Abstract
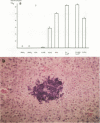
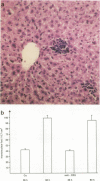
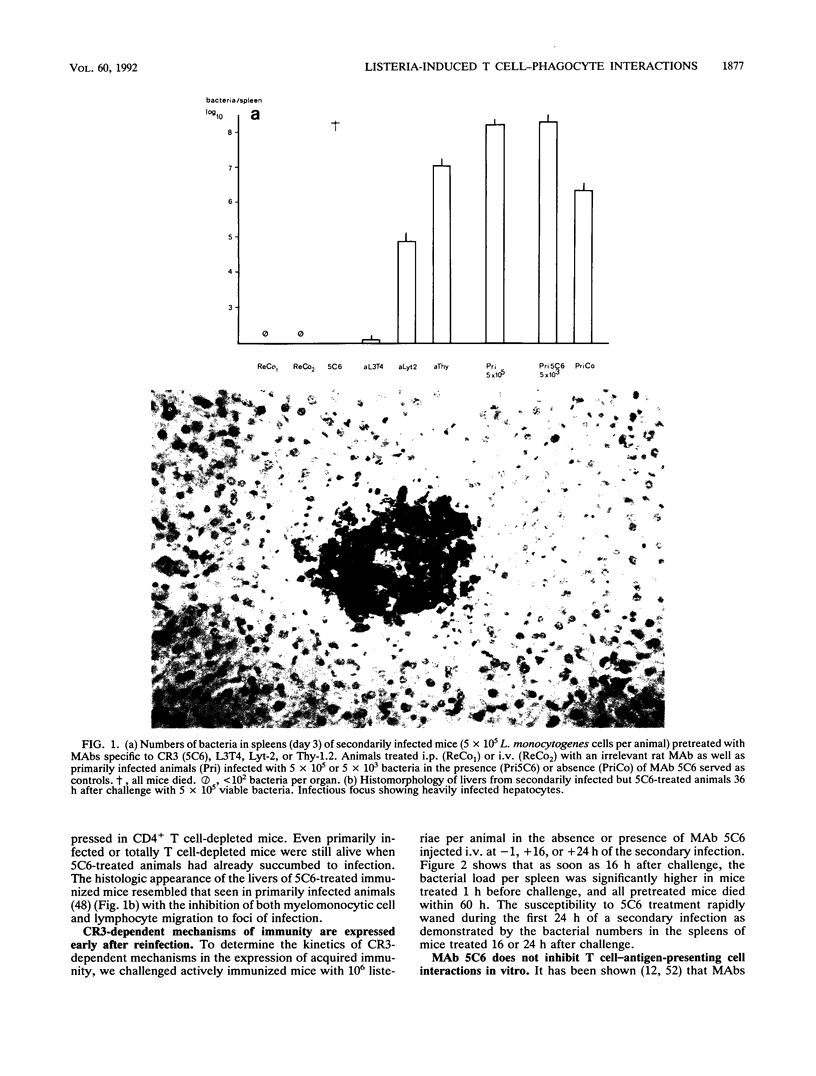
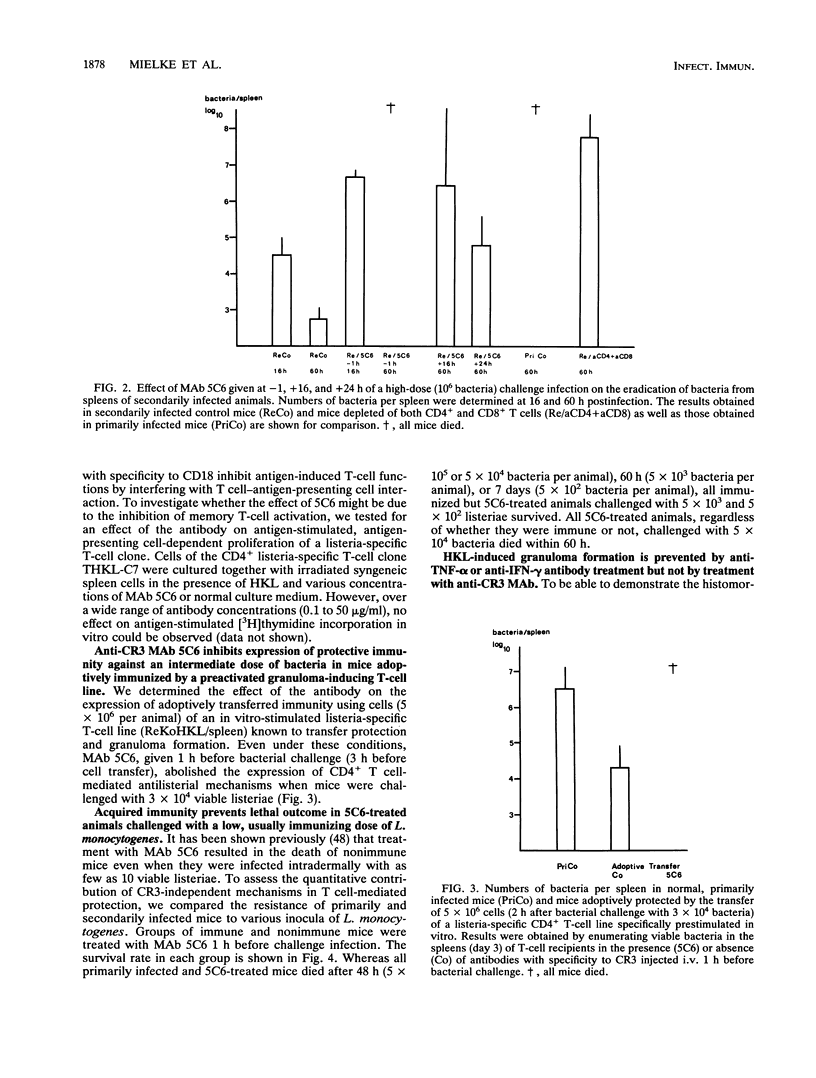
Listeria-immune mice are able to express protective immunity in the absence of CD4+ T cells and an apparent granulomatous inflammation. Using a monoclonal antibody (5C6) able to inhibit the recruitment of myelomonocytic cells into inflammatory foci by binding to complement receptor type 3 (CR3/CD11b), we could show that protective immunity and granuloma formation indeed depend on two distinct types of T cell-phagocyte interactions. Listeria-specific CD8+ T lymphocytes, possibly in collaboration with CD4- CD8- T cells, rapidly interact with myelomonocytic cells infiltrating infected tissues in a CR3/CD11b-dependent manner. This interaction results in potent antilisterial protection but not in granuloma formation. On the contrary, CD4+ T cells are able to induce adhesion mechanisms that allow the accumulation of monocytes in granulomatous lesions even in the presence of monoclonal antibody 5C6. However, the protective capacity of these CR3/CD11b-independent T cell-mediated immune mechanisms is low in listeriosis. Tumor necrosis factor alpha and gamma interferon, known to be essential for the expression of both resistance and acquired immunity, are shown to be necessarily involved in granuloma formation, too. It therefore remains to be explained why CD8+ T cells, able to secrete both cytokines, do not induce granuloma formation. The data point to the presence of an as yet undefined CD4+ T cell-derived granuloma-inducing factor and favor the hypothesis that CD8+ T cells, in collaboration with circulating phagocytes, mediate immunity by rapidly liberating listeriae from permissive cells or protecting them from becoming infected.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Brunt L. M., Portnoy D. A., Unanue E. R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990 Dec 1;145(11):3540–3546. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschryver-Kecskemeti K., Bancroft G. J., Bosma G. C., Bosma M. J., Unanue E. R. Pathology of Listeria infection in murine severe combined immunodeficiency. A study by immunohistochemistry and electron microscopy. Lab Invest. 1988 Jun;58(6):698–705. [PubMed] [Google Scholar]
- Dransfield I., Buckle A. M., Hogg N. Early events of the immune response mediated by leukocyte integrins. Immunol Rev. 1990 Apr;114:29–44. doi: 10.1111/j.1600-065x.1990.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Resolution of primary murine listeriosis and acquired resistance to lethal secondary infection can be mediated predominantly by Thy-1+ CD4- CD8- cells. J Infect Dis. 1991 Nov;164(5):869–877. doi: 10.1093/infdis/164.5.869. [DOI] [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Gervais F., Desforges C., Skamene E. The C5-sufficient A/J congenic mouse strain. Inflammatory response and resistance to Listeria monocytogenes. J Immunol. 1989 Mar 15;142(6):2057–2060. [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Hauser T., Frei K., Zinkernagel R. M., Leist T. P. Role of tumor necrosis factor in Listeria resistance of nude mice. Med Microbiol Immunol. 1990;179(2):95–104. doi: 10.1007/BF00198530. [DOI] [PubMed] [Google Scholar]
- Hausman M. S., Snyderman R., Mergenhagen S. E. Humoral mediators of chemotaxis of mononuclear leukocytes. J Infect Dis. 1972 Jun;125(6):595–602. doi: 10.1093/infdis/125.6.595. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991 Apr 1;173(4):793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Rodewald H. R., Hug E., De Libero G. Cloned Listeria monocytogenes specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J Immunol. 1988 May 1;140(9):3173–3179. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Krishnan V. L., Humphrey J. H. Inhibition of growth of Listeria monocytogenes in vitro, by immunologically activated mouse resident macrophages. Br J Exp Pathol. 1986 Dec;67(6):809–819. [PMC free article] [PubMed] [Google Scholar]
- Lukacs K., Kurlander R. Lyt-2+ T cell-mediated protection against listeriosis. Protection correlates with phagocyte depletion but not with IFN-gamma production. J Immunol. 1989 Apr 15;142(8):2879–2886. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Chen-Woan M. The cellular response to Listeria monocytogenes is mediated by a heterogeneous population of immunospecific T cells. Clin Invest Med. 1984;7(4):243–252. [PubMed] [Google Scholar]
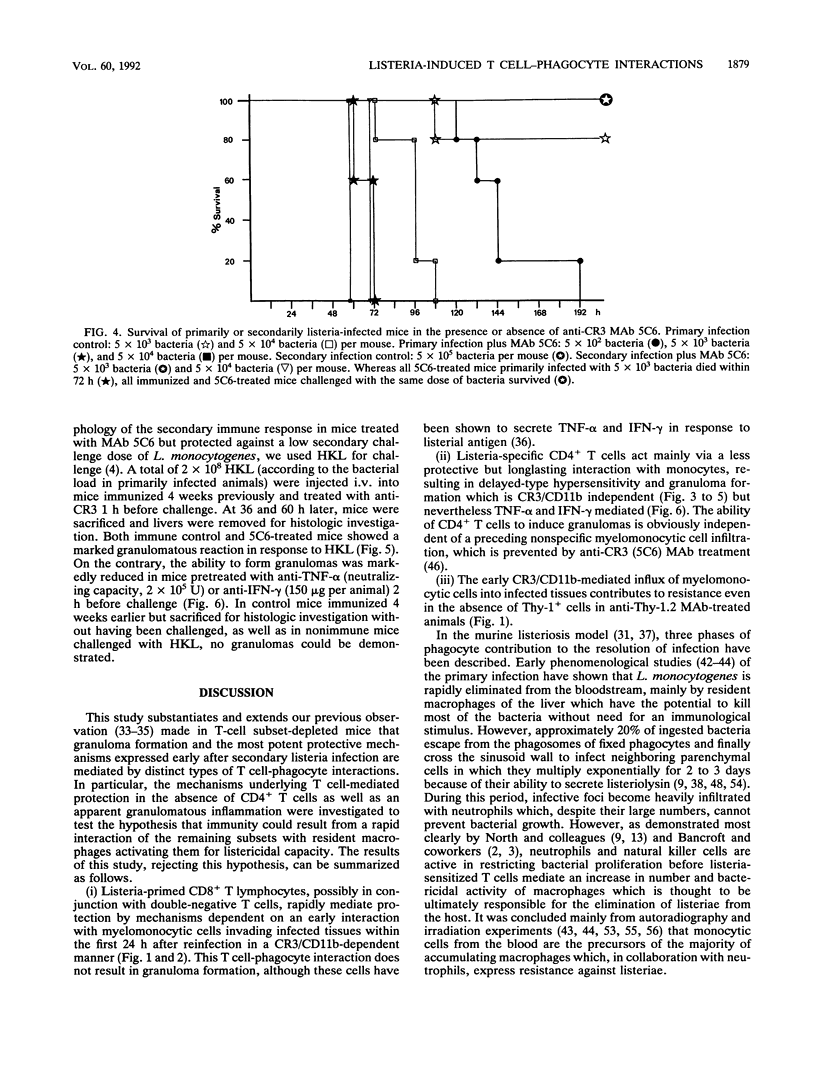
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
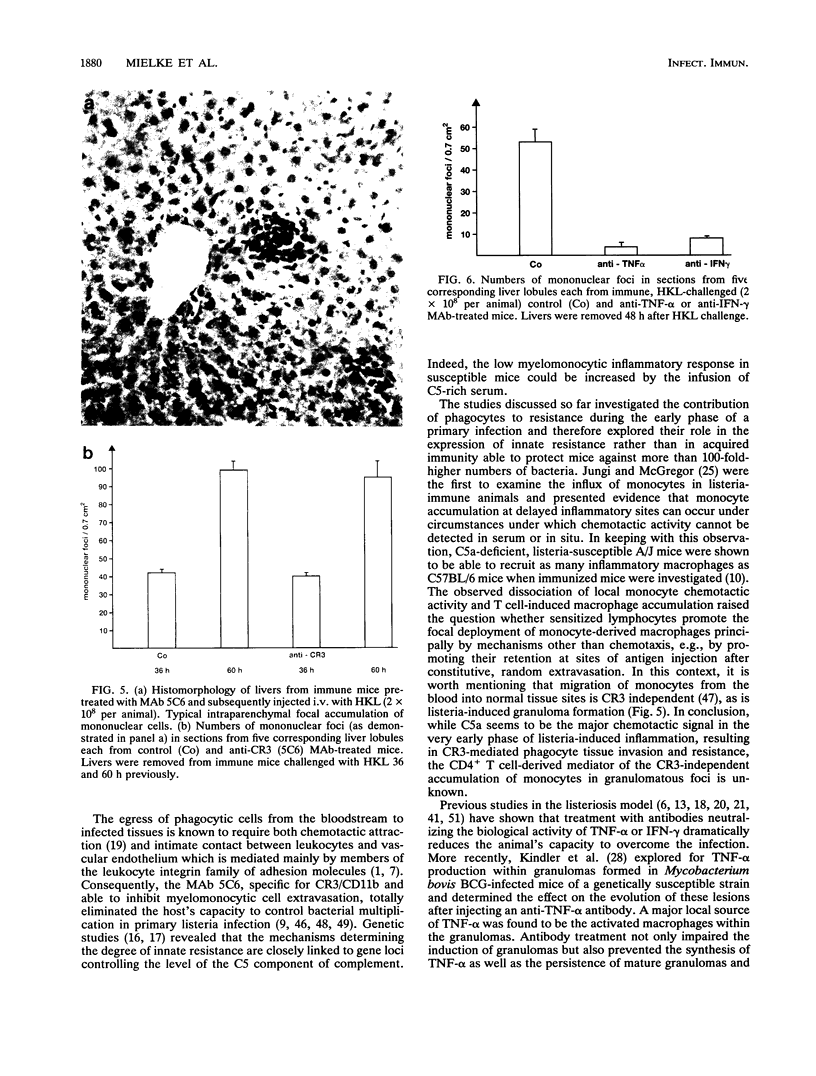
- Mielke M. E. T cell subsets in granulomatous inflammation and immunity to L. Monocytogenes and B. abortus. Behring Inst Mitt. 1991 Feb;(88):99–111. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Milon G., Louis J. T-cell responses during infections with Leishmania major. Behring Inst Mitt. 1991 Feb;(88):80–83. [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Roberts E. C., Demartini J. C., Orme I. M. Passive transfer of acquired resistance to Listeria monocytogenes infection is independent of mononuclear cell granuloma formation. Infect Immun. 1987 Dec;55(12):3215–3218. doi: 10.1128/iai.55.12.3215-3218.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Adoptive transfer of fluorescence-labeled cells shows that resident peritoneal macrophages are able to migrate into specialized lymphoid organs and inflammatory sites in the mouse. Eur J Immunol. 1990 Jun;20(6):1251–1258. doi: 10.1002/eji.1830200609. [DOI] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987 Dec 1;166(6):1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Law S. K. The leukocyte cell surface receptor(s) for the iC3b product of complement. Curr Top Microbiol Immunol. 1990;153:99–122. doi: 10.1007/978-3-642-74977-3_6. [DOI] [PubMed] [Google Scholar]
- Rosen H., Milon G., Gordon S. Antibody to the murine type 3 complement receptor inhibits T lymphocyte-dependent recruitment of myelomonocytic cells in vivo. J Exp Med. 1989 Feb 1;169(2):535–548. doi: 10.1084/jem.169.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D. Validation of a role for endogenously produced IFN gamma in resolution of Listeria monocytogenes infection in mice. Adv Exp Med Biol. 1988;239:185–192. doi: 10.1007/978-1-4757-5421-6_18. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. R., Mellouk S., Houghten R. A., Sedegah M., Kumar S., Good M. F., Berzofsky J. A., Miller L. H., Hoffman S. L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990 Mar 1;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-Den Dulk M. M. The kinetics of promonocytes and monocytes in the bone marrow. J Exp Med. 1970 Oct 1;132(4):813–828. doi: 10.1084/jem.132.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]