Abstract
Sildenafil, a potent inhibitor of phosphodiesterase-5 (PDE-5) induces powerful protection against myocardial ischemia-reperfusion injury. PDE-5 inhibition increases cGMP levels that activate cGMP-dependent protein kinase (PKG). However, the cause and effect relationship of PKG in sildenafil-induced cardioprotection and the downstream targets of PKG remain unclear. Adult ventricular myocytes were treated with sildenafil and subjected to simulated ischemia and reoxygenation. Sildenafil treatment significantly decreased cardiomyocyte necrosis and apoptosis. The PKG inhibitors, KT5823, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chloro-phenylthio) (Rp-8-pCPT-cGMPs), or DT-2 blocked the anti-necrotic and anti-apoptotic effect of sildenafil. Selective knockdown of PKG in cardiomyocytes with adenoviral vector containing short hairpin RNA of PKG also abolished sildenafil-induced protection. Furthermore, intra-coronary infusion of sildenafil in Langendorff-isolated mouse hearts prior to ischemia-reperfusion significantly reduced myocardial infarct size after 20 min ischemia and 30 min reperfusion, which was abrogated by KT5823. Sildenafil significantly increased PKG activity in intact hearts and cardiomyocytes. Sildenafil also enhanced the Bcl-2/Bax ratio, phosphorylation of Akt, ERK1/2, and glycogen synthase kinase 3β. All these changes (except Akt phosphorylation) were significantly blocked by KT5823 and short hairpin RNA of PKG. These studies provide the first evidence for an essential role of PKG in sildenafil-induced cardioprotection. Moreover, our results demonstrate that sildenafil activates a PKG-dependent novel signaling cascade that involves activation of ERK and inhibition of glycogen synthase kinase 3β leading to cytoprotection.
cGMP-dependent protein kinase or protein kinase G (PKG)2 is one of the major intracellular receptors for cGMP. This serine/threonine protein kinase has two isozymes (type I and type II; i.e. PKGI and PKGII) that are presented in eukaryotic cells. The N terminus of PKGI is encoded by two alternatively spliced exons that produce the isoforms, α and β (1-3). PKGIα is mainly found in lung, heart, platelets, and cerebellum, whereas PKGIβ is highly expressed with PKGIα in smooth muscles of uterus, vessels, intestine, and trachea (4, 5). More importantly, the activation of PKG phosphorylates numerous intracellular proteins that in turn regulate many important physiological functions such as control of vascular tone, cell differentiation and proliferation, and platelet aggregation (4). The role of PKG in cardioprotection was identified based on the findings that indicated a possible link between PKG activation, the opening of mitochondrial ATP-sensitive potassium (mito-KATP) channels (6-8), and blockade of pharmacological preconditioning by PKG inhibitors in cardiomyocytes (9, 10). Furthermore, we recently demonstrated that adenoviral gene transfer of PKGIα inhibited necrosis and apoptosis following simulated ischemia and reoxygenation (SI-RO) in adult rat cardiomyocytes (11). To translate these observations into the clinical setting, the use of a safe pharmacological agent that could selectively activate PKG and associated downstream signaling pathways (without the use of adenoviral gene transfer) would be an attractive approach for protecting the heart against ischemia-reperfusion injury as well as myocardial infarction-induced heart failure. In this respect, the potent phosphodiesterase-5 (PDE-5) inhibitors including sildenafil and other erectile dysfunction drugs are promising agents for stimulation of PKG in the heart. Because PDE-5 is a major enzyme that is responsible for catalytic break-down of cGMP in the vascular smooth muscle cells (12), its inhibition leads to increased cGMP accumulation and subsequent activation of PKG. Interestingly, our laboratory first discovered a cardioprotective effect of sildenafil that was dependent on enhanced nitric oxide (NO) generation through activation of endothelial/inducible NO synthase (eNOS/iNOS), activation of PKC, and opening of mito-KATP channels (13-15). We also demonstrated that PDE-5 inhibition attenuated cell death following simulated ischemia and reoxygenation through NO-dependent up-regulation of the Bcl-2/Bax ratio in adult cardiomyocytes (16). However, it is not known whether the protection afforded by sildenafil, and other PDE-5 inhibitors, against ischemia-reperfusion injury in the heart is causatively related with PKG activation. Moreover, the cardioprotective mechanisms, as well as downstream effectors triggered following activation of PKG with sildenafil are not completely understood. Accordingly, the primary goal of this investigation was to demonstrate a direct cause and effect relationship between PKG activation with sildenafil and protection against ischemia/reperfusion injury. The second goal was to determine the downstream targets of sildenafil-induced PKG activation including Akt, ERK, and JNK that were previously shown to be phosphorylated in the PKGIα-overexpressed cardiomyocytes and played an essential role in PKGIα-induced cytoprotection (11). Third, because PKG-induced phosphorylation and subsequent inhibition of GSK3β in osteoblasts (17) and GSK3β inhibition has been proposed as an essential mechanism for cardioprotection (18, 19), we also sought to investigate if sildenafil-induced protection involves PKG-dependent phosphorylation of GSK3β in cardiac cells. We performed the present study in our established models of simulated ischemia-reoxygenation in adult cardiomyocytes and isolated perfused heart subjected to global ischemia-reperfusion. Our data show that sildenafil induces cardioprotection through PKG and phosphorylation of ERK1/2 and GSK3β in conjunction with the increase in Bcl-2/Bax in cardiomyocytes and intact heart.
EXPERIMENTAL PROCEDURES
Isolation of Ventricular Cardiomyocytes—Adult male out-bred ICR mice (∼30 g) and adult male Wistar rats (∼300 g) were purchased from Harlan (Indianapolis, IN). Animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. The ventricular cardiomyocytes were isolated using an enzymatic technique as previously reported (11, 16). In brief, the mouse or rat was anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneal) and the heart was removed and retrograde perfused in a Langendorff system (37 °C) with a Ca2+-free bicarbonate-based buffer containing: 120 mm NaCl, 5.4 mm KCl, 1.2 mm MgSO4, 1.2 mm NaH2PO4, 5.6 mm glucose, 20 mm NaHCO3, 10 mm 2,3-butanedione monoxime, and 5 mm taurine, which was continuously gassed with 95% O2 + 5% CO2. The enzymatic digestion was commenced by adding collagenase type II (Worthington; 0.5 mg/ml) and protease type XIV (0.02 mg/ml) with 50 μg of CaCl2 to the perfusion buffer. The digested ventricular tissue was cut into chunks and aspirated with a transfer pipette for facilitating cell dissociation. The cell pellet was re-suspended for a three-step Ca2+ restoration procedure (i.e. 125, 250, and 500 μm CaCl2). The freshly isolated mouse cardiomyocytes were then plated with minimal essential medium M1018, pH 7.35-7.45, containing 1.2 mm Ca2+, 12 mm NaHCO3, 2.5% fetal bovine serum, and 1% penicillin-streptomycin onto slides or dishes that were precoated with 20 μg/ml mouse laminin for 1 h. The isolated cardiomyocytes were plated with Medium 199 containing 2 mm l-carnitine, 5 mm creatine, 5 mm taurine, 5 mm glucose, 0.1 μm insulin, 10% fetal bovine serum, and 1% penicillin-streptomycin. After 1 h of plating, the cardiomyocytes were infected with adenoviral vectors containing PKG-shRNA or control shRNA in serum-free growth medium for 24 h.
Experimental Protocols—The cultured cardiomyocytes (shown in Fig. 1A) were incubated at 37 °C and 5% CO2 for 1 h, with or without 1 or 10 μm sildenafil (a pure solid powder kindly provided by Pfizer, Inc., dissolved in water). A subgroup of cardiomyocytes were treated with PKG inhibitors KT5823 (2 μm) or Rp-8-pCPT-cGMPs (100 μm) (both purchased from Calbio-chem), or DT-2 trifluoroacetate (125 nm) (purchased from Sigma) with or without sildenafil for 1 h. The cardiomyocytes were then subjected to SI for 40 min by replacing the cell medium with an “ischemia buffer” that contained 118 mm NaCl, 24 mm NaHCO3, 1.0 mm NaH2PO4, 2.5 mm CaCl2-2H2O, 1.2 mm MgCl2, 20 mm sodium lactate, 16 mm KCl, 10 mm 2-de-oxyglucose (pH adjusted to 6.2) as reported previously (16). In addition, the cells were incubated under hypoxic conditions at 37 °C by adding N2 gas into a tri-gas incubator and maintained at 1-2% O2 and 5% CO2 during the entire SI period. Reoxygenation was accomplished by replacing the ischemic buffer with normal cell medium and resuming normoxic conditions. Cell necrosis and apoptosis were assessed after 1 or 18 h of reoxygenation, respectively.
FIGURE 1.
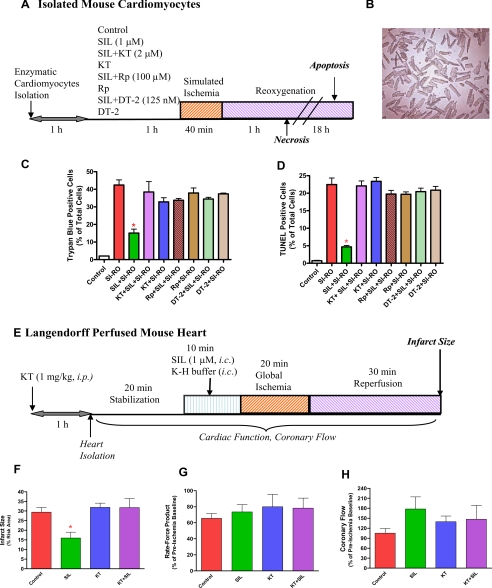
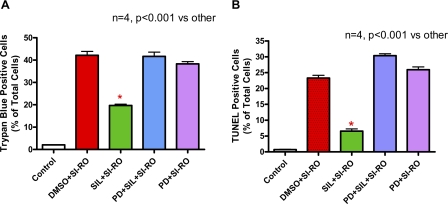
Experimental protocol. A, isolated mouse cardiomyocytes incubated for 1 h with or without sildenafil (SIL) (1 μm) in the presence or absence of protein kinase G (PKG) inhibitor, KT2358 (2 μm), Rp-8-pCPT-cGMPs (100 μm), or DT-2 (125 nm) and subjected to 40 min of SI and 1 or 18 h of RO. Cell necrosis and apoptosis were assessed after 1 or 18 h of reoxygenation, respectively. B, representative image of isolated mouse cardiomyocytes. C, quantitative data showing the effect of sildenafil (1 μm) with or without KT5823 (KT), Rp-8-pCPT-cGMPs (Rp), or DT-2 on cardiomyocyte necrosis following SI-RO as determined by trypan blue staining (* indicates p < 0.001 versus SI-RO; n = 4). D, quantitative data showing the effect of sildenafil (1 μm) with/without KT5823 (KT), Rp-8-pCPT-cGMPs (Rp), or DT-2 on cardiomyocyte apoptosis following SI-RO determined by TUNEL assay (* indicates p < 0.001 versus SI-RO; n = 4). E, isolated perfused hearts subjected to 10 min of intracoronary infusion of sildenafil (1 μm, at 0.25 ml/min pump speed) or Krebs-Henseleit (K-H) buffer followed by 20 min of no-flow global ischemia and 30 min of reperfusion. Myocardial infarct size was determined after the ischemia-reperfusion protocol. KT5823 (1 mg/kg) or volume-matched DMSO (solvent of KT5823) were administered (intraperitoneal) 1 h prior to the heart isolation. F, infarct size (% of risk area); G, double product of heart rate and ventricular developed force (% of pre-ischemic baseline); and H, coronary flow (% of pre-ischemic value). * indicates p < 0.05 versus all other groups.
Evaluation of Cell Viability and Apoptosis—Cell viability was assessed by trypan blue exclusion assay as reported previously (11, 16). Cardiomyocyte apoptosis was analyzed by TUNEL staining, using a commercial kit (BD Biosciences) that detects nuclear DNA fragmentation via a fluorescence assay as previously reported (11, 16). In brief, after SI and 18 h of reoxygenation, the cells in two chamber slides were fixed by 4% formaldehyde/phosphate-buffered saline at 4 °C for 25 min and subjected to TUNEL assay according to the manufacturer's protocol. The slides were then counterstained with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (a DNA intercalating dye for visualizing nuclei in fixed cells; catalogue number H-1200, Vector Laboratories). The stained cells were examined under an Olympus IX70 fluorescence microscope.
Langendorff-perfusued Mouse Heart—The methodology of isolated perfused mouse heart was described in detail in our previous publications (20, 21). In brief, the animal was anesthetized with heparinized sodium pentobarbital (100 mg/kg, 33 units of heparin, intraperitoneal), the heart was quickly removed from the thorax and the aortic opening of the heart rapidly cannulated (<3 min) and tied onto a 20-gauge blunt needle connected to Langendorff perfusion system. The heart was retrogradely perfused by modified Krebs-Henseleit solution containing: 118 mm NaCl, 24 mm NaHCO3, 2.5 mm CaCl2, 4.7 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 11 mm glucose, 0.5 mm EDTA, which was continuously gassed with 95% O2 + 5% CO2 (pH ∼ 7.4) and heart temperature was maintained at ∼37 °C. Ventricular function was measured by a force-displacement transducer (model FT03, Grass) attached to the apex with 5-0 surgical thread and metal hook and was continuously recorded with a PowerLab 8SP computerized data acquisition system. Coronary flow rate was measured by timed collection of the outflow of heart perfusate. The hearts were not electrically paced. Further details of the isolated heart experiment protocol are illustrated in Fig. 1E.
Measurement of Infarct Size—At the end of reperfusion, the heart was immediately removed from the Langendorff apparatus, weighed and frozen at -20 °C. The frozen heart was cut into six to seven transverse slices, stained by 10% tetrazolium chloride for 30 min at room temperature (∼22 °C), and subsequently fixed with 10% formalin for 2 to 4 h. The infarct area and risk zone was measured using computer morphometry (Bioquant 98). The risk area was calculated as total ventricular area minus the area of the cavities. The infarct size was presented as percentage of the risk area.
Construction of shRNA Adenoviral Expression Vectors—To knockdown the expression of PKG, we used siRNA, targeting the mouse cDNA of PKG type I (GenBank™ accession number NM_001013833): corresponding to bases 1593 to 1611, targeting the sequence 5′-GAACAAAGGCCATGACATT-3′, synthesized by Dharmacon Research Inc. (Lafayette, CO). A non-targeting scrambled RNA duplex siRNA control (NTSC, Dharmacon) containing 21-nucleotide sequences demonstrating no homology to murine genes was also used as a control for transfection. Transient transfections of duplex siRNAs (100 nm) were performed in H9C2 cells using siPORT™ amine (Ambion). After 48 h, RNA was isolated by Tri-Reagent (Molecular Research Center), and RT-PCR and quantitative real-time PCR were performed. After confirming the significant reduction of PKG expression in H9C2 cells using this duplex siRNA targeting the mouse PKGI, shRNA expression vector for PKG was constructed using the pSilencer™ adeno1.0-CMV system from Ambion (Adenoviral siRNA expression Vector System). The hairpin siRNA oligonucleotide (55-mer) sequence 5′-TCGAGGAACAAAGGCCATGACATTttcaagagaAATGTCATGGCCTTTGTTCAGA-3′ (mouse PKGI with sequence in capital letters and loop in lowercase italics) and its antisense with XhoI and SpeI were synthesized, annealed, and subcloned into the pSilencer adeno 1.0-CMV shuttle vector. HEK293 cells were transfected with linearized shRNA vector together with adenoviral LacZ backbone to generate a recombinant adenovirus.
PKG Activity Assay—PKG activity was assayed by colorimetric analysis with a CycLex cGMP-dependent protein kinase assay kit (MBL International, Woburn, MA) in the whole heart lysate as well as the myocyte lysate according to the manufacturer's protocol. PKG activity was further confirmed by measuring the phosphorylation levels of vasodilator-stimulated phosphoprotein (VASP) in the heart after treatment with sildenafil in the presence or absence of KT5823.
Western Blot Analysis—Total soluble protein was extracted from the myocytes with reporter lysis buffer (Promega) and from whole heart tissue with RIPA buffer. The homogenate was centrifuged at 14,000 × g for 15 min under 4 °C and the super-natant was recovered. 50 μg of protein from each sample was separated by SDS-PAGE and transferred onto nitrocellulose membrane (11, 16). The membrane was incubated with primary antibody at a dilution of 1:1000 for each of the respective proteins, i.e. PKG, actin (goat polyclonal), pAkt, Akt, pERK, ERK, pP38, P38, pJNK, JNK, Bcl-2, Bax (rabbit polyclonal) (Santa Cruz Biotechnology), pGSK (Ser9), GSK, pVASP (Ser239), and VASP (Cell Signaling Technology). The membrane was washed and incubated with horseradish peroxidase-conjugated secondary antibody (1:2000 dilution, 1 h at room temperature). The blots were developed using a chemiluminescent system (ECL Plus; Amersham Biosciences).
RT-PCR and Quantitative Real-time PCR—The total RNA was isolated from cardiomyocytes and the heart using Tri-Reagent (Molecular Research Center) (16). The reverse transcription and PCR amplification were performed using OneStep RT-PCR kit from Qiagen. The sequences of oligonucleotide primers used for RT-PCR were: forward, 5′-TCATCGAGGCTATGCCAAACTG-3′ and reverse, 5′-TCTGTGGGTGACGCAACACTTGG-3′ corresponding to the target sequence of mouse PKGI (GenBank accession number NM_001013833). β-Actin was used as an internal control and the primers used for RT-PCR were: forward, 5′-TGGGCCGCTCTAGGCACCA-3′ and reverse, 5′-TGGCCTTAGGGTTCAGGGG-3′ (GenBank accession number M12491). The transcript levels of PKG were quantified by real time PCR performed in the ABI prism 7900HT sequence detector system (Applied Biosystems, Foster City, CA) using the TaqMan® One Step PCR Master reagent kit (product number 4309169). All of the samples were processed in triplicate according to the manufacturers recommended conditions. The cycling conditions were: 48 °C for 30 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The cycle threshold was determined to provide the optimal standard curve values (0.98-1.0). The primers used for PKG were: forward, 5′-TGGTCACTAGGAATTCTGATGTATGAG-3′ and reverse, 5′-TGATATTGTAGGTTTTCATTGGATCTG-3′ and the TaqMan probe was: 5′-TCTGACTGGCAGCCCACCTTTCTCA-3′. The probes and primers were designed using the Primer Express® 2.0 version and synthesized in the Nucleic Acid Research Facilities of Virginia Commonwealth University. The probes were labeled in the 5′ end with FAM (6-carboxyfluorescein) and in the 3′ end with TAMRA (6-carboxytetramethylrhodamine). Ribosomal RNA (18S rRNA) from the predeveloped TaqMan Assay Reagents (product number 4310893E) was used as an endogenous control.
Data Analysis and Statistics—Data are presented as mean ± S.E. The differences between groups were analyzed with one-way analysis of variance followed by Student-Newman-Keuls post hoc test for pair-wise comparison. p < 0.05 was considered to be statistically significant.
RESULTS
Sildenafil Protects Cardiomyocytes against Necrosis through PKG—Our method for cell preparations yielded at least 85% of the cardiomyocytes with rod-shaped morphology (Fig. 1B). The myocytes were treated with or without 1 μm sildenafil for 1 h (Fig. 1A). Another group of cells were treated with PKG inhibitor, KT 5823, Rp-8-CPT-cGMPs or DT-2 for 1 h in the presence or absence of sildenafil. After 40 min of SI and 1 h of RO, the percentage of trypan blue positive (necrotic) cardiomyocytes increased to 42.4 ± 2.9% as compared with 2.0 ± 0.0% in non-ischemic controls (n = 4, p < 0.001; Fig. 1C). The percent of necrotic cardiomyocytes decreased from 42.4 ± 2.9 in the SI-RO group to 15.1 ± 2.2 in the sildenafil-treated group (n = 4, p < 0.001; Fig. 1C). KT5823, Rp-8-CPT-cGMPs, or DT-2 abolished the protective effect of sildenafil without having any further effect on cell death in the SI-RO cardiomyocytes (Fig. 1C).
After 40 min of SI and 18 h of RO, apoptotic nuclei increased from 0.7 ± 0.1% (in non-ischemic control group) to 22.5 ± 1.9% of total cells (p < 0.001, n = 4; Fig. 1D). Sildenafil reduced TUNEL-positive cells to 4.7 ± 0.4% (p < 0.001, n = 4; Fig. 1D). KT5823, Rp-8-CPT-cGMPs, as well as DT-2 blocked sildenafil-induced protection against apoptosis. These PKG inhibitors alone had no significant effect as compared with untreated SI-RO control group (Fig. 1D).
Role of PKG in Sildenafil-induced Protection in Mouse Heart—Intra-coronary administration of sildenafil (1 μm) prior to global ischemia-reperfusion reduced infarct size from 29.4 ± 2.4 to 16.0 ± 3.0% (p < 0.05; Fig. 1F). The infarct-limiting protection of sildenafil was abolished by KT5823 (infarct size 31.8 ± 4.7%), whereas KT5823 treatment alone had no effect on infarct size (31.9 ± 2.2%; Fig. 2F). In contrast, sildenafil (± KT5823) failed to improve the ventricular contractile function (Fig. 1G). Sildenafil increased post-ischemic coronary flow rate (178 ± 37% of pre-ischemia level), as compared with the control group (105 ± 14%), although the difference was not statistically significant due to the high intra-group variability (p > 0.05; Fig. 1H). This vasodilatory change induced by sildenafil was partially blunted by KT5823.
FIGURE 2.
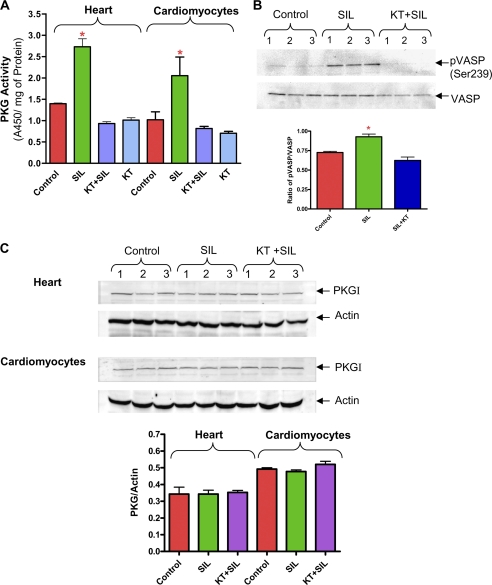
Effect of sildenafil (SIL) on PKG. A, PKG activity in the heart and cardiomyocytes following treatment with sildenafil (1 μm) and KT5823 (* indicates p < 0.05 versus respective control; n = 3). B, representative Western blot showing the phosphorylation of VASP protein and densitometry analysis of the ratio of pVASP/total VASP following treatment with sildenafil and KT5823 in mouse hearts (* indicates p < 0.05 versus control and SIL+KT; n = 3). C, expression of PKG protein in the heart and cardiomyocytes following treatment with sildenafil (n = 3, NS).
Effect of Sildenafil on PKG Activity—Sildenafil increased PKG activity in the heart and cardiomyocytes as compared with control groups (n = 3, p < 0.05, Fig. 2A). KT5823 inhibited sildenafil-induced PKG activity in the heart and in cardiomyocytes. Because phosphorylation of VASP at Ser239 is highly selective for PKG, we examined the level of VASP phosphorylation using a phosphospecific antibody that recognized the PKG-preferred site in VASP at the Ser239 position. Gambaryan et al. (22) previously reported that VASP protein expression in the mouse heart gradually decreased from the neonate to adult age. Our results showed that phosphorylation of VASP significantly increased after sildenafil treatment, which was completely blocked by KT5823 (Fig. 2B). VASP protein was undetectable in the isolated adult mouse cardiomyocytes. The protein level of PKG remained unchanged with sildenafil (Fig. 2C).
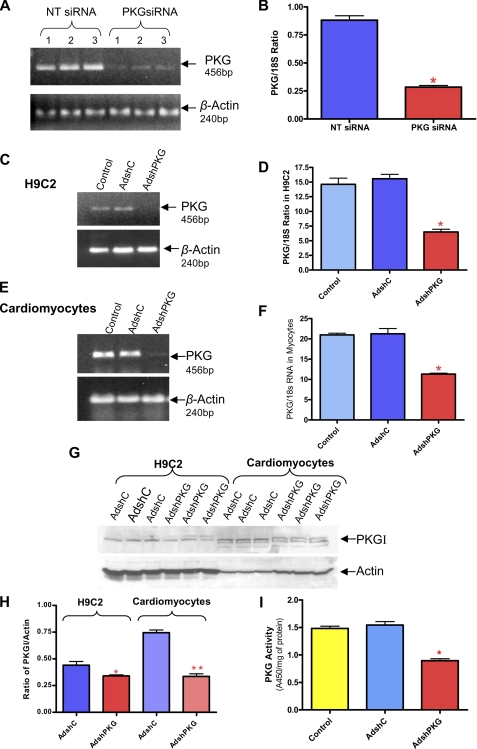
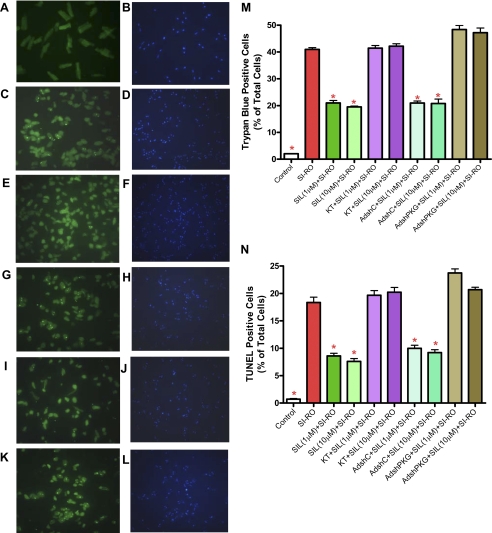
PKG Knockdown by siRNA and AdshRNA—RT-PCR (Fig. 3A) and quantitative real-time PCR (Fig. 3B) showed 70% knock-down of PKG expression after 72 h of transfection with duplex siRNA specific for PKG (siPKG) in H9C2 cells as compared with the scrambled siRNA controls (NT siRNA); (n = 3, p < 0.001). In addition, to create shRNA, we designed two complementary 55-mer oligonucleotides according to the same sequence of the 21-nucleotide siRNA specific to PKG, which we annealed and ligated into the shuttle vector to generate recombinant adenovirus. RT-PCR (Fig. 3, C and E) and real-time PCR (Fig. 3, D and F) confirmed significant knock-down of PKG mRNA in H9C2 cells after 48 h and in adult rat cardiomyocytes after 24 h of infection with AdshPKG as compared with non-infected controls or AdshC (cells infected with control adenovirus containing hairpin “scrambled” siRNA). Likewise, ∼60% knock-down of PKG in H9C2 cells (Fig. 3D) and 50% knock-down in adult cardiomyocytes (Fig. 3F) was achieved after infection with AdshPKG virus. Also, AdshPKG was successful in the knock-down of PKG protein expression in H9C2 cells and cardiomyocytes (Fig. 3, G and H). Moreover, PKG activity was inhibited with AdshPKG as compared with control as well as AdshC-infected rat cardiomyocytes (Fig. 3I). To determine the direct cause and effect relationship of PKG in sildenafil-induced cytoprotection, the AdshPKG-infected cardiomyocytes were treated with sildenafil (1 and 10 μm) before SI/RO. As expected, sildenafil-treated cells had reduced necrosis, i.e. 41.0 ± 0.7% (SI-RO) versus 21.0 ± 0.9% (1 μm sildenafil) and 19.5 ± 0.3% (10 μm sildenafil) of total cells (n = 4, p < 0.001, Fig. 4M). The anti-necrotic effect of sildenafil was inhibited by KT5823. Moreover, the protective effect of sildenafil against necrosis was blunted with AdshPKG as compared with AdshC-infected control cardiomyocytes. Similarly, AdshPKG abrogated the protective effect of sildenafil against apoptosis following SI-RO (n = 4; Fig. 4, K-N). Control cardiomyocytes infected with AdshC did not interfere with the protective effect of sildenafil against apoptosis (n = 4, p < 0.001; Fig. 4, I, J, and N).
FIGURE 3.
Selective knock-down of PKG by siRNA and AdshRNA. A, representative gel showing the transcript levels of PKG and β-actin in H9C2 cells after 48 h of transfection by NT siRNA (scrambled siRNA) or PKG-specific siRNA (PKG siRNA). B, bar diagram showing quantitative changes in PKG mRNA measured by real-time PCR (* indicates p < 0.001 versus NT siRNA; n = 3). C and E, representative gels showing the transcript levels of PKG and β-actin in H9C2 cells and cardiomyocytes after 24 h of infection with adenovirus expressing scrambled shRNA (AdshC) or PKG-specific shRNA (AdshPKG). D and F, bar diagram showing the quantitative change of PKG mRNA assessed by real-time PCR (* indicates p < 0.001 versus other groups; n = 3). G, representative Western blot showing the expression levels of PKG and actin in H9C2 and rat cardiomyocytes after 24 h of infection with AdshC or AdshPKG. H, bar diagram showing the ratio of PKG to actin expression (* indicates p < 0.01 versus respective AdshC; ** indicates p < 0.001 versus respective AdshC; n = 3). I, PKG activity in the isolated adult rat myocytes after 24 h of infection with AdshC or AdshPKG.
FIGURE 4.
Effect of PKG silencing on anti-necrotic and anti-apoptotic effects of SIL in adult rat cardiomyocytes. * indicates p < 0.001 versus SI-RO; n = 4). A, C, E, G, I, and K, TUNEL-positive nuclei are stained in green fluorescent color. B, D, F, H, J, and L, total nuclei stained with 4′,6′-diamidino-2-phenylindole. A and B, non-ischemic control (normal) cardiomyocytes; C and D, cardiomyocytes subjected to 90 min of SI and 18 h of RO; E and F, cardiomyocytes treated with 1 μm sildenafil (SIL) before SI-RO; G and H, cardiomyocytes treated with 1 μm sildenafil and 2 μm KT5823 before SI-RO; I and J, cardiomyocytes infected with AdshC followed by treatment with 1 μm SIL before SI-RO; K and L, cardiomyocytes infected with AdshPKG followed by treatment with 1 μm sildenafil before SI-RO. M and N, bar diagram showing quantitative data from four independent experiments of necrosis and apoptosis (* indicates p < 0.001 versus SI-RO; n = 4).
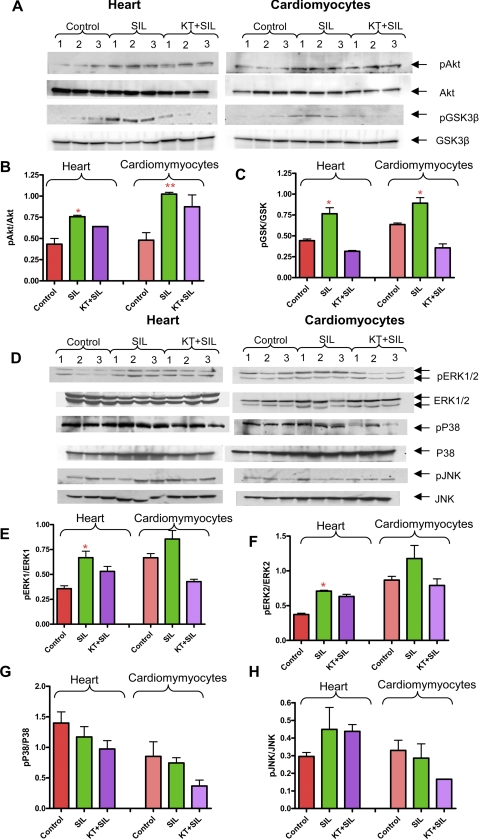
Sildenafil Induces Phosphorylation of Akt and GSK3β: Role of PKG—Phosphorylation of Akt at Ser473 is important for its full activation. Sildenafil increased phosphorylation of Akt in the heart after 10 min of infusion and cardiomyocytes following 1 h of incubation (Fig. 5, A and B). Akt phosphorylation was not completely blunted by KT5823 (Fig. 5, A and B, NS versus sildenafil). One of the downstream targets activated by Akt is GSK3β (18, 23). Our data show that sildenafil enhanced phosphorylation of GSK3β in the heart and cardiomyocytes, which was blocked by KT5823 (Fig. 5, A-C). These results suggest that phosphorylation of GSK3β by sildenafil is mediated by a PKG-dependent pathway in the heart and cardiomyocytes, but phosphorylation of Akt by sildenafil is not completely dependent on PKG.
FIGURE 5.
Phosphorylation of Akt, GSK3β, and MAPKs with sildenafil (SIL). A and D, isolated mouse hearts infused with sildenafil (1 μm) for 10 min in the presence or absence of KT5823 (1 mg/kg) and mouse cardiomyocytes treated with sildenafil (1 μm) for 1 h in the absence or presence of KT5823 (2 μm) for 1 h. B, densitometry scanning analysis of ratio of pAkt/total Akt (* indicates p < 0.05 versus control and KT + sildenafil; ** indicates p < 0.05 versus control; n = 3). C, densitometry scanning analysis of ratio of pGSK3β/total GSK3β (* indicates p < 0.01 versus control and KT + sildenafil; n = 3). E, densitometry scanning analysis showing ratio of pERK1/total ERK1 (* indicates p < 0.05 versus control; n = 3); F, pERK2/total ERK2 (* indicates p < 0.05 versus control; n = 3); G, pP38/total p38 (n = 3; NS); H, pJNK/total JNK (n = 3; NS).
Sildenafil Causes PKG-dependent Phosphorylation of ERK—Sildenafil induced phosphorylation of ERK1/2 in the heart and cardiomyocytes, which was inhibited by KT5823 (Fig. 5, D-F). However, no significant alteration in phosphorylation of p38 and JNK was observed following treatment with sildenafil in the absence or presence of KT5823 (Fig. 5, D, G, and H). The expression level of total p38 and JNK were increased with sildenafil + KT5823 as compared with control. As a result, the ratio of phosphorylated p38/total p38 and pJNK to JNK was decreased although the differences were nonsignificant (Fig. 5, G and H).
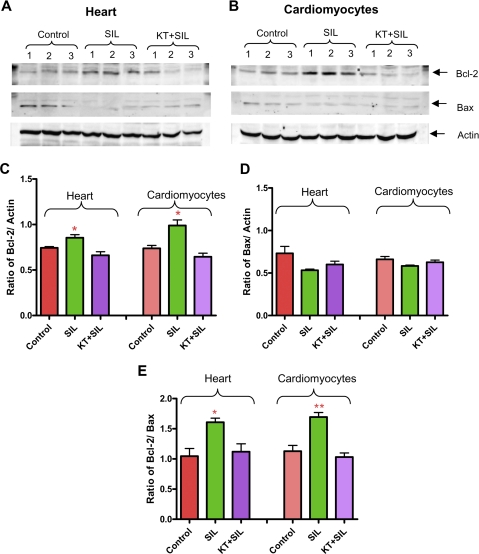
Sildenafil Increases PKG-dependent Expression of Bcl-2—Sildenafil enhanced the expression of Bcl-2 in heart and cardiomyocytes (n = 3, p < 0.05; Fig. 6, A-C) which was inhibited by KT5823. The expression of the proapoptotic protein Bax was not altered by sildenafil (Fig. 6 A, B, and D). Nevertheless, the ratio of Bcl-2 to Bax was increased with sildenafil treatment, and was diminished by co-treatment with KT5823 (Fig. 6E).
FIGURE 6.
Role of PKG in sildenafil (SIL)-induced expression of Bcl-2 and Bax. A, isolated mouse hearts infused with sildenafil (1 μm) for 10 min in the absence or presence of KT5823 (1 mg/kg). B, mouse cardiomyocytes treated with sildenafil (1 μm) in the absence or presence of KT5823 (2 μm) for 1 h. C, densitometry analysis of the Bcl-2/actin ratio (* indicates p < 0.05 versus control and KT + sildenafil; n = 3); D, Bax/actin ratio (n = 3; NS); E, Bcl-2/Bax ratio (* indicates p < 0.05 versus control and KT + sildenafil; ** indicates p < 0.01 versus control and KT + sildenafil; n = 3).
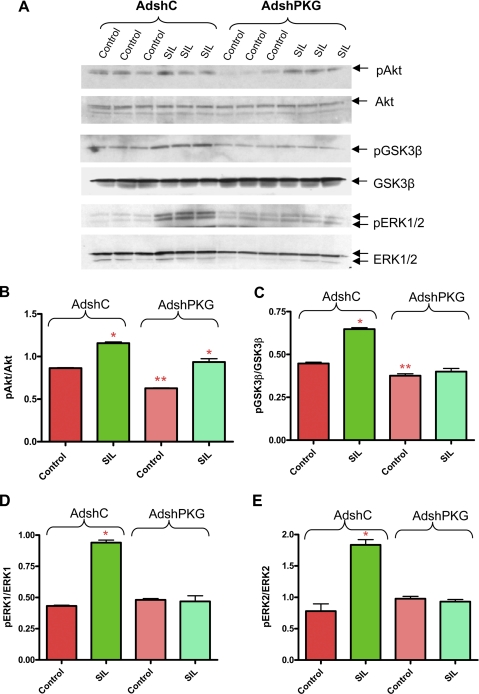
Effect of PKG Knockdown on Sildenafil-induced Phosphorylation of Akt, GSK3β, and ERK—The phosphorylation of Akt was significantly reduced in adult cardiomyocytes after infection with AdshPKG as compared with AdshC (Fig. 7, A and B). Nevertheless, sildenafil treatment was still able to increase Akt phosphorylation in myocytes infected with either AdshC or AdshPKG (Fig. 7, A and B). These results indicate that the phosphorylation of Akt was partially dependent on PKG and the enhancement of phosphorylation of Akt by sildenafil in the AdshPKG-infected cardiomyocytes was not sufficient to elicit cytoprotection (Fig. 4). In contrast, the phosphorylation of GSK3β and ERK1/2 in AdshC-infected myocytes was increased after sildenafil treatment and was completely inhibited after PKG silencing in cardiomyocytes (Fig. 7, A and C-E).
FIGURE 7.
Phosphorylation of Akt, GSK3β, and ERK1/2 after silencing of PKG in sildenafil (SIL)-treated cardiomyocytes. A, Western blotting analysis showing the phosphorylation level of Akt, GSK3β, and ERK1/2. B, densitometry analysis of the ratio pAkt/total Akt (* indicates p < 0.001 versus control; ** indicates p < 0.01 versus control of AdshC; n = 3). C, pGSK3β/total GSK3β (* indicates p < 0.001 versus control of AdshC; ** indicates p < 0.01 versus control of AdshC; n = 3); D, pERK1/ERK1 (* indicates p < 0.001 versus control of AdshC; n = 3); E, pERK2/ERK2 (* indicates p < 0.001 versus control of AdshC; n = 3).
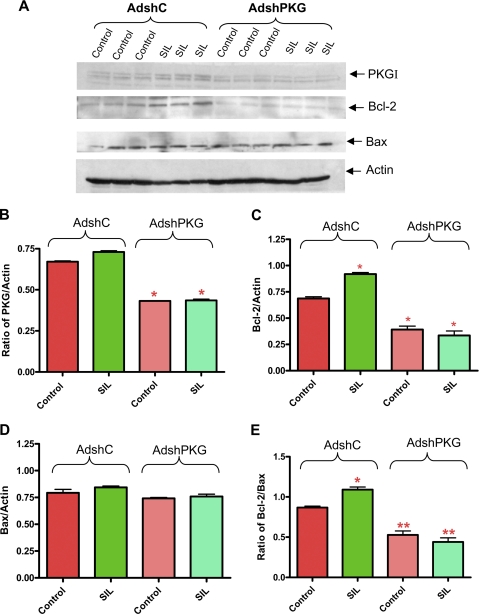
PKG Silencing Blocks Sildenafil-induced Up-regulation of Bcl-2—AdshPKG infection caused 55% knock-down of PKG expression in cardiomyocytes as compared with AdshC (Fig. 8, A and B). Bcl-2 expression was increased by sildenafil in cardiomyocytes infected with AdshC, which was completely inhibited by AdshPKG (Fig. 8, A and C). PKG knock-down had no effect on the expression of Bax and treatment with sildenafil did not alter Bax level as well (Fig. 8, A and D). However, the Bcl-2/Bax ratio was significantly increased after sildenafil treatment in the AdshC-infected cardiomyocytes. PKG silencing with AdshPKG completely blunted the increase of the Bcl-2/Bax ratio induced by sildenafil (Fig. 8E).
FIGURE 8.
Expression of Bcl-2 and Bax after silencing of PKG in cardiomyocytes. A, expression of PKGI, Bcl-2, and Bax after sildenafil (SIL) treatment. B, densitometry analysis of ratio of PKGI to actin (* indicates p < 0.001 versus control of AdshC; n = 3); C, Bcl-2 to actin (* indicates p < 0.001 versus control AdshC; n = 3); D, Bax to actin (n = 3; NS); and E, Bcl-2 to Bax (* indicates p < 0.01 versus control of AdshC; ** indicates p < 0.01 versus control of AdshC; n = 3).
ERK Inhibitor Abrogates Sildenafil-induced Protection in Cardiomyocytes—To elucidate the important role of ERK in sildenafil-induced protection, we treated mouse myocytes for 1 h with ERK inhibitor, PD98059 (20 μm; Calbio-chem), in the presence or absence of sildenafil. After 40 min of SI and 1 h of RO, the antinecrotic effect of sildenafil was inhibited by PD98059 (Fig. 9A). Similarly, PD98059 blocked the sildenafil-induced protection against apoptosis (Fig. 9B).
FIGURE 9.
Effect of ERK inhibitor, PD98059, on anti-necrotic and anti-apoptotic effects of SIL in mouse cardiomyocytes. Bar diagram showing quantitative data of necrosis (A) and apoptosis (B) (* indicates p < 0.05 versus DMSO + SI-RO control; n = 4). PD98059 was dissolved in DMSO and myocytes were treated with DMSO (as vehicle control) or PD98059 (20 μm) for 1 h prior to 40 min of SI and 1 h of RO (for necrosis) and 18 h of RO (for apoptosis). SIL, sildenafil.
DISCUSSION
PDE-5 inhibitors are a class of vasoactive drugs that have been developed for treatment of erectile dysfunction in men (24, 25). Their mechanism of action involves inhibition of the PDE-5 enzyme and the resulting increase in the cGMP level, which leads to smooth muscle relaxation in the penis. The PDE enzymes, of at least 11 subtypes, are ubiquitous throughout the body, and perform a variety of functions. We and other investigators have provided evidence for the protein expression and localization of PDE-5 in the ventricular tissue and isolated cardiomyocytes in mice (16, 26) and dogs (27). Since 2002, a number of studies from our laboratory have demonstrated the cardioprotective effect of PDE-5 inhibitors against ischemia-reperfusion injury in the intact heart and cardiomyocytes (13-16). We first hypothesized that vasodilatory action of PDE-5 inhibitors could potentially release endogenous mediators of cardioprotection including adenosine and bradykinin that may trigger the signaling cascade (through the action of kinases including PKC and MAPKs) and generation of NO by phosphorylation of eNOS. NO activates guanlylate cyclase resulting in enhanced formation of cGMP, which activates PKG. PKG can subsequently open mito-KATP channels (6-8) that exert cardioprotective effects against ischemia-reperfusion injury. The current investigation has further elucidated the direct cause and effect relationship of PKG in protection against ischemia-reperfusion injury with sildenafil and demonstrated the novel downstream signaling pathways associated with cardioprotection. Using RNA interference and pharmacological inhibitors of PKG and ERK, we have shown that sildenafil-induced cardioprotection was associated with PKG-dependent phosphorylation of ERK, GSK3β, and increased expression of Bcl-2 in cardiomyocytes. Although sildenafil caused a PKG-independent increase in Akt phosphorylation, it was not sufficient to protect the ischemic cardiomyocytes. These results provide a new signaling mechanism underlying the cardioprotective effects of sildenafil and possibly other PDE-5 inhibitors that are currently in use for treatment of ED in men. It is noteworthy that our current results are conceptually supported by a recent study, which demonstrated that another PDE-5 inhibitor, vardenafil, administered at reperfusion in an isolated rat heart model induced cardioprotection against ischemia-reperfusion injury through activation of guanylyl cyclase and enhancement of PKG activity (28).
As shown in Fig. 1, C and D, sildenafil significantly attenuated necrosis and apoptosis in cardiomyocytes following simulated ischemia-reoxygenation and also reduced infarct size in isolated heart following global ischemia-reperfusion (Fig. 1F). PKG inhibitors, KT5823, Rp-8-pCPT-cGMPs, or DT-2 blocked this protection in cardiomyocytes (Fig. 1, C and D). KT5823 also attenuated the sildenafil-induced protection in the intact heart (Fig. 1F). Considering the potential nonspecific effects of pharmacological inhibitors of PKG, we designed siRNA to specifically knockdown the expression of PKG. We observed that transfection of H9C2 cells with siRNA targeting PKG resulted in 70% reduction of PKG mRNA expression (Fig. 3, A and B). Additionally, we prepared an adenoviral expression vector containing shRNA of PKG (using hairpin forming oligo sequence identical to the above-mentioned siRNA of PKG, AdshPKG) and successfully reduced the expression of PKG as well as PKG activity in the primary cultures of cardiomyocytes (Fig. 3, E-I). The knock-down of PKG expression completely abrogated the protective effect of sildenafil against necrosis and apoptosis following simulated ischemia and reoxygenation in cardiomyocytes (Fig. 4).
A distinct NO-cGMP-PKG pathway that leads to direct phosphorylation and activation of mito-KATP channels has been proposed in a model of isolated rabbit ventricular myocytes (6, 7). Our previous studies showed acute and delayed cardioprotection with sildenafil via opening of mito-KATP in rabbits (14) and mice (29). The NOS-derived NO could trigger a signal transduction cascade that involves activation of guanylyl cyclase, cGMP production, activation of PKG, and opening of mito-KATP channels (8). This signaling scenario appears to be consistent with the present results showing PKG dependence and induction of iNOS/eNOS that were associated with sildenafil-induced cardioprotection in mouse heart (15) and cardiomyocytes (16). More recent evidence also supports the notion that PKG may be the upstream regulator of the signaling cascade that results in mito-KATP channel opening and cardioprotection in rats (30). Costa et al. (10) proposed that activated PKG phosphorylates some target protein that shuttles the cardioprotective signal to PKCε residing in the intermembrane space of mitochondria. The basis for this compartmentalization model was that exogenous PKG + cGMP activated mito-KATP in isolated mitochondria, whereas cGMP alone could not.
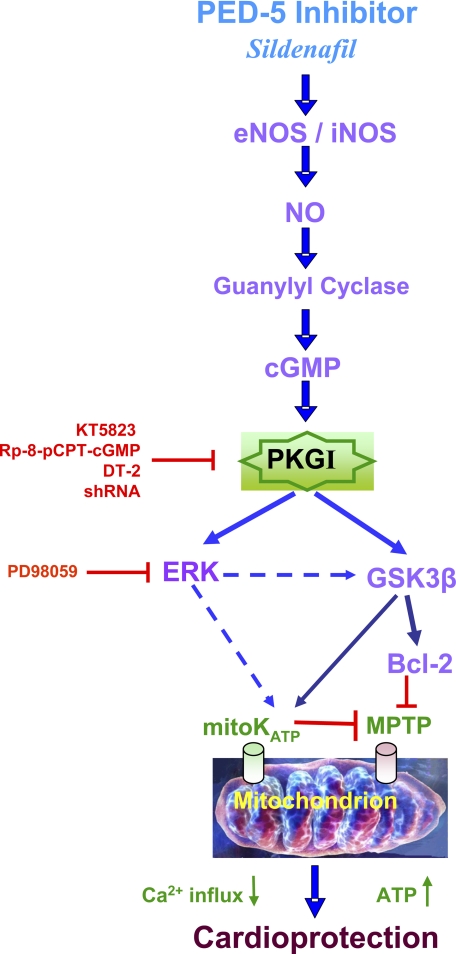
More importantly, we have identified a novel PKG-dependent cytoprotective mechanism of sildenafil involving phosphorylation of ERK, and GSK3β, and induction of Bcl-2 (as summarized in Fig. 10). We hypothesized that the so-called survival kinases including ERK and phosphatidylinositol 3-kinase/Akt could be the potential downstream targets of PKG following its activation by sildenafil and may play an important role in cardioprotection. It has been shown that PKGI can modulate gene expression in various cell types by modulating ERK1/2 (4, 31). PKGIα also induced ERK1/2 via activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) in smooth muscle cells (2). Furthermore, we recently demonstrated that increased expression of PKGIα by adenoviral gene transfer in cardiomyocytes in the absence of sildenafil or other pathophysiological stimuli (such as ischemic preconditioning) resulted in a cytoprotective phenotype that was associated with the phosphorylation of Akt, ERK, and JNK and increased Bcl-2 expression (11). The present results show that sildenafil-induced PKG-dependent phosphorylation/induction of ERK, GSK3β, and Bcl-2 in both isolated cardiomyocytes and heart (Figs. 5 and 6). Moreover, the ERK inhibitor, PD98059, abolished sildenafil-induced protection against SI-RO in cardiomyocytes thereby further supporting the critical role of ERK in the protective signaling pathway (Fig. 9). These data also provide a novel PKG-dependent signaling pathway in addition to the previously reported PKC-ERK cascade (32). Interestingly, we observed that the sildenafil-induced increase in phosphorylation of Akt was only partially (insignificantly) attenuated by either KT5823 (Fig. 5) or shRNA-PKG (Fig. 7). These results suggest that Akt phosphorylation with sildenafil was not primarily dependent on PKG. Moreover, sildenafil-elicited Akt activation alone was not sufficient to protect ischemic heart or cardiomyocytes when PKG was inhibited. These data are different from the published studies where acute activation of Akt has been shown to exert a protective effect against both myocardial infarction and ventricular dysfunction after ischemia-reperfusion in vivo and in vitro (33, 34). Clearly, Akt-independent signaling (including phosphorylation of GSK3β and ERK) appears to be more important in the cardioprotective effect of sildenafil.
FIGURE 10.
Proposed signaling pathways of PKG-dependent cardioprotection by sildenafil against myocardial ischemia-reperfusion injury.
The present study also provided new evidence for PKG-dependent phosphorylation of GSK3β in cardiomyocytes and the intact heart (Figs. 5 and 7). A number of studies have shown that GSK3β is the phosphorylation target of phosphatidylinositol 3-kinase/Akt (35, 36). Activation of GSK3β was shown to induce myocardial apoptosis (37) and GSK3β becomes inactivated when phosphorylated at Ser9 (an N-terminal serine residue) by Akt (38). Persistent inhibition of GSK3β induces compensatory hypertrophy, inhibits apoptosis and fibrosis, and increases cardiac contractility (39). Ischemic preconditioning, an endogenously triggered cardioprotective stimulus has also been shown to enhance phosphorylation of Akt that further leads to phosphorylation and subsequent inactivation of GSK3β (18, 23). Hypoxic preconditioning, which also activates PKC, was shown to phosphorylate and inhibit GSK3β and cause protection in adult cardiomyocytes, through its ability to modulate mitochondrial permeability transition, a key component of mitochondria-mediated cardiac cell death following ischemia-reperfusion (19). Activation of several signal transduction pathways, including insulin activation of the phosphatidylinositol 3-kinase/Akt pathway, cAMP activation of the cAMP-dependent protein kinase (40, 41), or Ras activation of ERK1/2 could also lead to phosphorylation and inhibition of GSK3β (42-46). The role of GSK3β in modulating cell death and survival has been suggested by several studies. For example, GSK3β promotes apoptosis in neuronal cells (41, 47) and vascular smooth muscle cells (38, 41, 47, 48). GSK3β also inhibits cytoprotective proteins such as heat shock factor-1 and hsp70 (41, 47, 49, 50). Recently GSK3β has been shown to promote apoptosis by phosphorylation of MCL-1, an antiapoptotic Bcl-2 family member, leading to its degradation by a ubiquitin proteasome pathway and in turn facilitating cytochrome c release and apoptosis (51). There is also an integral role for GSK3β and β-catenin signaling in regulating VEGF, Bcl-2, and survivin, which may be critical for cardioprotection by ischemic preconditioning (23). We predict that sildenafil (and potentially other PDE-5 inhibitors) may induce survival through PKG-dependent modulation of one or more of these signaling molecules/proteins through GSK3β phosphorylation.
During the past 19 years, sildenafil has evolved from a potential anti-angina drug to an on-demand treatment for erectile dysfunction and more recently as a new treatment for pulmonary hypertension (52). In addition, animal studies suggest that the drug has powerful cardioprotective effects against ischemia-reperfusion injury (13-16), doxorubicin-induced cardiomyopathy (53), and post-infarction heart failure (54). Sildenafil also exerts an anti-hypertensive effect against chronic inhibition of nitric-oxide synthase (55). In the present study, we have identified a novel mechanism by which sildenafil induces a PKG-dependent protective pathway in cardiomyocytes and isolated hearts subjected to ischemia-reperfusion injury. The sildenafil treatment caused PKG-dependent phosphorylation of ERK1/2 and GSK3β in conjunction with an increase in the Bcl-2/Bax ratio in cardiomyocytes and in the intact heart. Thus the PKG-dependent phosphorylation of GSK3β with sildenafil or possibly other PDE-5 inhibitors may have significant therapeutic implications in cytoprotection against ischemia-reperfusion injury as well as myocardial-infarction-induced heart failure.
This work was supported, in whole or in part, by National Institutes of Health Grants HL51045, HL59469, HL79424, and HL093685 (to R. C. K.). This work was also supported by American Heart Association Mid-Atlantic Affiliate Beginning Grant-in-Aid 0765273U (to A. D.) and National Scientist Development Grant 0530157N (to L. X.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PKG, cGMP-dependent protein kinase; AdshC, control recombinant adenovirus containing hairpin scrambled siRNA; AdshPKG, adenovirus expressing a hairpin siRNA that targets mouse PKG; Akt, protein kinase B; GSK3β, glycogen synthase kinase-3β; PDE-5, phosphodiesterase type 5; mito-KATP, mitochondrial ATP-sensitive potassium channel; RO, reoxygenation; Rp-8-pCPT-cGMPs, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio); SI, simulated ischemia; VASP, vasodialator-stimulated phosphoprotein; MAPK, mitogen-activated protein kinase; NO, nitric oxide; NOS, nitric-oxide synthase; PKC, protein kinase C; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase; shRNA, short hairpin; siRNA, small interfering RNA; RT, reverse transcriptase.
References
- 1.Hofmann, F. (2005) J. Biol. Chem. 280 1-4 [DOI] [PubMed] [Google Scholar]
- 2.Komalavilas, P., Shah, P. K., Jo, H., and Lincoln, T. M. (1999) J. Biol. Chem. 274 34301-34309 [DOI] [PubMed] [Google Scholar]
- 3.Lincoln, T. M., and Cornwell, T. L. (1993) FASEB J. 7 328-338 [DOI] [PubMed] [Google Scholar]
- 4.Hofmann, F., Ammendola, A., and Schlossmann, J. (2000) J. Cell Sci. 113 1671-1676 [DOI] [PubMed] [Google Scholar]
- 5.Keilbach, A., Ruth, P., and Hofmann, F. (1992) Eur. J. Biochem. 208 467-473 [DOI] [PubMed] [Google Scholar]
- 6.Han, J., Kim, N., Kim, E., Ho, W. K., and Earm, Y. E. (2001) J. Biol. Chem. 276 22140-22147 [DOI] [PubMed] [Google Scholar]
- 7.Han, J., Kim, N., Joo, H., Kim, E., and Earm, Y. E. (2002) Am. J. Physiol. 283 H1545-H1554 [DOI] [PubMed] [Google Scholar]
- 8.Qin, Q., Yang, X. M., Cui, L., Critz, S. D., Cohen, M. V., Browner, N. C., Lincoln, T. M., and Downey, J. M. (2004) Am. J. Physiol. 287 H712-H718 [DOI] [PubMed] [Google Scholar]
- 9.Krieg, T., Philipp, S., Cui, L., Dostmann, W. R., Downey, J. M., and Cohen, M. V. (2005) Am. J. Physiol. 288 H1976-H1981 [DOI] [PubMed] [Google Scholar]
- 10.Costa, A. D., Garlid, K. D., West, I. C., Lincoln, T. M., Downey, J. M., Cohen, M. V., and Critz, S. D. (2005) Circ. Res. 97 329-336 [DOI] [PubMed] [Google Scholar]
- 11.Das, A., Smolenski, A., Lohmann, S. M., and Kukreja, R. C. (2006) J. Biol. Chem. 281 38644-38652 [DOI] [PubMed] [Google Scholar]
- 12.Bender, A. T., and Beavo, J. A. (2006) Pharmacol. Rev. 58 488-520 [DOI] [PubMed] [Google Scholar]
- 13.Das, A., Ockaili, R., Salloum, F., and Kukreja, R. C. (2004) Am. J. Physiol. 286 H1455-H1460 [DOI] [PubMed] [Google Scholar]
- 14.Ockaili, R., Salloum, F., Hawkins, J., and Kukreja, R. C. (2002) Am. J. Physiol. 283 H1263-H1269 [DOI] [PubMed] [Google Scholar]
- 15.Salloum, F., Yin, C., Xi, L., and Kukreja, R. C. (2003) Circ. Res. 92 595-597 [DOI] [PubMed] [Google Scholar]
- 16.Das, A., Xi, L., and Kukreja, R. C. (2005) J. Biol. Chem. 280 12944-12955 [DOI] [PubMed] [Google Scholar]
- 17.Zhao, X., Zhuang, S., Chen, Y., Boss, G. R., and Pilz, R. B. (2005) J. Biol. Chem. 280 32683-32692 [DOI] [PubMed] [Google Scholar]
- 18.Tong, H., Imahashi, K., Steenbergen, C., and Murphy, E. (2002) Circ. Res. 90 377-379 [DOI] [PubMed] [Google Scholar]
- 19.Juhaszova, M., Zorov, D. B., Kim, S. H., Pepe, S., Fu, Q., Fishbein, K. W., Ziman, B. D., Wang, S., Ytrehus, K., Antos, C. L., Olson, E. N., and Sollott, S. J. (2004) J. Clin. Investig. 113 1535-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xi, L., Hess, M. L., and Kukreja, R. C. (1998) Mol. Cell Biochem. 186 69-77 [PubMed] [Google Scholar]
- 21.Xi, L., Jarrett, N. C., Hess, M. L., and Kukreja, R. C. (1999) Circulation 99 2157-2163 [DOI] [PubMed] [Google Scholar]
- 22.Gambaryan, S., Hauser, W., Kobsar, A., Glazova, M., and Walter, U. (2001) Histochem. Cell Biol. 116 535-543 [DOI] [PubMed] [Google Scholar]
- 23.Kaga, S., Zhan, L., Altaf, E., and Maulik, N. (2006) J. Mol. Cell Cardiol. 40 138-147 [DOI] [PubMed] [Google Scholar]
- 24.Boolell, M., Allen, M. J., Ballard, S. A., Gepi-Attee, S., Muirhead, G. J., Naylor, A. M., Osterloh, I. H., and Gingell, C. (1996) Int. J. Impot. Res. 8 47-52 [PubMed] [Google Scholar]
- 25.Porst, H., Rosen, R., Padma-Nathan, H., Goldstein, I., Giuliano, F., Ulbrich, E., and Bandel, T. (2001) Int. J. Impot. Res. 13 192-199 [DOI] [PubMed] [Google Scholar]
- 26.Takimoto, E., Champion, H. C., Belardi, D., Moslehi, J., Mongillo, M., Mergia, E., Montrose, D. C., Isoda, T., Aufiero, K., Zaccolo, M., Dostmann, W. R., Smith, C. J., and Kass, D. A. (2005) Circ. Res. 96 100-109 [DOI] [PubMed] [Google Scholar]
- 27.Senzaki, H., Smith, C. J., Juang, G. J., Isoda, T., Mayer, S. P., Ohler, A., Paolocci, N., Tomaselli, G. F., Hare, J. M., and Kass, D. A. (2001) FASEB J. 15 1718-1726 [DOI] [PubMed] [Google Scholar]
- 28.Maas, O., Donat, U., Frenzel, M., Rutz, T., Kroemer, H. K., Felix, S. B., and Krieg, T. (2008) Br. J. Pharmacol. 154 25-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, X., Fisher, P. W., Xi, L., and Kukreja, R. C. (2008) J. Mol. Cell Cardiol. 44 105-113 [DOI] [PubMed] [Google Scholar]
- 30.Cuong, D. V., Kim, N., Youm, J. B., Joo, H., Warda, M., Lee, J. W., Park, W. S., Kim, T., Kang, S., Kim, H., and Han, J. (2006) Am. J. Physiol. 290 H1808-H1817 [DOI] [PubMed] [Google Scholar]
- 31.Pilz, R. B., and Casteel, D. E. (2003) Circ. Res. 93 1034-1046 [DOI] [PubMed] [Google Scholar]
- 32.Ping, P., Zhang, J., Cao, X., Li, R. C., Kong, D., Tang, X. L., Qiu, Y., Manchikalapudi, S., Auchampach, J. A., Black, R. G., and Bolli, R. (1999) Am. J. Physiol. 276 H1468-H1481 [DOI] [PubMed] [Google Scholar]
- 33.Fujio, Y., Nguyen, T., Wencker, D., Kitsis, R. N., and Walsh, K. (2000) Circulation 101 660-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsui, T., Tao, J., del Monte, F., Lee, K. H., Li, L., Picard, M., Force, T. L., Franke, T. F., Hajjar, R. J., and Rosenzweig, A. (2001) Circulation 104 330-335 [DOI] [PubMed] [Google Scholar]
- 35.Badorff, C., Ruetten, H., Mueller, S., Stahmer, M., Gehring, D., Jung, F., Ihling, C., Zeiher, A. M., and Dimmeler, S. (2002) J. Clin. Investig. 109 373-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M., and Hemmings, B. A. (1995) Nature 378 785-789 [DOI] [PubMed] [Google Scholar]
- 37.Bergmann, M. W., Rechner, C., Freund, C., Baurand, A., El Jamali, A., and Dietz, R. (2004) J. Mol. Cell Cardiol. 37 681-690 [DOI] [PubMed] [Google Scholar]
- 38.Hardt, S. E., and Sadoshima, J. (2002) Circ. Res. 90 1055-1063 [DOI] [PubMed] [Google Scholar]
- 39.Hirotani, S., Zhai, P., Tomita, H., Galeotti, J., Marquez, J. P., Gao, S., Hong, C., Yatani, A., Avila, J., and Sadoshima, J. (2007) Circ. Res. 101 1164-1174 [DOI] [PubMed] [Google Scholar]
- 40.Fang, X., Yu, S. X., Lu, Y., Bast, R. C., Jr., Woodgett, J. R., and Mills, G. B. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 11960-11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, M., Wang, X., Meintzer, M. K., Laessig, T., Birnbaum, M. J., and Hei-denreich, K. A. (2000) Mol. Cell. Biol. 20 9356-9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali, A., Hoeflich, K. P., and Woodgett, J. R. (2001) Chem. Rev. 101 2527-2540 [DOI] [PubMed] [Google Scholar]
- 43.Cohen, P., and Frame, S. (2001) Nat. Rev. Mol. Cell. Biol. 2 769-776 [DOI] [PubMed] [Google Scholar]
- 44.Eldar-Finkelman, H., Seger, R., Vandenheede, J. R., and Krebs, E. G. (1995) J. Biol. Chem. 270 987-990 [DOI] [PubMed] [Google Scholar]
- 45.Hetman, M., Hsuan, S. L., Habas, A., Higgins, M. J., and Xia, Z. (2002) J. Biol. Chem. 277 49577-49584 [DOI] [PubMed] [Google Scholar]
- 46.Stambolic, V., and Woodgett, J. R. (1994) Biochem. J. 303 701-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pap, M., and Cooper, G. M. (1998) J. Biol. Chem. 273 19929-19932 [DOI] [PubMed] [Google Scholar]
- 48.Hall, J. L., Chatham, J. C., Eldar-Finkelman, H., and Gibbons, G. H. (2001) Diabetes 50 1171-1179 [DOI] [PubMed] [Google Scholar]
- 49.He, B., Meng, Y. H., and Mivechi, N. F. (1998) Mol. Cell. Biol. 18 6624-6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xavier, I. J., Mercier, P. A., McLoughlin, C. M., Ali, A., Woodgett, J. R., and Ovsenek, N. (2000) J. Biol. Chem. 275 29147-29152 [DOI] [PubMed] [Google Scholar]
- 51.Maurer, U., Charvet, C., Wagman, A. S., Dejardin, E., and Green, D. R. (2006) Mol. Cell 21 749-760 [DOI] [PubMed] [Google Scholar]
- 52.Ghofrani, H. A., Osterloh, I. H., and Grimminger, F. (2006) Nat. Rev. Drug Discov. 5 689-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher, P. W., Salloum, F., Das, A., Hyder, H., and Kukreja, R. C. (2005) Circulation 111 1601-1610 [DOI] [PubMed] [Google Scholar]
- 54.Salloum, F. N., Abbate, A., Das, A., Houser, J. E., Mudrick, C. A., Qureshi, I. Z., Hoke, N. N., Roy, S. K., Brown, W. R., Prabhakar, S., and Kukreja, R. C. (2008) Am. J. Physiol. 294 H1398-H1406 [DOI] [PubMed] [Google Scholar]
- 55.Kukreja, R. C. (2007) Br. J. Pharmacol. 150 538-540 [DOI] [PMC free article] [PubMed] [Google Scholar]