Abstract
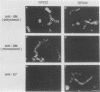
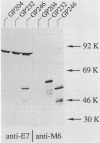
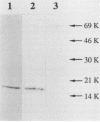
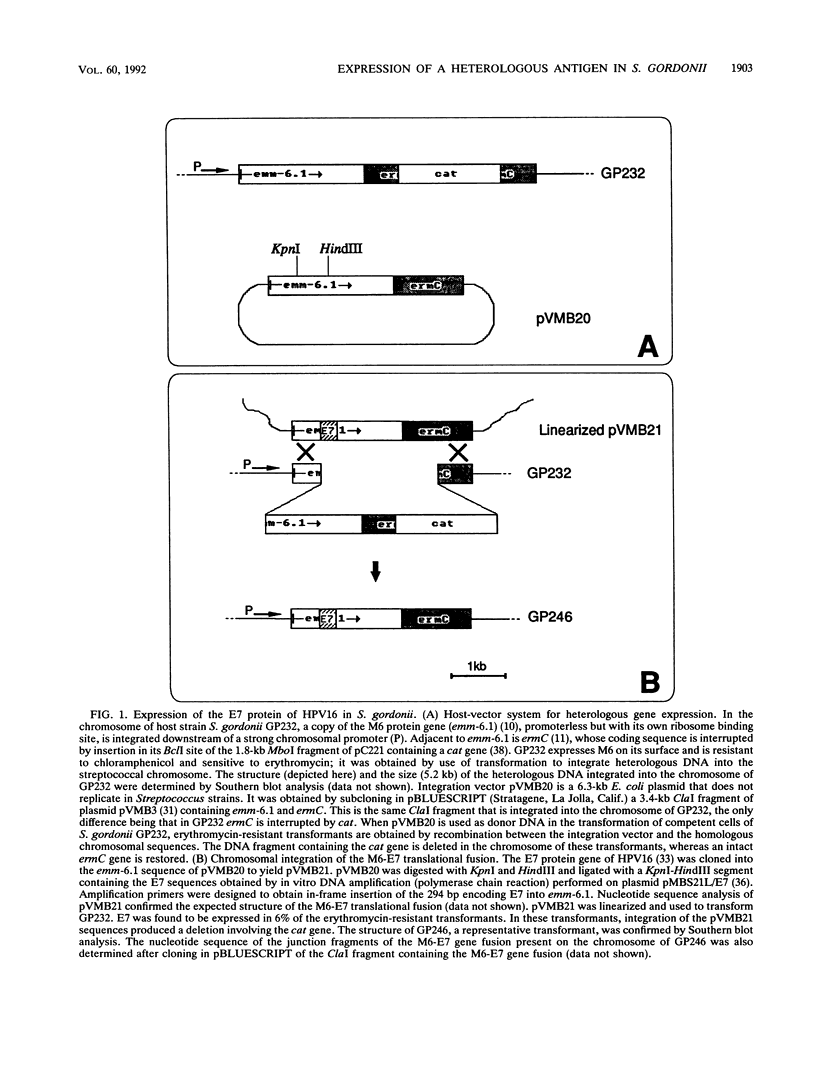
We have developed a system in which a foreign antigen replaces nearly all of the surface-exposed region of the fibrillar M protein from Streptococcus pyogenes and is fused to the C-terminal attachment motif of the M molecule. The fusion protein is thus expressed on the surface of Streptococcus gordonii, a commensal organism of the oral cavity. The antigen chosen to be expressed within the context of the M6 molecule was the E7 protein (98 amino acids) of human papillomavirus type 16. Stable recombinant streptococci were obtained by integrating genetic constructs into the chromosome, exploiting in vivo homologous recombination. The M6-E7 fusion protein expressed on the S. gordonii surface was shown to be immunogenic in mice. This is the first step in the construction of recombinant live vaccines in which nonpathogenic streptococci as well as other gram-positive bacteria may be used as vectors to deliver heterologous antigens to the immune system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Sobczak E., Michel M. L., Molla A., Tiollais P., Hofnung M. Presentation of two epitopes of the preS2 region of hepatitis B virus on live recombinant bacteria. J Immunol. 1987 Sep 1;139(5):1658–1664. [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Pancholi V., Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990 Sep;4(9):1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Parry D. A., Trus B. L., Hollingshead S. K., Scott J. R., Manjula B. N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3(1):60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein. Sci Am. 1991 Jun;264(6):58–65. doi: 10.1038/scientificamerican0691-58. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Schneewind O., Wilson E. M., Fischetti V. A. Assembly and analysis of a functional vaccinia virus "amplicon" containing the C-repeat region from the M protein of Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3190–3194. doi: 10.1073/pnas.88.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochmus-Kudielka I., Schneider A., Braun R., Kimmig R., Koldovsky U., Schneweis K. E., Seedorf K., Gissmann L. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989 Nov 15;81(22):1698–1704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- Jones K. F., Khan S. A., Erickson B. W., Hollingshead S. K., Scott J. R., Fischetti V. A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986 Oct 1;164(4):1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Yoshiike K. Immortalization of primary rat cells by human papillomavirus type 16 subgenomic DNA fragments controlled by the SV40 promoter. Virology. 1988 Jul;165(1):321–325. doi: 10.1016/0042-6822(88)90694-0. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980 Jun;28(3):692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzi G., Cerni C., Kieny M. P., Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991 Mar;181(1):62–69. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Oggioni M. R., Pozzi G. Conjugative mobilization of the cloned M6 protein gene from Streptococcus pneumoniae to Streptococcus pyogenes. Microbiologica. 1990 Oct;13(4):273–281. [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkus M. E., Piccini A., Lipinskas B. R., Paoletti E. Recombinant vaccinia virus: immunization against multiple pathogens. Science. 1985 Sep 6;229(4717):981–984. doi: 10.1126/science.2992092. [DOI] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Guild W. R. Modes of integration of heterologous plasmid DNA into the chromosome of Streptococcus pneumoniae. J Bacteriol. 1985 Mar;161(3):909–912. doi: 10.1128/jb.161.3.909-912.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Musmanno R. A., Lievens P. M., Oggioni M. R., Plevani P., Manganelli R. Method and parameters for genetic transformation of Streptococcus sanguis Challis. Res Microbiol. 1990 Jul-Aug;141(6):659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- Pozzi G., Musmanno R. A., Renzoni E. A., Oggioni M. R., Cusi M. G. Host-vector system for integration of recombinant DNA into chromosomes of transformable and nontransformable streptococci. J Bacteriol. 1988 Apr;170(4):1969–1972. doi: 10.1128/jb.170.4.1969-1972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Strugnell R. A., Maskell D., Fairweather N., Pickard D., Cockayne A., Penn C., Dougan G. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene. 1990 Mar 30;88(1):57–63. doi: 10.1016/0378-1119(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Tommasino M., Contorni M., Scarlato V., Bugnoli M., Maundrell K., Cavalieri F. Synthesis, phosphorylation, and nuclear localization of human papillomavirus E7 protein in Schizosaccharomyces pombe. Gene. 1990 Sep 14;93(2):265–270. doi: 10.1016/0378-1119(90)90234-i. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Yoshiike K. Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol. 1989 Feb;63(2):965–969. doi: 10.1128/jvi.63.2.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]