Abstract
Nitric oxide (NO) is a short lived secondary messenger, synthesized by nitric-oxide synthases (NOS). It is believed that the activity of inducible NOS (iNOS) is regulated primarily at the transcription level by inducing expression of iNOS mRNA and protein, which then continuously produces NO, until its degradation. Platelets do not have the nuclear transcriptional regulatory mechanisms of the iNOS gene and are believed to generate NO in response to agonist stimulation via endothelial NOS (eNOS). However, here we show that agonist-induced NO production is only partially eNOS-dependent and is also mediated by iNOS. Platelet agonist-induced NO production is significantly reduced in iNOS-knockout platelets. Platelet NO production occurs within seconds after agonist addition and is not accompanied by changes in iNOS protein levels, indicating a signaling-mediated functional activation mechanism of iNOS. Importantly, iNOS knock-out and iNOS inhibitors reduce agonist-induced platelet secretion and aggregation and cGMP levels, indicating that iNOS activation is important in stimulating platelets via the newly identified NO-cGMP-dependent platelet secretion pathway. Furthermore, iNOS knock-out mice have prolonged bleeding time, suggesting that this novel mode of regulation of iNOS activity plays a physiologically relevant role in hemostasis.
Nitric oxide (NO)4 is involved in various biological processes, such as vasodilation, host defense, tumor development, hemostasis, and thrombosis (1). It is synthesized from l-arginine by the family of enzymes known as nitric-oxide synthases (NOS). There are three isoforms of NOS enzymes: NOS1 (neuronal NOS, nNOS), NOS2 (inducible NOS, iNOS), and NOS3 (endothelial NOS or eNOS) (2). nNOS and eNOS are constitutively expressed in cells, and the enzymatic activities of eNOS and nNOS are regulated by intracellular Ca2+ levels and protein phosphorylation (3). iNOS is currently described as a Ca2+-independent enzyme that is not normally expressed in resting cells (2). Upon stimulation of several different cell types (such as endothelial cells and macrophages) with various cytokines or bacterial lipopolysaccharide, iNOS transcription is induced, and, within several hours, iNOS protein is expressed. It is believed that once induced to express, iNOS is always active and produces NO until the protein is degraded (4, 5). Interestingly, constitutive expression of low levels of iNOS has been reported in various tissues, such as kidneys (6), paranasal sinuses (7), and blood platelets (8, 9). It is unclear, however, whether this constitutively expressed iNOS is active and how its function is regulated.
Blood platelets do not have nuclei and thus do not have the ability to regulate protein expression transcriptionally. Upon exposure to subendothelium-bound agonists, such as von Willebrand factor or collagen, or soluble agonists, such as thrombin, thromboxane A2, and ADP, at sites of vascular injury, platelets are rapidly activated to form primary thrombi. Agonist-activated platelets generate NO (10, 11). We have recently shown that platelet agonists induce activation of phosphoinositide 3-kinase and Akt, which activates eNOS (12) and that eNOS plays an important role in NO synthesis during platelet activation and in stimulating cyclic guanosine monophosphate (cGMP)-dependent platelet secretion and secretion-dependent platelet aggregation (13, 14). Here, we show that NO synthesis during platelet activation not only involves eNOS, but is also mediated by the constitutively expressed platelet iNOS. Inducible NOS-derived NO is also important in promoting platelet activation, both ex vivo and in vivo, via the cGMP-dependent mechanism. Thus, our data reveal that iNOS can be activated by a physiologically important mechanism that is posttranslational and independent of previously identified transcriptional regulation.
EXPERIMENTAL PROCEDURES
Materials—Protein synthesis inhibitor puromycin was from Sigma. iNOS inhibitors, 1400W and aminoguanidine, and cGMP analog 8-bromo-cGMP were from Calbiochem. Human α-thrombin was purchased from Enzyme Research Laboratories. Luciferin/luciferase reagent and collagen were purchased from Chronolog. INOS- and eNOS-deficient mice, backcrossed for more than 10 generations to C57BL background, and C57BL/6 control mice were obtained from Jackson laboratory, and colonies were maintained at the University of Illinois Biological Resources Laboratory.
Preparation of Washed Platelets—Fresh blood from healthy volunteers was anticoagulated with 1/7 volume of acid-citrate dextrose as described (15). For the preparation of mouse platelets, 8–12-week-old mice of either sex were anesthetized with an intraperitoneal injection of pentobarbital, and blood was drawn from the inferior vena cava (15). Blood from five to six mice of either genotype was pooled, and platelets were isolated by differential centrifugation as described (15). Platelets were washed two times with CGS buffer (0.12 m sodium chloride, 0.0129 m trisodium citrate, and 0.03 m d-glucose, pH 6.5), resuspended in modified Tyrode's buffer, and allowed to rest for at least 1 h at room temperature before use (16).
Platelet Aggregation and Secretion—Platelet aggregation was measured in a turbidometric platelet aggregometer (Chronolog) at 37 °C with stirring (1,000 rpm). Platelets were preincubated for 5 min at 37 °C with various concentrations of inhibitors or corresponding vehicle controls before the addition of platelet agonists. In some experiments, 8-bromo-cGMP was added immediately after the addition of platelet agonist. Platelet secretion was monitored in parallel with platelet aggregation as ATP release in a platelet lumiaggregometer (Chronolog) with the addition of luciferin/luciferase reagent to the platelet suspension. Quantification was performed using the ATP standard. Statistical analysis was performed using a paired t test.
NO Measurements—Washed mouse platelets in Tyrode's buffer were stimulated with thrombin under stirring conditions at 37 °C. NO release was measured electrochemically in real time using nafion-coated porphyrinic microsensor, calibrated with standard NO solutions (17). In some experiments, platelets were preincubated with 200 μm puromycin or vehicle control for 10 min. These measurements were done using commercial free radical analyzer Apollo 4000 and nitric oxide sensor ISO-NOPF (World Precision Instruments) calibrated with NO donor, S-nitroso-N-acetyl-d,l-penicillamine solutions in 0.1 m copper(II)-sulfate, according to the manufacturer's instructions. Statistical analysis was performed using a t test.
Measurement of Platelet cGMP Levels—Washed mouse platelets, resuspended in modified Tyrode's buffer, were stimulated with collagen for 5 min in a platelet aggregometer with stirring (1,000 rpm) at 37 °C. Washed human platelets were preincubated with iNOS inhibitors for 5 min or an equal volume of vehicle control at 37 °C before the addition of agonist and incubated in a platelet aggregometer with stirring (1,000 rpm) for 5 min. The reaction was stopped by the addition of ice-cold 12% (w/v) trichloroacetic acid, the samples were centrifuged at 2,000 × g for 15 min at 4 °C, and the supernatant was extracted four times with 5 volumes of water-saturated diethyl ether. The samples were lyophilized, and cGMP concentrations were determined using a cGMP enzyme immunoassay kit from Amersham Biosciences-Pharmacia Biotech. Results are expressed as mean ± S.E. Statistical significance between groups was determined by a t test.
iNOS Detection in Platelets—RNA was isolated from C57 black or iNOS knock-out mouse platelets with the total RNA extraction kit from Promega. Total RNA was reverse-transcribed using Thermoscript RT-PCR (Invitrogen), following the instructions of the manufacturer. One-tenth of each reaction was used as a cDNA template for the PCR. iNOS cDNA was amplified over 45 cycles with a forward primer 5′-CCAAGGTTGTCTGCATGGA-3′ and reverse primer 5′-TGTCATGAGCAAAGGCGCA-3′. PCR products (expected size, 220 bp) were separated on 2% agarose gels containing ethidium bromide and visualized under a UV lamp. A control PCR was done from same cDNA preparations using primers specific for glyceraldehyde-3-phosphate dehydrogenase. To exclude the possible contamination from leukocytes, RNA template was isolated from washed leukocytes, and RT-PCR was performed using identical primers and PCR conditions as described for platelets. Isolation of leukocytes was performed as described (18).
To detect iNOS protein in mouse platelets, washed platelets (2 × 109 platelets/ml) resuspended in modified Tyrode's buffer were solubilized with an equal amount of solubilization buffer (0.1 m Tris, 10 mm EGTA, 150 mm NaCl, 2% Triton X-100, pH 7.4) containing 0.2 mm E64, 2 mm phenylmethylsulfonyl fluoride, 0.08 unit/ml aprotinin, 2 mm NaF and incubated on ice for 20 min. After centrifuging for 15 min at 13,000 × g at 4 °C, both supernatant and pellets were respectively solubilized in SDS-PAGE sample buffer and analyzed by SDS-PAGE and Western blotting using an anti-iNOS monoclonal antibody (BD Transduction Laboratories). In most experiments, iNOS protein was only detected in the Triton X-100-insoluble pellet. For detection of iNOS protein in human platelets, 4 × 109/ml platelets were solubilized as above. After centrifugation, platelet lysates were immunoprecipitated with a monoclonal anti-iNOS antibody or control IgG. Immunoprecipitates were analyzed by SDS-PAGE and Western blotting with the rabbit polyclonal anti-iNOS antibody.
eNOS Detection in Platelets—Washed platelets resuspended in modified Tyrode's buffer were solubilized with 50 mm β-octylglucoside, 10 mm Hepes, 10 mm EDTA, 0.15 m NaCl, 1 mm phenylmethylsulfonyl fluoride, 0.1 mm E64, pH 7.4. After centrifugation, the lysates were incubated with 2′,5′-ADP-agarose beads, and bound proteins were analyzed by SDS-PAGE and Western blotting with eNOS-specific antibody (Transduction Laboratories).
Bleeding Time Analysis—Mice of either sex (10–15 weeks old, 24–30 g) were anesthetized with pentobarbital (100 μg/g). Tails were cut 0.5 cm from the tip and immediately immersed in 0.15 m NaCl at 37 °C. Bleeding was followed visually, and time to stable cessation of bleeding (no rebleeding within 1 min) was recorded. Data were analyzed by Mann-Whitney and log-rank tests.
RESULTS
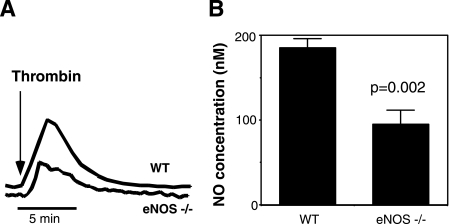
Role of iNOS in Agonist-induced Platelet NO Production—To investigate which NOS isoform is responsible for agonist-induced NO production in platelets, wild-type or eNOS–/– platelets were stimulated with thrombin and analyzed for NO release in real time using an electrochemical detection method. NO production in eNOS–/– platelets was reduced only by approximately 50%, compared with wild-type platelets (Fig. 1). Partial reduction in NO production in eNOS–/– platelets implies the presence of another NOS isoform. This result is consistent with our recent data that eNOS knock-out platelets showed partial reduction in agonist-induced cGMP elevation (13).
FIGURE 1.
Partial inhibition of thrombin-induced NO production by eNOS knock-out. Equal numbers of washed mouse platelets (109/ml) isolated from wild-type C57 black mice (WT) or eNOS knock-out (eNOS–/–) mice were stimulated with 0.1 unit/ml thrombin. NO release was measured in real time with a porphyrinic microsensor. Shown are representative traces (A) and quantitative data from four experiments (B).
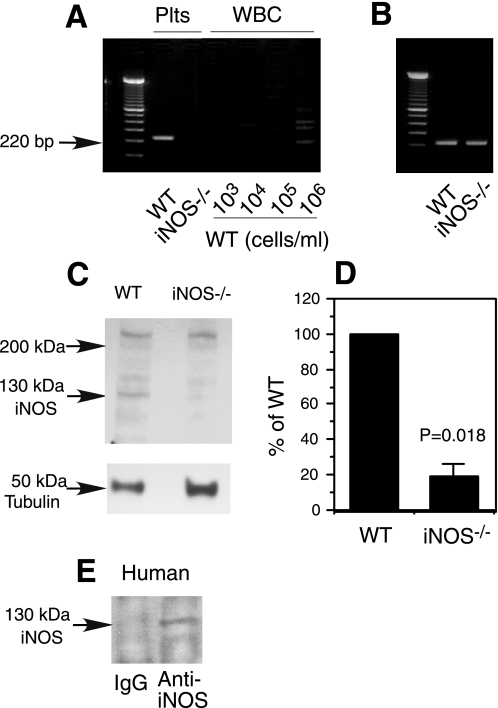
Previous reports have detected low levels of iNOS, but not neuronal NOS in human platelets (8, 9), although its functional state remained unknown. Indeed, we have detected the expression of iNOS mRNA (Fig. 2A) in wild-type but not iNOS–/– mouse platelets by RT-PCR. The PCR amplicon of iNOS mRNA fragment is unlikely to be from contaminating leukocytes in platelet preparation because this specific 220-bp band was not detected in RNA isolated from blood leukocyte preparations containing up to 50 times higher concentration of leukocytes than the concentration of contaminating leukocytes in the platelet preparation (106/ml leukocytes versus 2 × 104/ml leukocytes in platelet preparation) (Fig. 2A). This indicates that the observed iNOS mRNA was derived from platelets. Consistent with the expression of iNOS mRNA in wild-type platelets, we also detected low levels of iNOS protein expression in wild-type but not in iNOS–/– platelets by Western blotting (Fig. 2, C and D). These data suggest that resting mouse platelets constitutively express iNOS. iNOS is also detected in human platelets (Fig. 2E).
FIGURE 2.
Expression of iNOS in platelets. A and B, mouse platelet RNA was isolated from 2.5 × 109 platelets/ml wild-type (WT) or iNOS knock-out platelets (iNOS–/–), and RT-PCR was performed with primers specific for iNOS (A) or a housekeeping gene, GAPDH (B). Leukocyte contamination of platelet preparation was 2 × 104/ml. RNA isolated from WT mouse leukocytes (WBC) of indicated concentrations was also analyzed by RT-PCR using iNOS-specific primers under the same conditions as for platelet preparations to verify that iNOS fragment was not from leukocyte contamination (A). C, Western blotting detection of iNOS protein in the Triton X-100-insoluble fraction of WT or iNOS–/– mouse platelets. A mouse monoclonal anti-iNOS antibody was used to detect iNOS. An antibody againstα-tubulin was used to monitor loading. D, Western blotting results from each of three experiments described in C were scanned and quantitated for uncalibrated optical density using National Institutes of Health ImageJ. Optical density of iNOS band was compared between wild-type and iNOS–/– platelets and expressed as percentage of wild type. Statistical significance was analyzed using a paired t test. The percentage value of the iNOS–/– lane reflects the background level optical density. E, washed human platelet lysates were incubated with iNOS-specific antibody or IgG control. Immunoprecipitates were Western blotted for iNOS.
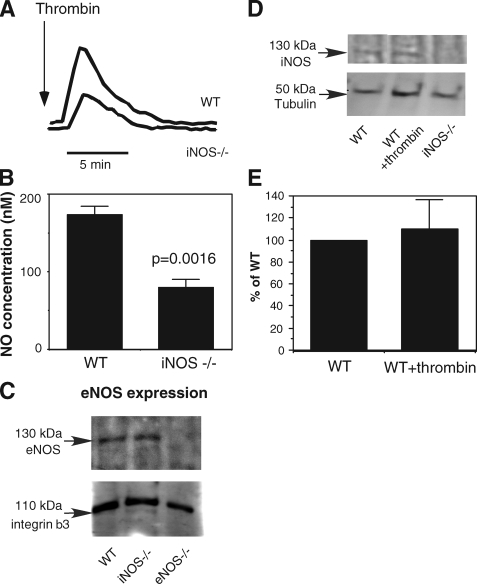
To investigate whether platelet iNOS is functional, wild-type or iNOS–/– platelets were stimulated with thrombin, and NO production was measured. Platelets from iNOS-knock-out mice showed significantly reduced NO production compared with wild-type platelets (Fig. 3, A and B). Because iNOS-knockout platelets express levels of eNOS comparable with wild-type platelets (Fig. 3C), the reduction in NO production in iNOS-knock-out platelets suggests that agonist stimulation of platelets induces iNOS-dependent NO synthesis.
FIGURE 3.
Activation of iNOS-dependent NO production by platelet agonists. A and B, equal numbers of washed mouse platelets isolated from wild-type mice (WT) or iNOS–/– mice were stimulated with 0.1 unit/ml thrombin. NO release was measured in real time with a porphyrinic microsensor. Shown are representative traces (A) and quantitative data from four experiments (B). C, platelet lysates from wild-type (WT), iNOS knockout (iNOS–/–), or eNOS knock-out (eNOS–/–) mice were incubated with ADP-agarose beads. The bead-bound proteins were then immunoblotted with a monoclonal anti-eNOS antibody as described (13). To confirm that equal numbers of platelets were used in the assay, platelet lysates were also immunoblotted with an anti-integrin β3 antibody. D, washed mouse platelets isolated from wild-type (WT) or iNOS–/– mice were stimulated with or without 0.1 unit/ml thrombin. iNOS was then detected by Western blotting as described under “Experimental Procedures.” Equal loading was monitored by immunoblotting with an anti-tubulin antibody. E, Western blotting results from each of three experiments as shown in D were scanned and quantitated using National Institutes of Health ImageJ for uncalibrated optical density. The ratio of optical density of iNOS band in thrombin-stimulated wild-type platelets to that of resting wild-type platelets is expressed as percentage of wild type.
iNOS Activation Does Not Result from Increased iNOS Protein Expression—Agonist-induced NO production in platelets occurs within seconds after thrombin stimulation and peaks at approximately 2–3 min (Figs. 1A and 3A). This type of signal is indicative of transient activation of enzymatic function of already expressed iNOS protein, which, unlike the transcriptional regulation of iNOS, has not been known previously. Platelets do not have a nucleus. Although platelets contain a significant amount of mRNA and have the potential for de novo protein synthesis (19, 20), significant protein synthesis occurs in response to agonist stimulation only after much longer incubation (21). To exclude the possibility that agonist-stimulated iNOS activity results from de novo iNOS protein synthesis, we have compared the protein expression levels between resting and thrombin-stimulated platelets. Indeed, we show that iNOS protein levels are similar before and after 5 min of agonist stimulation (Fig. 3, D and E), indicating that agonist-stimulated iNOS activation is independent of changes in the levels of iNOS protein expression. As a further support to this conclusion, we also obtained data that preincubation with a protein synthesis inhibitor puromycin under conditions shown previously to abolish protein translation (22, 23) did not affect the thrombin-induced NO production in wild-type or eNOS knock-out platelets (data not shown). Consistent with these results, we have also obtained data that there is no significant de novo synthesis of Bcl-3, a protein shown to be synthesized after prolonged platelet activation, after 5 min of agonist stimulation of mouse platelets under the same experimental conditions that iNOS-dependent NO synthesis was demonstrated (data not shown). Thus, it appears that agonist-induced NO production does not involve increased iNOS protein levels. Consequently, these results indicate that platelet iNOS is activated by a platelet agonist-induced signaling mechanism that regulates the enzymatic function of constitutively expressed iNOS protein.
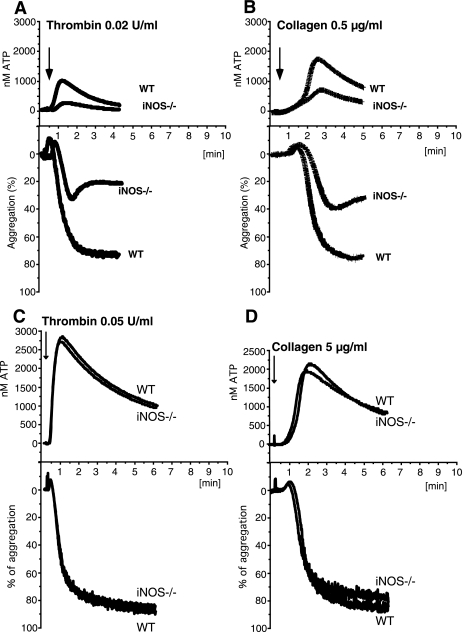
Role of iNOS in Platelet Activation and Hemostasis—To determine whether agonist-induced activation of iNOS is important in regulating platelet activation, platelets from wild-type or iNOS-deficient mice were stimulated with low concentrations of thrombin and assayed for platelet aggregation (Fig. 4A). iNOS–/– platelets showed significantly inhibited aggregation response, indicating that iNOS plays an important role in platelet activation. The inhibitory effect of iNOS knock-out is characteristic of a defect in platelet secretion. To determine whether iNOS knockout affects platelet secretion, ATP release was measured concomitantly with platelet aggregation. Low dose thrombin-induced platelet secretion was inhibited significantly in iNOS–/– platelets (Fig. 4A). To determine whether iNOS activation also plays a role in platelet activation induced by other platelet agonists, wild-type or iNOS-deficient platelets were stimulated with a low dose of collagen. Similar to thrombin-stimulated platelets, iNOS–/– platelets showed decreased collagen-induced secretion and aggregation response (Fig. 4B). Thus, iNOS participates in mouse platelet activation induced by different platelet agonists. The defect in platelet aggregation of iNOS knock-out platelets could be overcome at higher agonist concentrations (Fig. 4, C and D). This is not unexpected because eNOS also mediates agonist induced NO production and contributes to platelet activation (13).
FIGURE 4.
Stimulatory role of iNOS in mouse platelet aggregation and secretion. Representative aggregation and secretion traces of washed wild-type (WT) or iNOS–/– mouse platelets stimulated with 0.02 unit/ml thrombin (A), 0.5 μg/ml collagen (B), 0.05 unit/ml thrombin (C), or 5 μg/ml collagen (D). The statistical difference between WT and iNOS–/– platelets was analyzed using a paired t test with following results: A, aggregation p = 0.0053, n = 5; secretion p = 0.0086, n = 5; B, aggregation p = 0.0106, n = 6; secretion p = 0.0052, n = 4.
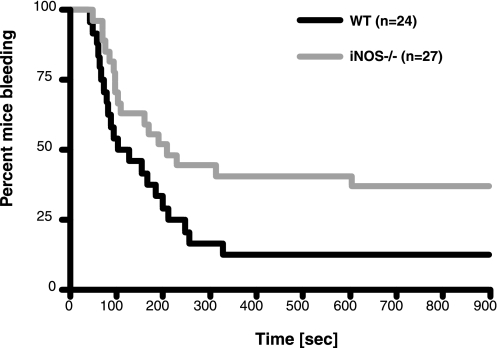
To test the effect of iNOS knock-out on hemostasis in vivo, we have determined bleeding time in a standard tail bleeding assay in mice. Consistent with the data from ex vivo studies, we found that iNOS knock-out mice had significantly prolonged bleeding time compared with wild-type controls. Median bleeding time for iNOS–/– mice was 208 s, whereas wild-type mice had a median bleeding time of 115.5 s, p < 0.05 (Mann-Whitney test). The effect of iNOS knock-out on bleeding time was also significant by a log rank test (p < 0.05) (Fig. 5). Taken together, the studies on iNOS knock-out suggest that the described posttranslational activation of iNOS occurs under physiological conditions and plays an important role in platelet activation and hemostasis.
FIGURE 5.
Effect of iNOS deficiency on bleeding time. Tail bleeding time experiments were performed as described under “Experimental Procedures.” Shown is the percentage of mice still bleeding as a function of time. The difference between wild-type (WT)(black line) and iNOS-deficient mice (iNOS–/–)(gray line) was significant by a log rank test (p = 0.031).
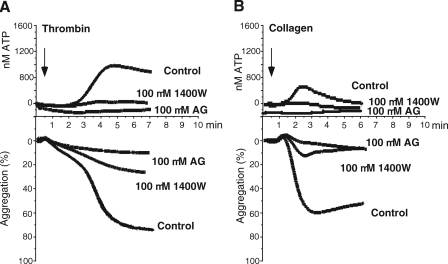
Human platelets also express low levels of iNOS protein (8, 9) (Fig. 2D). To investigate the role of iNOS in human platelet activation, washed human platelets were preincubated with iNOS-specific inhibitors 1400W (24) or aminoguanidine (25) and stimulated with a low concentration of agonists. Both agents inhibited platelet secretion and the second wave of platelet aggregation induced by low dose thrombin (Fig. 6A). These iNOS inhibitors also reduced collagen-stimulated human platelet secretion and aggregation (Fig. 6B). The inhibitory effects of both inhibitors are dose-dependent, with marked inhibition already observed at 25 μm (supplemental Fig. 1). It has been reported that aminoguanidine is 10 times more selective for iNOS over eNOS with an IC50 of 330 μm for eNOS, whereas 1400W exhibits higher than 4,000-fold selectivity for iNOS over eNOS and an IC50 of 1,000 μm for eNOS (2). It has also been reported that 300 μm 1400W had only a slight effect on eNOS activity in a tissue-based activity assay (24). Therefore, although we do not exclude very limited effects of these inhibitors on NO production generated by other enzymes, it is likely that the inhibitory actions of these agents result predominantly from iNOS inhibition. Thus, iNOS-mediated NO production plays an important role in low dose agonist-induced human platelet secretion and the secretion-dependent second wave of platelet aggregation.
FIGURE 6.
Stimulatory role of iNOS in human platelet aggregation and secretion. A and B, representative aggregation and secretion traces of washed human platelets preincubated for 5 min at 37 °C with 100 μm iNOS-specific inhibitors 1400W or aminoguanidine (AG) and stimulated with 0.02 unit/ml thrombin (A) or 0.7 μg/ml collagen (B). The statistical difference between control and inhibitor-treated platelets was analyzed using a paired t test with following results: A, 1400W aggregation p = 0.0024, n = 4, 1400W secretion p = 0.0442, n = 4; aminoguanidine aggregation p = 0.0018, n = 3, aminoguanidine secretion p = 0.0227, n = 3; B, 1400W aggregation p = 0.0025, n = 4, 1400W secretion p = 0.0374, n = 4; aminoguanidine aggregation p = 0.0138, n = 4, aminoguanidine secretion p = 0.0136, n = 3.
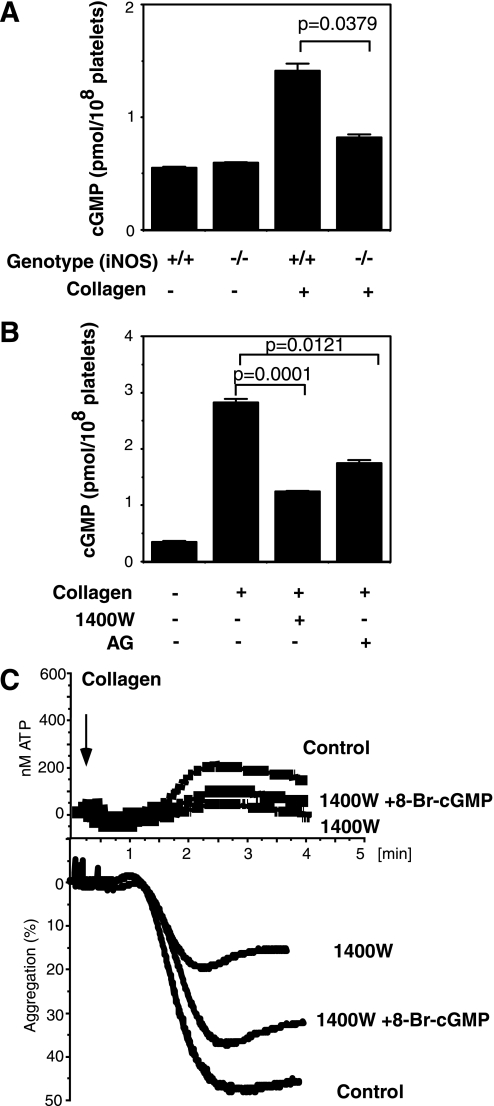
iNOS Promotes Platelet Activation in a cGMP-dependent Manner—NO regulates cellular functions by cGMP-dependent and -independent signaling pathways. In the cGMP-dependent pathway, agonist-stimulated NO activates soluble guanylyl cyclase and induces cGMP synthesis, which then further activates the cGMP-dependent protein kinase. To investigate the role of iNOS in cGMP production, we examined the effect of iNOS knock-out or iNOS inhibitors on collagen-induced cGMP production. Consistent with previous studies, collagen induced cGMP elevation (26). Collagen-induced cGMP elevation was significantly inhibited by iNOS-knock-out (Fig. 7A) or iNOS inhibitors (Fig. 7B), suggesting that NO produced by the agonist-activated iNOS plays an important role in agonist-stimulated cGMP elevation. Inhibition of cGMP production by iNOS knock-out was partial, which is consistent with our previous results that eNOS also plays a role in agonist-induced platelet NO production and cGMP elevation (13). Thus, both eNOS and iNOS are important in stimulating the cGMP-dependent signaling pathway, which explains why the respective knockouts show relatively mild defects in platelet activation.
FIGURE 7.
Roles of cGMP in iNOS-mediated platelet activation. A and B, wild-type (iNOS+/+) and iNOS-deficient (iNOS–/–) mouse platelets (A) or human platelets pretreated with or without iNOS inhibitors 1400W (0.1 mm) and aminoguanidine (AG) (0.1 mm)(B) were stimulated with 0.5 μg/ml collagen for 5 min. cGMP levels (mean ± S.E., n = 3) were then determined. C, washed human platelets pretreated with 100 μm 1400W or buffer (Control) were stimulated with 0.5 μg/ml collagen followed by the addition of 1 μm 8-Br-cGMP or vehicle. Note that the addition of a low concentration of 8-Br-cGMP partially reversed the inhibitory effect of the iNOS inhibitor on platelet aggregation (p = 0.0111, n = 3) and secretion (p = 0.0351, n = 3).
To determine whether the role of agonist-induced iNOS activation in stimulating platelet activation is mediated via the cGMP-dependent signaling pathway, we investigated whether a membrane-permeable cGMP analog could reverse the inhibitory effect of the iNOS inhibitor 1400W on platelet activation. Low concentrations of cell-permeable 8-bromo-cGMP reversed the inhibitory effect of the iNOS inhibitor on platelet aggregation and secretion (Fig. 7C). Therefore, agonist-induced iNOS activation is upstream of the cGMP/protein kinase G pathway, which was recently found to play a stimulatory role in platelet activation (14, 27).
DISCUSSION
The data presented in this study demonstrate the novel activation of iNOS protein by intracellular signals. These data also indicate that constitutively expressed low levels of iNOS serve as a “low output” NO production mechanism that is physiologically important in mediating platelet secretion, aggregation, and primary hemostasis.
The role of NO in many physiological and pathological processes has been the subject of a great many controversies. However, it is becoming increasingly evident that NO plays biphasic roles both physiologically and pathophysiologically in many cells. Thus, the effects of low concentrations of NO on a physiological or pathological event often contradict the effects of high concentrations of NO. Biphasic roles of NO have been shown in leukocytes and macrophages (28–30). Similarly, NO exerts both stimulatory and inhibitory effects during tumor development depending on NO concentrations (31). In heart, liver, and kidney, NO has been shown to be both proapoptotic and antiapoptotic, depending on the amount of synthesized NO and the redox environment (32–34). In platelets, it has been known for many years that high concentrations of NO donors (micromolar range) inhibit platelet function (35, 36). However, we have shown recently that low concentrations of NO (low nanomolar range) stimulate platelet activation via the cGMP-dependent protein kinase pathway, again indicating a biphasic effect of the NO-cGMP pathway in platelet activation (12, 13, 27, 37). It is important to note that the controversy on the role of NO in platelets has been complicated by the fact that investigators in some studies used platelet preparations that are desensitized and thus no longer responsive to low concentrations of agonists (such as 0.1 unit/ml thrombin) (38). Studies using desensitized platelets are naturally unable to show the stimulatory effect of NO on platelet activation induced by low concentrations of agonists. Our study is consistent with the biphasic effect of NO in platelets and further shows that low concentrations of NO can be produced by iNOS during platelet activation.
Cells regulate NO levels by regulating the activity and expression of different isoforms of NOS. Traditionally, eNOS has been considered to be a low output enzyme that synthesizes low levels of NO, believed to serve the physiological role of a secondary messenger. Conversely, it is believed that iNOS is a high output NOS whose expression is inducible at the transcriptional level and that upon expression, iNOS is always active and synthesizes high levels of NO (4, 5). Catalytic activity of iNOS is almost 10 times higher than eNOS (39, 40). However, it has been shown recently that the principal NOS isoform mediating NO overproduction responsible for cardiovascular dysfunction during anaphylactic shock is eNOS rather than iNOS (41), indicating that isoforms other than iNOS can serve as a high output source of NO. However, constitutive expression of low levels of iNOS has been reported in different tissues, including platelets (8, 9), but the functions of this constitutively expressed iNOS were previously unknown. Our data clearly show that constitutively expressed iNOS protein in platelets can be activated by agonist-induced intracellular signaling to produce NO and that iNOS appears to contribute to approximately half of the NO produced during platelet activation. The significant contribution of the barely detectable levels of iNOS in platelet NO synthesis is probably because iNOS protein has much higher catalytic efficiency than eNOS protein (40). More importantly, our study uncovers a novel nontranscriptional regulatory mechanism that activates the function of constitutively expressed iNOS.
Our data demonstrate that low concentrations of NO derived from platelet iNOS, similar to eNOS-derived NO, activate soluble guanylyl cyclase to produce cGMP, which stimulates platelet secretion and thus aggregation. As discussed above, because of the presence of eNOS, the functional defects of iNOS knock-out mice are mild and are only shown when platelets are stimulated with very low concentrations of agonists. However, to our surprise, iNOS knock-out mice showed prolonged bleeding time. NO is a known vessel dilator, and thus the effect of reduced NO production by iNOS knock-out on vascular wall should favor a shortened bleeding time. Thus, our results suggest that NO generated by platelet iNOS may play a stimulatory role in in vivo hemostasis. Interestingly, a previous study showed that when iNOS expression in vascular wall was induced by tumor necrosis factor-α, the high concentrations of NO it produced were associated with a reduction in injury-induced thrombosis in vivo (42). Our data, when combined with these results, suggest a biphasic role for iNOS in vasculature. Constitutively expressed iNOS can be activated during platelet activation by posttranslational signaling and thus serve as a low output NOS that promotes platelet activation and hemostasis, but high levels of iNOS transcriptionally induced to express in vascular wall cells by cytokines such as tumor necrosis factor-α during inflammation serve as a high output NOS that is associated with the inhibitory effect on thrombosis (42).
Taken together, our results are not only significant to our understanding of the roles of NO signaling pathway in platelets, but are also of general significance for the following reasons. First, these data for the first time indicate that iNOS can be expressed as a relatively inactive form in cells which becomes activated in response to stimuli. Second, they indicate that there are two different iNOS activation mechanisms: a fast functional activation in which iNOS responds to stimuli immediately without requiring the induction of protein synthesis, and the slow transcriptional regulation that leads to the production of a large quantity of NO. Finally, our data indicate that iNOS can function both as a low output and a high output NOS and can be responsible for the biphasic roles of NO in cellular functions. It would be very interesting to understand further how iNOS function is activated by intracellular signaling.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL062350, HL068819, and HL080264 (to X. D.) and HL60678 (to R. A. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: NO, nitric oxide; NOS, nitric-oxide synthase; eNOS, endothelial nitric-oxide synthase; iNOS, inducible nitric-oxide synthase; nNOS, neuronal nitric-oxide synthase; RT, reverse transcription.
References
- 1.Schlossmann, J., Feil, R., and Hofmann, F. (2003) Ann. Med. 35 21–27 [DOI] [PubMed] [Google Scholar]
- 2.Alderton, W. K., Cooper, C. E., and Knowles, R. G. (2001) Biochem. J. 357 593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming, I., and Busse, R. (2003) Am. J. Physiol. 284 R1–R12 [DOI] [PubMed] [Google Scholar]
- 4.MacMicking, J., Xie, Q. W., and Nathan, C. (1997) Annu. Rev. Immunol. 15 323–350 [DOI] [PubMed] [Google Scholar]
- 5.Kleinert, H., Pautz, A., Linker, K., and Schwarz, P. M. (2004) Eur. J. Pharmacol. 500 255–266 [DOI] [PubMed] [Google Scholar]
- 6.Tojo, A., Gross, S. S., Zhang, L., Tisher, C. C., Schmidt, H. H., Wilcox, C. S., and Madsen, K. M. (1994) J. Am Soc. Nephrol. 4 1438–1447 [DOI] [PubMed] [Google Scholar]
- 7.Lundberg, J. O., Farkas-Szallasi, T., Weitzberg, E., Rinder, J., Lidholm, J., Anggaard, A., Hokfelt, T., Lundberg, J. M., and Alving, K. (1995) Nat. Med. 1 370–373 [DOI] [PubMed] [Google Scholar]
- 8.Chen, L. Y., and Mehta, J. L. (1996) J. Cardiovasc. Pharmacol. 27 154–158 [DOI] [PubMed] [Google Scholar]
- 9.Wallerath, T., Gath, I., Aulitzky, W. E., Pollock, J. S., Kleinert, H., and Forstermann, U. (1997) Thromb. Haemostasis 77 163–167 [PubMed] [Google Scholar]
- 10.Malinski, T., Radomski, M. W., Taha, Z., and Moncada, S. (1993) Biochem. Biophys. Res. Commun. 194 960–965 [DOI] [PubMed] [Google Scholar]
- 11.Freedman, J. E., Sauter, R., Battinelli, E. M., Ault, K., Knowles, C., Huang, P. L., and Loscalzo, J. (1999) Circ. Res. 84 1416–1421 [DOI] [PubMed] [Google Scholar]
- 12.Stojanovic, A., Marjanovic, J. A., Brovkovych, V. M., Peng, X., Hay, N., Skidgel, R. A., and Du, X. (2006) J. Biol. Chem. 281 16333–16339 [DOI] [PubMed] [Google Scholar]
- 13.Marjanovic, J. A., Li, Z., Stojanovic, A., and Du, X. (2005) J. Biol. Chem. 280 37430–37438 [DOI] [PubMed] [Google Scholar]
- 14.Li, Z., Zhang, G., Marjanovic, J. A., Ruan, C., and Du, X. (2004) J. Biol. Chem. 279 42469–42475 [DOI] [PubMed] [Google Scholar]
- 15.Li, Z., Zhang, G., Le Breton, G. C., Gao, X., Malik, A. B., and Du, X. (2003) J. Biol. Chem. 278 30725–30731 [DOI] [PubMed] [Google Scholar]
- 16.Du, X. P., Plow, E. F., Frelinger, A. L., 3rd, O'Toole, T. E., Loftus, J. C., and Ginsberg, M. H. (1991) Cell 65 409–416 [DOI] [PubMed] [Google Scholar]
- 17.Brovkovych, V., Stolarczyk, E., Oman, J., Tomboulian, P., and Malinski, T. (1999) J. Pharm. Biomed. Anal. 19 135–143 [DOI] [PubMed] [Google Scholar]
- 18.Ulmer, A. J., and Flad, H. D. (1979) J. Immunol. Methods 30 1–10 [DOI] [PubMed] [Google Scholar]
- 19.Warshaw, A. L., Laster, L., and Shulman, N. R. (1967) J. Biol. Chem. 242 2094–2097 [PubMed] [Google Scholar]
- 20.Kieffer, N., Guichard, J., Farcet, J. P., Vainchenker, W., and Breton-Gorius, J. (1987) Eur. J. Biochem. 164 189–195 [DOI] [PubMed] [Google Scholar]
- 21.Weyrich, A. S., Dixon, D. A., Pabla, R., Elstad, M. R., McIntyre, T. M., Prescott, S. M., and Zimmerman, G. A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 5556–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seiser, R. M., and Nicchitta, C. V. (2000) J. Biol. Chem. 275 33820–33827 [DOI] [PubMed] [Google Scholar]
- 23.Roy, A., and Wonderlin, W. F. (2003) J. Biol. Chem. 278 4397–4403 [DOI] [PubMed] [Google Scholar]
- 24.Garvey, E. P., Oplinger, J. A., Furfine, E. S., Kiff, R. J., Laszlo, F., Whittle, B. J., and Knowles, R. G. (1997) J. Biol. Chem. 272 4959–4963 [DOI] [PubMed] [Google Scholar]
- 25.Griffiths, M. J., Messent, M., MacAllister, R. J., and Evans, T. W. (1993) Br. J. Pharmacol. 110 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haslam, R. J., and McClenaghan, M. D. (1974) Biochem. J. 138 317–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Z., Xi, X., Gu, M., Feil, R., Ye, R. D., Eigenthaler, M., Hofmann, F., and Du, X. (2003) Cell 112 77–86 [DOI] [PubMed] [Google Scholar]
- 28.Browning, D. D., McShane, M. P., Marty, C., and Ye, R. D. (2000) J. Biol. Chem. 275 2811–2816 [DOI] [PubMed] [Google Scholar]
- 29.Browning, D. D., Windes, N. D., and Ye, R. D. (1999) J. Biol. Chem. 274 537–542 [DOI] [PubMed] [Google Scholar]
- 30.Connelly, L., Palacios-Callender, M., Ameixa, C., Moncada, S., and Hobbs, A. J. (2001) J. Immunol. 166 3873–3881 [DOI] [PubMed] [Google Scholar]
- 31.Lechner, M., Lirk, P., and Rieder, J. (2005) Semin. Cancer Biol. 15 277–289 [DOI] [PubMed] [Google Scholar]
- 32.Razavi, H. M., Hamilton, J. A., and Feng, Q. (2005) Pharmacol. Ther. 106 147–162 [DOI] [PubMed] [Google Scholar]
- 33.Vodovotz, Y., Kim, P. K., Bagci, E. Z., Ermentrout, G. B., Chow, C. C., Bahar, I., and Billiar, T. R. (2004) Curr. Mol. Med. 4 753–762 [DOI] [PubMed] [Google Scholar]
- 34.Brune, B. (2002) Kidney Int. 61 786–789 [DOI] [PubMed] [Google Scholar]
- 35.Mellion, B. T., Ignarro, L. J., Ohlstein, E. H., Pontecorvo, E. G., Hyman, A. L., and Kadowitz, P. J. (1981) Blood 57 946–955 [PubMed] [Google Scholar]
- 36.Jang, E. K., Azzam, J. E., Dickinson, N. T., Davidson, M. M., and Haslam, R. J. (2002) Br. J. Haematol. 117 664–675 [DOI] [PubMed] [Google Scholar]
- 37.Li, Z., Ajdic, J., Eigenthaler, M., and Du, X. (2003) Blood 101 4423–4429 [DOI] [PubMed] [Google Scholar]
- 38.Morrell, C. N., Matsushita, K., Chiles, K., Scharpf, R. B., Yamakuchi, M., Mason, R. J., Bergmeier, W., Mankowski, J. L., Baldwin, W. M., 3rd, Faraday, N., and Lowenstein, C. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 3782–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santolini, J., Meade, A. L., and Stuehr, D. J. (2001) J. Biol. Chem. 276 48887–48898 [DOI] [PubMed] [Google Scholar]
- 40.Stuehr, D. J., Santolini, J., Wang, Z. Q., Wei, C. C., and Adak, S. (2004) J. Biol. Chem. 279 36167–36170 [DOI] [PubMed] [Google Scholar]
- 41.Cauwels, A., Janssen, B., Buys, E., Sips, P., and Brouckaert, P. (2006) J. Clin. Invest. 116 2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambien, B., Bergmeier, W., Saffaripour, S., Mitchell, H. A., and Wagner, D. D. (2003) J. Clin. Invest. 112 1589–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.