Abstract
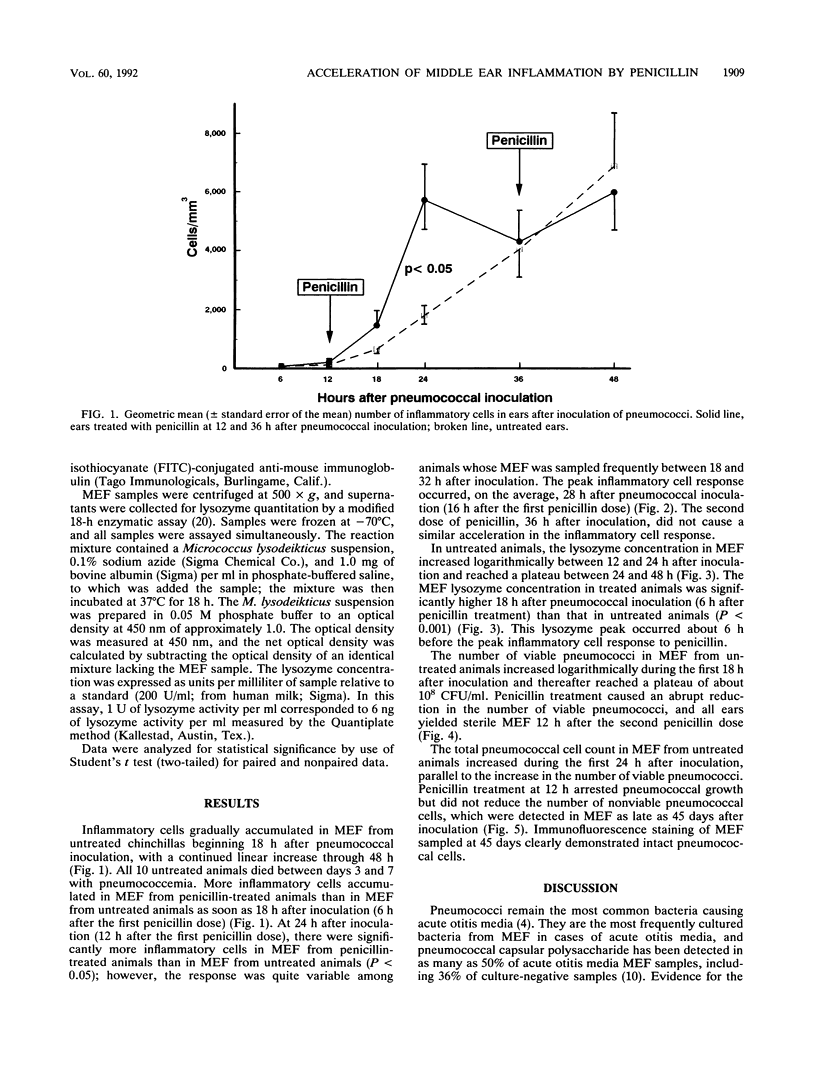
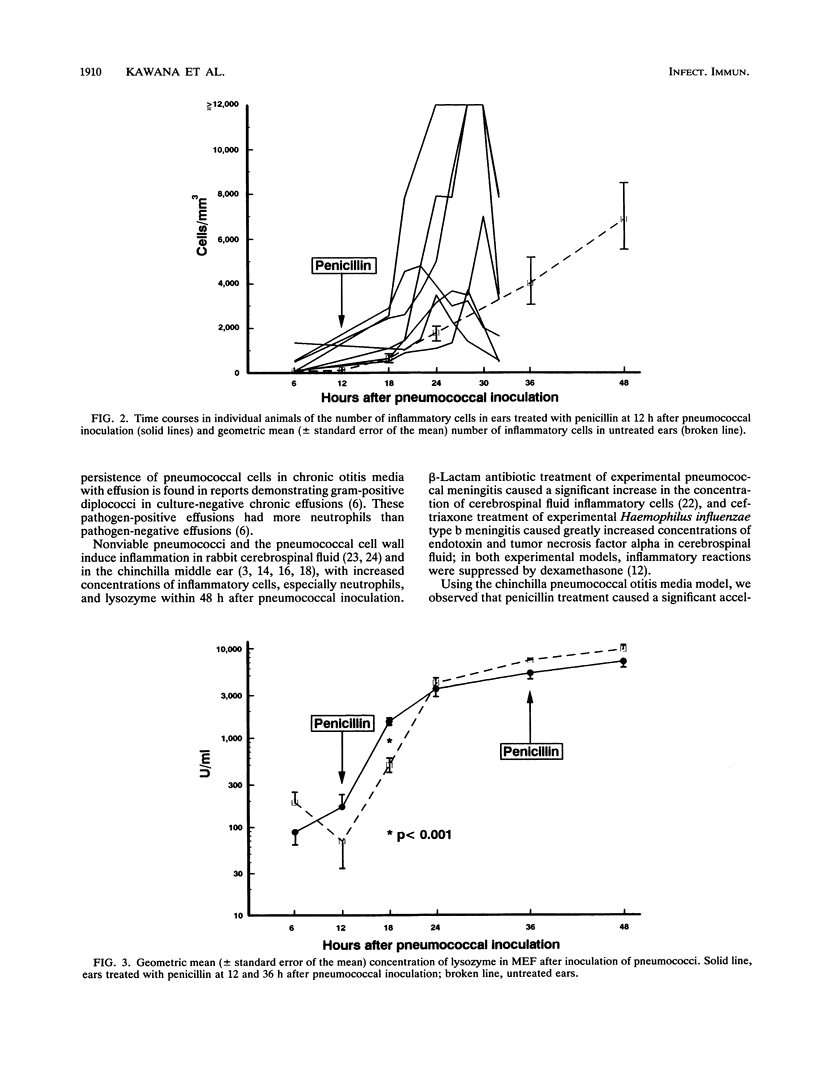
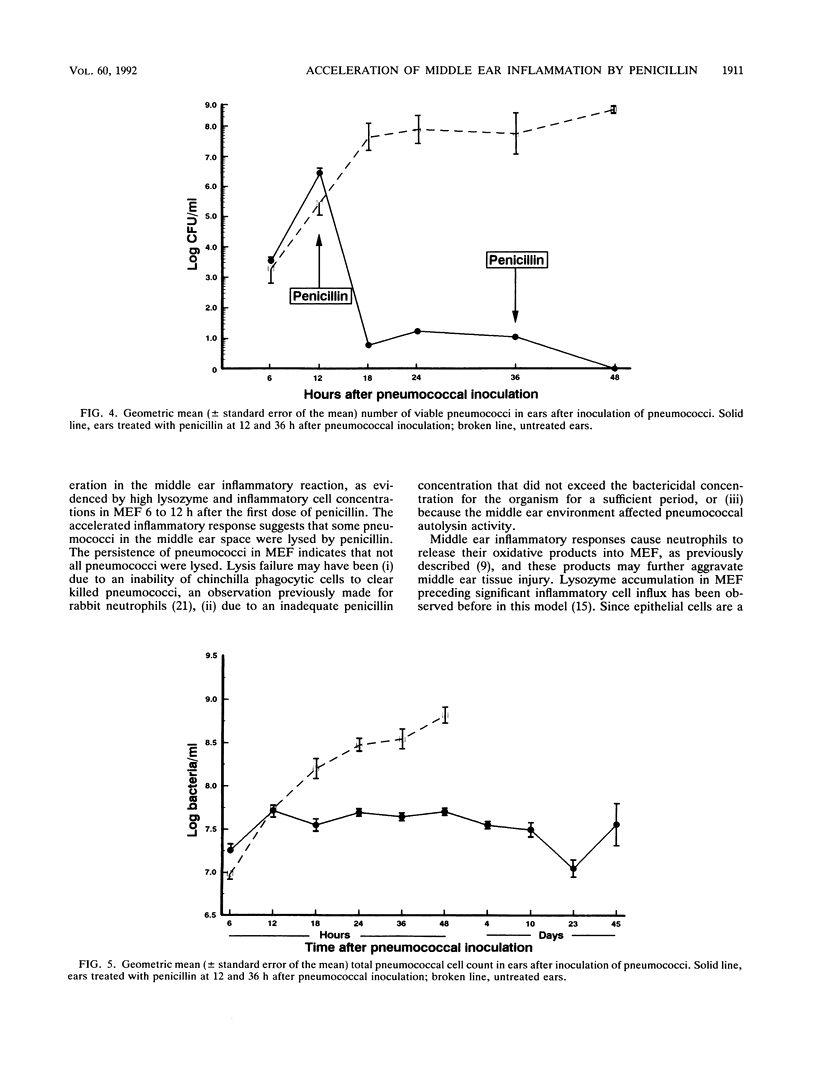
Most Streptococcus pneumoniae strains are killed by very low concentrations of penicillin and other beta-lactam antibiotics, yet middle ear inflammation and effusion persist for days to weeks after treatment in most cases of pneumococcal otitis media. To study the effect of beta-lactam antibiotic treatment on pneumococci and the middle ear inflammatory response during pneumococcal otitis media, we measured concentrations of pneumococci, inflammatory cells, and lysozyme in middle ear fluid (MEF) by using the chinchilla model. Procaine penicillin G given intramuscularly 12 and 36 h after inoculation of pneumococci into the middle ear caused a significant acceleration in the MEF inflammatory cell concentration compared with that in untreated controls, with a significant peak in the inflammatory cell concentration 24 h after pneumococcal inoculation. The lysozyme concentration in MEF also increased more rapidly in treated than in control animals. Viable pneumococci were not detected in MEF after the second dose of penicillin, but the total pneumococcal cell concentration remained unchanged for at least 45 days. Therefore, penicillin treatment accelerated middle ear inflammation while killing pneumococci, but treatment did not accelerate clearance of the nonviable pneumococcal cells from MEF. Further studies will need to define the contribution of these responses to acute and chronic tissue injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman S., Grose K., Zerbe G. O. Medical management of chronic middle-ear effusion. Results of a clinical trial of prednisone combined with sulfamethoxazole and trimethoprim. Am J Dis Child. 1987 Jun;141(6):690–694. doi: 10.1001/archpedi.1987.04460060106048. [DOI] [PubMed] [Google Scholar]
- Canafax D. M., Nonomura N., Erdmann G. R., Le C. T., Juhn S. K., Giebink G. S. Experimental animal models for studying antimicrobial pharmacokinetics in otitis media. Pharm Res. 1989 Apr;6(4):279–285. doi: 10.1023/a:1015938205892. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Batalden P. B., Le C. T., Lassman F. M., Buran D. J., Seltz A. E. A controlled trial comparing three treatments for chronic otitis media with effusion. Pediatr Infect Dis J. 1990 Jan;9(1):33–40. doi: 10.1097/00006454-199001000-00008. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Juhn S. K., Weber M. L., Le C. T. The bacteriology and cytology of chronic otitis media with effusion. Pediatr Infect Dis. 1982 Mar-Apr;1(2):98–103. doi: 10.1097/00006454-198203000-00007. [DOI] [PubMed] [Google Scholar]
- Giebink G. S. The microbiology of otitis media. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S18–S20. [PubMed] [Google Scholar]
- Hanamure Y., Lim D. J. Normal distribution of lysozyme- and lactoferrin-secreting cells in the chinchilla tubotympanum. Am J Otolaryngol. 1986 Nov-Dec;7(6):410–425. doi: 10.1016/s0196-0709(86)80017-5. [DOI] [PubMed] [Google Scholar]
- Kawana M., Kawana C., Yokoo T., Quie P. G., Giebink G. S. Oxidative metabolic products released from polymorphonuclear leukocytes in middle ear fluid during experimental pneumococcal otitis media. Infect Immun. 1991 Nov;59(11):4084–4088. doi: 10.1128/iai.59.11.4084-4088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luotonen J., Herva E., Karma P., Timonen M., Leinonen M., Mäkelä P. H. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13(3):177–183. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Waltman W. D., 2nd, Gray B., Briles D. E. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987 Oct;3(4):249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Juhn S. K., Harada T., Aeppli D. Pathophysiology of Streptococcus pneumoniae otitis media: kinetics of the middle ear biochemical and cytologic host responses. Ann Otol Rhinol Laryngol. 1991 Mar;100(3):236–243. doi: 10.1177/000348949110000313. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Zelterman D., Harada T., Juhn S. K. Early biochemical events in pneumococcal otitis media: arachidonic acid metabolites in middle ear fluid. Ann Otol Rhinol Laryngol. 1991 May;100(5 Pt 1):385–388. doi: 10.1177/000348949110000507. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Zelterman D., Harada T., Juhn S. K. Middle ear fluid lysozyme source in experimental pneumococcal otitis media. Ann Otol Rhinol Laryngol. 1991 Jul;100(7):593–596. doi: 10.1177/000348949110000715. [DOI] [PubMed] [Google Scholar]
- Persico M., Podoshin L., Fradis M. Otitis media with effusion: a steroid and antibiotic therapeutic trial before surgery. Ann Otol Rhinol Laryngol. 1978 Mar-Apr;87(2 Pt 1):191–196. doi: 10.1177/000348947808700208. [DOI] [PubMed] [Google Scholar]
- Ripley-Petzoldt M. L., Giebink G. S., Juhn S. K., Aeppli D., Tomasz A., Tuomanen E. The contribution of pneumococcal cell wall to the pathogenesis of experimental otitis media. J Infect Dis. 1988 Feb;157(2):245–255. doi: 10.1093/infdis/157.2.245. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Puglese J., Schwartz D. M. Use of a short course of prednisone for treating middle ear effusion. A double-blind crossover study. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):296–300. doi: 10.1177/00034894800890s369. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Martinez R. J. A simple and ultrasensitive enzymatic assay for the quantitative determination of lysozyme in the picogram range. Anal Biochem. 1980 Nov 15;109(1):67–70. doi: 10.1016/0003-2697(80)90011-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Beckerdite S., McDonnell M., Elsbach P. The activity of the pneumococcal autolytic system and the fate of the bacterium during ingestion by rabbit polymorphonuclear leukocytes. J Cell Physiol. 1977 Aug;92(2):155–160. doi: 10.1002/jcp.1040920203. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Rich R., Bray M. A., Zak O., Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987 May;155(5):985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Tomasz A., Hengstler B., Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985 Mar;151(3):535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]


