with antibodies specific for SUMO-1, SUMO-2/3, or the FLAG epitope (Fig. 7A). Consistent with previous findings, high molecular mass conjugates of CENP-E were detected with SUMO-2/3 antibodies, but not with SUMO-1 antibodies (9). Also consistent with previous findings for full-length endogenous BLM (25), high molecular mass conjugates of BLM-(1-458/NLS) could be detected with antibodies for both SUMO-1 and SUMO-2/3, but the level of SUMO-2/3 conjugates detected was significantly greater (antibodies were used at dilutions that produced equivalent signals with equivalent amounts of monomeric SUMO-1 and SUMO-2/3). Importantly, high molecular mass conjugates were not detected in cells transfected with the double SIM mutant protein or the empty vector.
FIGURE 7.
SUMO binding is required for paralog-selective sumoylation of BLM in vivo and for its localization to PML nuclear bodies. A, 293T cells were transfected with empty plasmid (Control) and plasmids coding for FLAG-tagged CENP-E tail domain (CENP-E), wild-type BLM (1-458/NLS) (BLM WT), and FLAG-tagged BLM (1-458/NLS) double SIM mutant (BLM DM). FLAG-tagged proteins were immunopurified and analyzed by immunoblotting with FLAG-, SUMO-1-, or SUMO-2/3-specific antibodies as indicated. Antibody heavy chains (h.c.) and light chains (l.c.) are indicated. B, HeLa cells were transfected with plasmids coding for green fluorescent protein-tagged wild-type BLM (1-458/NLS) or the double SIM mutant protein. Cells were fixed, permeabilized, and stained with 4′,6-diamidino-2-phenylindole and antibodies to PML. Cells were visualized by fluorescence microscopy. Bar, 10 μm.
We also compared the localization of wild-type and double SIM mutant BLM-(1-458/NLS) proteins using immunofluorescence microscopy. As expected, wild-type BLM-(1-458/NLS) localized to the nucleus, where it could be detected throughout the nucleoplasm and also concentrated in several foci that colocalized with PML (Fig. 7B). In contrast, the double SIM mutant BLM-(1-458/NLS) was only detected in the nucleoplasm and could not be found in PML nuclear bodies. These findings indicate the physiological significance of non-covalent SUMO binding in mediating BLM SUMO-2/3 modification and localization to PML nuclear bodies.
Sumoylation of HIPK2 Is Dependent on Non-covalent SUMO Binding—To obtain evidence that non-covalent SUMO binding may be required for sumoylation of proteins in addition to BLM, we investigated HIPK2. Amino acid sequence analysis revealed that HIPK2 contains a single SIM between amino acids 872 and 892 (Fig. 8A). Constructs coding for a C-terminal fragment (amino acids 800-1049) of wild-type HIPK2 and a SIM-mutant HIPK2 (with amino acid substitutions at Val-878, Val-880, and Ile-881) were expressed in vitro, and binding assays were performed using GST and GST-tagged SUMO-1 and SUMO-2 (Fig. 8B). Wild-type HIPK2-(800-1049) bound equally well to both SUMO-1 and SUMO-2, whereas the HIPK2 SIM mutant showed no binding activity above background (Fig. 8B). We next investigated the ability of HIPK2 to be modified by SUMO-1 and SUMO-2 and also the dependence of modification on non-covalent SUMO binding. Wild-type HIPK2-(800-1049) and the SIM mutant protein were again expressed in vitro and incubated in reactions containing either SUMO-1, SUMO-2, or SUMO-2(QFI). Consistent with SUMO binding being essential for covalent modification, HIPK2 (800-1049) was modified equally well by SUMO-1 and SUMO-2 (consistent with its equal affinity for SUMO-1 and SUMO-2), but it was not modified by the SUMO-2(QFI) mutant (Fig. 8C). Also consistent with non-covalent binding being essential of modification, wild-type HIPK2-(800-1049) was modified by SUMO-1 and SUMO-2, whereas modification of the HIPK2 SIM mutant protein was undetectable.
FIGURE 8.
SUMO binding represents a general mechanism for SUMO substrate recognition and modification. A, HIPK2 contains an identifiable SIM in its C terminus. Substitutions were introduced into the hydrophobic patch to produce a SIM mutant protein, as indicated. B, C-terminal fragments (amino acids 800-1049) of wild-type HIPK2 (HIPK2 WT) or SIM mutant HIPK2 (HIPK2 M1) were expressed in vitro. Translation products (Input) were incubated with GST or GST-tagged SUMO-1 or SUMO-2. Bound proteins were eluted with SDS-sample buffer and analyzed by SDS-PAGE and autoradiography. Binding assays contained equivalent amounts of GST and GST-tagged SUMO proteins as determined by immunoblot analysis of eluted proteins with an anti-GST antibody. C, in vitro expressed C-terminal fragments (amino acids 800-1049) of wild-type HIPK2 (HIPK2 WT) or SIM mutant HIPK2 (HIPK2 M1) were incubated in modification reactions containing either SUMO-1, SUMO-2, or SUMO-2(QFI) for 60 min. Reactions were resolved by SDS-PAGE and analyzed by autoradiography. D, a proposed mechanism for sumoylation mediated by non-covalent SUMO binding. A SIM-containing protein binds to SUMO that is covalently linked to the active site cysteine of Ubc9. Binding enhances the local concentration of Ubc9 in the vicinity of the substrate, allowing Ubc9 to recognize lysine residues not necessarily contained within optimal consensus sumoylation motifs. SUMO is subsequently covalently conjugated to the substrate, and Ubc9 is released. Paralog-selective sumoylation is determined by the relative affinities of the SIM for SUMO-1 or SUMO-2/3.
DISCUSSION
SIM-mediated Sumoylation of BLM and HIPK2—Using BLM as a model substrate, we have demonstrated that paralog-selective sumoylation can be determined by properties intrinsic to the modified protein itself. BLM was found to contain two SIMs that mediate non-covalent interactions with SUMO. Our findings revealed that the SIMs in BLM bind preferentially to SUMO-2 relative to SUMO-1 and that selective modification of BLM both in vitro and in vivo is determined by this preferential SUMO-binding activity. Evidence supporting this conclusion included: 1) competition studies demonstrating the BLM modification is inhibited by excess free SUMO, 2) analysis of BLM and SUMO mutants, demonstrating that modification is inhibited by mutations that block non-covalent interactions, and 3) analysis of a SUMO-1/2 chimera, demonstrating that paralog-selective modification of BLM is determined by the affinities of its non-covalent interactions with SUMO-1 and SUMO-2.
We also demonstrated that sumoylation of a C-terminal fragment of HIPK2 is dependent on non-covalent SUMO binding and that, like BLM, the ability of this fragment to be modified by SUMO-1 and SUMO-2 correlated with its SUMO-binding activity. Whereas BLM bound preferentially to SUMO-2 and was modified preferentially by SUMO-2, HIPK2 bound SUMO-1 and SUMO-2 equally well and was modified equally well by both paralogs. Thus, our findings have broad implications for the role of SIMs as mediators of sumoylation and, importantly, as possible determinants of paralog-selective sumoylation.
The Generality of SIM-dependent sumoylation—Many SUMO-modified proteins characterized to date are modified at lysine residues that exist within the ΨKXE consensus sequence (where Ψ is a hydrophobic residue, K is the modified lysine, X is any amino acid, and E is glutamic acid). Biochemical and structural studies have revealed that this consensus sequence is recognized directly by Ubc9, interacting with a pocket near the active site of the enzyme (30, 32, 33). Thus, direct Ubc9 binding represents a well characterized molecular mechanism for SUMO substrate recognition and modification site selection that, in many cases, is likely to operate independent of non-covalent SUMO binding. Sumoylation of RanGAP1, for example, is mediated by direct Ubc9 binding and does not involve non-covalent interactions with SUMO (Figs. 4E and 6E).
Based on our studies of BLM and HIPK2, we propose that non-covalent SUMO binding represents an alternative mechanism for determining SUMO substrate recognition and modification. Importantly, this mechanism provides an explanation for paralog selectivity that is not afforded by direct Ubc9 binding. In addition to BLM and HIPK2, sumoylation of several additional proteins have been found to be SIM-dependent, suggesting that non-covalent SUMO binding may represent a general mechanism for substrate selection and modification. SUMO binding has, for example, been shown to be required for sumoylation of Daxx both in vitro an in vivo (34). Broad-based deletion analysis of thymine DNA glycosylase also revealed a correlation between loss of SUMO-binding activity and loss of sumoylation (35). Finally, SUMO binding has been proposed to facilitate paralog-selective auto-sumoylation of the SUMO E3 ligase domain of Nup358/RanBP2 in vitro (36).
Although these findings indicate that non-covalent SUMO binding may represent a common mechanism for recognition and sumoylation of SIM-containing proteins, how Ubc9 ultimately recognizes specific lysines as sites for modification within these proteins is not known. Notably, the N-terminal domain of BLM and the C-terminal fragment of HIPK2 do not contain consensus sumoylation sites and modification of BLM occurs at multiple “preferred” lysines C-terminal to the SIMs (lysines 317, 331, 344, and 347). Mutagenesis studies of BLM indicate that lysine selection is promiscuous, because lysine to arginine substitutions at preferred modification sites result in enhanced modification at secondary sites (25). Similar promiscuous modification site selection has also been observed in studies of Daxx (34).
Based on these observations, we propose a mechanism whereby SIMs facilitate sumoylation by mediating interactions with the SUMO moiety of charged Ubc9, thereby increasing the local concentration of the E2 enzyme in the vicinity of target proteins (Fig. 8D). In principle, BLM could interact with a SUMO moiety that is bound covalently to Ubc9 through the active site cysteine, or non-covalently to a SUMO-binding surface on the opposite face of the enzyme (37, 38). We do not favor a model in which the SUMO moiety is covalently linked to a lysine residue in Ubc9, because appreciable sumoylation of Ubc9 did not occur under assay conditions sufficient for BLM modification. Preferred modification sites within the target protein may correspond to lysine residues that are in close spatial proximity to the SIMs and/or that are most exposed and accessible to Ubc9 binding following initial SIM interactions. Characterization of other proteins whose modification depends on SUMO binding could help to identify rules defining conjugation site selection. Future structural studies of a substrate/Ubc9·SUMO complex would also provide valuable insights and support for the proposed model.
Similarities to UIM-mediated Ubiquitination—Relevant to our findings, many proteins that bind non-covalently to ubiquitin are also substrates for covalent ubiquitination (39). In several cases, ubiquitin binding has in fact been shown to mediate ubiquitination. Eps15, for example, contains a ubiquitin-interacting motif (UIM) that enables it to recruit the Parkin and Nedd4 E3 ligases. In the case of Parkin, Eps15 binds to a ubiquitin-like domain in the E3 protein using its UIM, thereby facilitating ubiquitination (40). Interaction of Eps15 with Nedd4 on the other hand is dependent on monoubiquitination of Nedd4 and subsequent UIM-mediated binding and Eps15 modification (41). More recent studies have demonstrated that proteins containing UIMs can also be modified through a mechanism that is independent of E3 ligases and that involves direct recruitment of Ub-charged E2 conjugating enzymes (42). Notably, this mechanism is similar to our proposed model for SIM-dependent sumoylation (Fig. 8D). Although the exact function of ubiquitination of UIM-containing proteins is not fully understood, it has been hypothesized that this may enable a regulatory intramolecular interaction that prevents intermolecular interactions with other ubiquitinated proteins (43). Thus in the future it will be interesting to investigate whether the sumoylation of SIM-containing proteins might similarly affect intramolecular binding and regulation of interactions with downstream factors.
SUMO Binding and BLM Function—Regulated sumoylation of BLM has been proposed to control its association with PML nuclear bodies and DNA repair foci (25). Consistent with this proposal, we found that BLM SIM mutants are not covalently sumoylated and also fail to localize to PML nuclear bodies in vivo. These findings provide evidence for the physiological significance of SIM-mediated sumoylation in vivo. Although clearly essential for sumoylation, non-covalent SUMO binding may also play a dual role by helping to tether BLM to other sumoylated proteins within PML nuclear bodies. Consistent with this possibility, fusing SUMO-2 to BLM SIM mutants does not restore localization to PML nuclear bodies (data not shown), suggesting that both covalent sumoylation and SUMO binding may be essential for efficient targeting. A combination of covalent sumoylation and non-covalent SUMO binding has been proposed as a general mechanism for the assembly of PML nuclear bodies (44). How sumoylation and retention or release of BLM from PML nuclear bodies are regulated in normal cells and in cells with DNA damage is of great interest. Our findings indicate that sumoylation of BLM could be regulated at the level of its SUMO-binding activity. Notably, residues within the SIMs of BLM include serines and threonines that could serve as sites for phosphorylation and control of SUMO-binding activity. Whether these residues are differentially phosphorylated in vivo in response to DNA damage, and how phosphorylation affects SUMO-binding activity, are important questions for future studies. In addition, it will also be of interest to investigate and understand the functional significance of the SUMO-2/3-selective modification of BLM. BLM interacts with a large number of proteins that is likely to be involved in its association both with PML nuclear bodies and DNA repair foci (22). It is therefore possible that one or more BLM-interacting proteins contain SUMO-2/3-selective SIMs that mediate interactions with modified BLM. SUMO-2/3-selective modification of BLM-interacting proteins could further facilitate interactions with BLM through formation of a network of interactions dependent on SUMO modification and binding (45).
In conclusion, our studies have revealed that non-covalent SUMO binding is able to mediate substrate recognition and covalent paralog-selective sumoylation. It is anticipated that sumoylation of SIM-containing proteins by this mechanism could have multiple effects, including auto-inhibition of SUMO-binding activity or promotion of interactions with downstream SIM-containing proteins. Elucidation of the full range of effects awaits identification and characterization of other SIM-containing SUMO substrates, of which there are likely to be many.
Supplementary Material
Acknowledgments
We thank all members of the Matunis laboratory and Drs. Robert Cohen and Cynthia Wolberger for insightful discussions during the course of this work. We are also particularly grateful to Josh Sims for technical assistance and advice with protein binding assays.
This work was supported, in whole or in part, by National Institutes of Health Grants GM060980 and U54RR020839. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: SUMO, small ubiquitin-related modifier; SIM, SUMO-interacting motif; E1, ubiquitin-activating enzyme; E2, ubiquitin carrier protein; E3, ubiquitin-protein isopeptide ligase; PML, promyelocytic leukemia; HIPK2, homeodomain interacting protein kinase 2; GST, glutathione S-transferase; UIM, ubiquitin-interacting motif.
References
- 1.Geiss-Friedlander, R., and Melchior, F. (2007) Nat. Rev. Mol. Cell. Biol. 8 947-956 [DOI] [PubMed] [Google Scholar]
- 2.Johnson, E. S. (2004) Annu. Rev. Biochem. 73 355-382 [DOI] [PubMed] [Google Scholar]
- 3.Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P., and Dikic, I. (2006) J. Biol. Chem. 281 16117-16127 [DOI] [PubMed] [Google Scholar]
- 4.Kerscher, O. (2007) EMBO Rep. 8 550-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minty, A., Dumont, X., Kaghad, M., and Caput, D. (2000) J. Biol. Chem. 275 36316-36323 [DOI] [PubMed] [Google Scholar]
- 6.Song, J., Durrin, L. K., Wilkinson, T. A., Krontiris, T. G., and Chen, Y. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 14373-14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas-Acosta, G., Russell, W. K., Deyrieux, A., Russell, D. H., and Wilson, V. G. (2005) Mol. Cell Proteomics 4 56-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vertegaal, A. C., Andersen, J. S., Ogg, S. C., Hay, R. T., Mann, M., and Lamond, A. I. (2006) Mol. Cell Proteomics 5 2298-2310 [DOI] [PubMed] [Google Scholar]
- 9.Zhang, X. D., Goeres, J., Zhang, H., Yen, T. J., Porter, A. C., and Matunis, M. J. (2008) Mol. Cell 29 729-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma, Y., Arnaoutov, A., and Dasso, M. (2003) J. Cell Biol. 163 477-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayaydin, F., and Dasso, M. (2004) Mol. Biol. Cell 15 5208-5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitoh, H., and Hinchey, J. (2000) J. Biol. Chem. 275 6252-6258 [DOI] [PubMed] [Google Scholar]
- 13.Yang, W., Sheng, H., Warner, D. S., and Paschen, W. (2008) J. Cereb. Blood Flow Metab. 28 269-279 [DOI] [PubMed] [Google Scholar]
- 14.Alkuraya, F. S., Saadi, I., Lund, J. J., Turbe-Doan, A., Morton, C. C., and Maas, R. L. (2006) Science 313 1751. [DOI] [PubMed] [Google Scholar]
- 15.Pauws, E., and Stanier, P. (2007) Trends Genet. 23 631-640 [DOI] [PubMed] [Google Scholar]
- 16.Desterro, J. M., Rodriguez, M. S., Kemp, G. D., and Hay, R. T. (1999) J. Biol. Chem. 274 10618-10624 [DOI] [PubMed] [Google Scholar]
- 17.Johnson, E. S., Schwienhorst, I., Dohmen, R. J., and Blobel, G. (1997) EMBO J. 16 5509-5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desterro, J. M., Thomson, J., and Hay, R. T. (1997) FEBS Lett. 417 297-300 [DOI] [PubMed] [Google Scholar]
- 19.Johnson, E. S., and Blobel, G. (1997) J. Biol. Chem. 272 26799-26802 [DOI] [PubMed] [Google Scholar]
- 20.Lee, G. W., Melchior, F., Matunis, M. J., Mahajan, R., Tian, Q., and Anderson, P. (1998) J. Biol. Chem. 273 6503-6507 [DOI] [PubMed] [Google Scholar]
- 21.Ellis, N. A., Groden, J., Ye, T. Z., Straughen, J., Lennon, D. J., Ciocci, S., Proytcheva, M., and German, J. (1995) Cell 83 655-666 [DOI] [PubMed] [Google Scholar]
- 22.Cheok, C. F., Bachrati, C. Z., Chan, K. L., Ralf, C., Wu, L., and Hickson, I. D. (2005) Biochem. Soc. Trans. 33 1456-1459 [DOI] [PubMed] [Google Scholar]
- 23.Sung, P., and Klein, H. (2006) Nat. Rev. Mol. Cell. Biol. 7 739-750 [DOI] [PubMed] [Google Scholar]
- 24.Wu, L. (2007) DNA Repair (Amst.) 6 936-944 [DOI] [PubMed] [Google Scholar]
- 25.Eladad, S., Ye, T. Z., Hu, P., Leversha, M., Beresten, S., Matunis, M. J., and Ellis, N. A. (2005) Hum. Mol. Genet 14 1351-1365 [DOI] [PubMed] [Google Scholar]
- 26.Matunis, M. J., Wu, J., and Blobel, G. (1998) J. Cell Biol. 140 499-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beresten, S. F., Stan, R., van Brabant, A. J., Ye, T., Naureckiene, S., and Ellis, N. A. (1999) Protein Expr. Purif. 17 239-248 [DOI] [PubMed] [Google Scholar]
- 28.Zhang, H., Saitoh, H., and Matunis, M. J. (2002) Mol. Cell. Biol. 22 6498-6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatham, M. H., Kim, S., Yu, B., Jaffray, E., Song, J., Zheng, J., Rodriguez, M. S., Hay, R. T., and Chen, Y. (2003) Biochemistry 42 9959-9969 [DOI] [PubMed] [Google Scholar]
- 30.Bernier-Villamor, V., Sampson, D. A., Matunis, M. J., and Lima, C. D. (2002) Cell 108 345-356 [DOI] [PubMed] [Google Scholar]
- 31.Sun, H., Leverson, J. D., and Hunter, T. (2007) EMBO J. 26 4102-4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez, M. S., Dargemont, C., and Hay, R. T. (2001) J. Biol. Chem. 276 12654-12659 [DOI] [PubMed] [Google Scholar]
- 33.Sampson, D. A., Wang, M., and Matunis, M. J. (2001) J. Biol. Chem. 276 21664-21669 [DOI] [PubMed] [Google Scholar]
- 34.Lin, D. Y., Huang, Y. S., Jeng, J. C., Kuo, H. Y., Chang, C. C., Chao, T. T., Ho, C. C., Chen, Y. C., Lin, T. P., Fang, H. I., Hung, C. C., Suen, C. S., Hwang, M. J., Chang, K. S., Maul, G. G., and Shih, H. M. (2006) Mol. Cell 24 341-354 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi, H., Hatakeyama, S., Saitoh, H., and Nakayama, K. I. (2005) J. Biol. Chem. 280 5611-5621 [DOI] [PubMed] [Google Scholar]
- 36.Tatham, M. H., Kim, S., Jaffray, E., Song, J., Chen, Y., and Hay, R. T. (2005) Nat. Struct. Mol. Biol. 12 67-74 [DOI] [PubMed] [Google Scholar]
- 37.Capili, A. D., and Lima, C. D. (2007) J. Mol. Biol. 369 608-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knipscheer, P., van Dijk, W. J., Olsen, J. V., Mann, M., and Sixma, T. K. (2007) EMBO J. 26 2797-2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Fiore, P. P., Polo, S., and Hofmann, K. (2003) Nat. Rev. Mol. Cell. Biol. 4 491-497 [DOI] [PubMed] [Google Scholar]
- 40.Fallon, L., Belanger, C. M., Corera, A. T., Kontogiannea, M., Regan-Klapisz, E., Moreau, F., Voortman, J., Haber, M., Rouleau, G., Thorarinsdottir, T., Brice, A., van Bergen En Henegouwen, P. M., and Fon, E. A. (2006) Nat. Cell Biol. 8 834-842 [DOI] [PubMed] [Google Scholar]
- 41.Woelk, T., Oldrini, B., Maspero, E., Confalonieri, S., Cavallaro, E., Di Fiore, P. P., and Polo, S. (2006) Nat. Cell Biol. 8 1246-1254 [DOI] [PubMed] [Google Scholar]
- 42.Hoeller, D., Hecker, C. M., Wagner, S., Rogov, V., Dotsch, V., and Dikic, I. (2007) Mol. Cell 26 891-898 [DOI] [PubMed] [Google Scholar]
- 43.Hoeller, D., Crosetto, N., Blagoev, B., Raiborg, C., Tikkanen, R., Wagner, S., Kowanetz, K., Breitling, R., Mann, M., Stenmark, H., and Dikic, I. (2006) Nat. Cell Biol. 8 163-169 [DOI] [PubMed] [Google Scholar]
- 44.Shen, T. H., Lin, H. K., Scaglioni, P. P., Yung, T. M., and Pandolfi, P. P. (2006) Mol. Cell 24 331-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matunis, M. J., Zhang, X. D., and Ellis, N. A. (2006) Dev. Cell 11 596-597 [DOI] [PubMed] [Google Scholar]
- 46.Reverter, D., and Lima, C. D. (2005) Nature 435 687-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.