Such isolates are widespread around clinical centers and are associated with malignancies that cause fewer deaths than other C. tropicalis isolates.
Keywords: Candida tropicalis, candidemia, flucytosine, antifungal drug resistance, polymorphic microsatellite markers, multilocus sequence typing, molecular typing, URA3, epidemiology, research
Abstract
Candida tropicalis is a diploid ascomycetes yeast responsible for 4%–24% of candidemia. Resistance to flucytosine is rarely described for this species but was observed for 45 (35%) of 130 C. tropicalis isolates recovered from blood cultures in the Paris area in a 4-year survey. The aims of this study were to test the hypothesis that the flucytosine-resistant isolates could represent a subgroup and to determine the relationship between epidemiologic and genomic data. Epidemiologic data and gene sequences were analyzed, and molecular typing was performed. Our results suggest that a clone of flucytosine-resistant isolates, associated with malignancies and a lower mortality than that for other C. tropicalis isolates, is widespread in the Paris area. We propose the analysis of 2 polymorphic microsatellite markers coupled with URA3 sequencing to track the clone.
Candida tropicalis is a diploid ascomycetes yeast commonly found on the skin and in digestive tracts of healthy human hosts worldwide (1). Infections caused by C. tropicalis are reported in 4%–24% of patients with candidemia, depending on the country of study, underlying risk factors, and period of study (2). Primary resistance to flucytosine (5FC) occurs in <5% of all Candida species except for C. krusei, in which it is detected in up to 28% of isolates (3). It was thus unexpected to observe 35% resistance to 5FC among C. tropicalis isolates recovered from blood cultures in the active surveillance program on yeast-related fungemia implemented by the French National Reference Center for Mycoses and Antifungals (NRCMA) in the Paris area. The YEASTS program is designed to analyze the epidemiologic trends of yeast fungemia by collecting isolates and epidemiologic and clinical data. The second objective is to study the clinical isolates in terms of species, antifungal susceptibility profiles, and genetic diversity to look for associations between subtypes of isolates and epidemiologic/clinical parameters. To test the hypothesis that the 5FC resistant (R5FC) isolates could represent a different species or a subgroup, the R5FC and susceptible (S5FC) isolates were compared on the basis of several phenotypic and molecular features.
Materials and Methods
Strains
Clinical isolates of C. tropicalis recovered from blood cultures during the YEASTS program from October 1, 2002, through September 30, 2006, were selected for the study. Epidemiologic and clinical data concerning the patients were collected by using a standardized electronic form. Isolates (1 isolate/patient) were sent to NRCMA for identification and MIC determination (see below). All isolates were stored frozen in 40% glycerol at –80°C.
The type strain of C. tropicalis CBS 94 (ATCC 750, S5FC) was included in the study as a reference. In addition, 29 strains of taxonomic synonyms available at the Centraalbureau voor Schimmelcultures (CBS, Utrecht, the Netherlands) were studied.
Phenotypic Characterization of All C. tropicalis Isolates
All isolates were identified at the species level by using the assimilation patterns obtained with the commercialized strips ID32C (bioMérieux, Marcy-l’Etoile, France). MICs to 9 systemic antifungal agents were determined for all clinical isolates and the type strain by using the EUCAST microdilution method (4). For nonclinical isolates, only MICs of 5FC and fluconazole were determined.
Additional Studies on Selected Isolates
Growth Characteristics
For the first 16 S5FC and 14 R5FC consecutive isolates of C. tropicalis and for CBS 94 other studies were performed. Additional carbon sources were tested by using the commercial strips CH50 (bioMérieux). Maximal temperature of growth (42°C or 45°C) was determined on Sabouraud dextrose agar. Growth in hyperosmolar medium (50% glucose or 10% NaCl) was also evaluated.
Nucleotide Sequence Determination
After 24 hours of incubation at 27°C on Sabouraud agar plates, single colonies were discharged in 1 mL of distilled water in a microcentrifuge tube, and DNA extraction was performed by using the High Pure PCR Template Preparation Kit (Roche Applied Science, Mannheim, Germany) according to manufacturer’s instructions. Universal fungal primers were used for the amplification of the internal transcribed spacer 1 (ITS1)–5.8S-ITS2 (primers V9D and LS266 [5,6]) and the 26S (primers NL1 and NL4 [7]) rDNA regions. Primers (Table 1) were designed to amplify a partial sequence of the actin gene (GenBank accession nos. AJ389059 and AJ508499) and the 14-α-demethylase gene (GenBank accession nos. AY942646 and M23673). Reaction volumes of 20 μL contained 1 μL of genomic DNA, 1.25 U of AmpliTaq Gold, 2 μL of PCR buffer 10×, 2 μL of 25 mmol/L MgCl2 2 μL of 2.5 mmol/L deoxyribonucleoside triphosphates (dNTPs) (Roche), and 1 μL (10 μM) of primers. The PCR products were amplified by using the ICycler Thermocycler (Bio-Rad, Marnes-La-Coquette, France) set up with a first cycle of denaturation for 10 min at 95°C, followed by 30 cycles of denaturation at 94°C for 30 s, 30 s at the relevant annealing temperature, elongation at 72°C for 30 s, and a final extension step of 10 min at 72°C. Both strands of purified amplified fragments were sequenced at the Genopole of the Pasteur Institute, on an ABI Prism 3700 DNA Analyzer (Applied Biosystems, Courtaboeuf, France), with the same primers that were used in the PCR step. Sequences were edited with Chromas Pro version 1.33 (Technelysium Pty Ltd, Helensvale, Queensland, Australia). Multiple sequences alignments were performed with Clustal W version 1.8 (www.ebi.ac.uk/clustalw/).
Table 1. Primers sequences and amplification parameters used in the present study.
| Locus | Primer | 5′ labeling | Sequence (5′ → 3′)* | Annealing temperature, °C | |
|---|---|---|---|---|---|
| 14αDM† | DMC1 | >TGGGTGGTCAACATACTTC | 50 | ||
| DMC2‡ | <CATCTRTGTCTACCACCACC | ||||
| Actin | ACTa | >AAGGTATTATGGTTGGTATGG | 55 | ||
| ACTb | <TCGAAATCTAAAGCAACGTAA | ||||
| URA3 | URAF | HEX§ | >ATTGGATAGTCCCTCTAAACTCACTACTA | 55 | |
| URAr | <AGCATTAGTTATATCACTCCACGATGAA | ||||
| URAF2 | >TGCCGATATTGGAAATACAGTTA | 50 | |||
| URAr2 | <AATCAACTATTCAAGTTGACCG | ||||
| CTU2 | <GTTGGAACATCAATTGATGCACATAAAT | 55 | |||
| Unknown | CT14a | 6FAM¶ | >GTAAATCTTGTATACCGTGGA | 55 | |
| CT14b | <TAGCCCATTTTCTAGTTTTGC | ||||
| FCY1 | CTCDf | >ATCATTAGTTCAGATGGTAAAGTCTTG | 58 | ||
| CTCDr | <CCTTTTTAGTAACATGTCTATTCTCCA | ||||
| FCY2 | CTCP1f | >TGCCCATAAATTAAATGCAGAA | 58 | ||
| CTCP1r | <GGAAGCAACAAACCCAAAAA | ||||
| FCY2 | CTCP2f | >TGCTGCCGATTATGTTGTTT | 58 | ||
| CTCP2r | <GTGAAAACGAGCCAATCCAT | ||||
| FUR1 | FUR1f | >TCATCAAAACCATGTCTGCTG | 58 | ||
| FUR1r | <AAGTGTATGTAGTGATAATTGCTATGC | ||||
*>, Sense primer; <, antisense primer. †14 α demethylase. ‡R = G or A. §4, 7, 2′,4′, 5′,7′-hexachloro-6-carboxyfluorescein. ¶6-carboxyfluorescein.
Following preliminary results, primers were then designed to amplify the complete sequence of the orotidine-5′-phosphate decarboxylase gene (URA3, GenBank accession no. AF040702) (Table 1). Furthermore, the complete sequences of FCY1 (coding for the cytosine deaminase), FCY2 (coding for the purine cytosine permease), and FUR1 (coding for the uracil phosphoribosyl transferase) were determined. Primers were designed by using sequences from the Broad Institute C. tropicalis database genome (locus CTRG_02927.3 for FCY1, locus CTRG_02059.3 for FCY2, and locus CTRG_02689.3 for FUR1) (Table 1). The sequences were amplified as described above (except for the duration of annealing and elongation [1 min] when using the primers set CTCP1f/CTCP1r). The sequences were translated with the standard genetic code (www.bioinformatics.org/sms/index.html). The resulting protein sequences were aligned with the BioloMICS software (BioloMICS, version 7.2.5, BioAware S.A., Hannut, Belgium).
Microsatellite Selection
C. tropicalis genome sequences available from GenBank databases and from the Broad Institute (www.broad.mit.edu/annotation/fungi/candida_tropicalis) were studied to identify sequences containing microsatellite repeats. Two polymorphic microsatellite markers (PMMs) were selected, 1 upstream of the URA3 gene (URA3 PMM) and 1 on a nonannotated sequence (CT14 PMM). Oligonucleotide primers were designed from the sequence of the corresponding flanking regions to obtain PCR products ranging in size from 100 bp to 200 bp. One primer of each set was 5′ labeled with different dyes (Table 1). PCR was conducted independently for the 2 loci in a 20-μL reaction volume containing 2 μL of extracted DNA, 1.25 U of AmpliTaq Gold, 2 μL of PCR Buffer 10×, 4 μL of 25 mmol/L MgCl2 , 2 μL of 2 mmol/L dNTPs, and 0.2 μL (10 μM) of primers. PCR amplifications were performed for a total of 27 cycles by using the following conditions: denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, and a final extension step of 5 min at 72°C. Two microliters of each PCR product mixed with 20 μL of formamide and 0.5 μL of an internal standard labeled with 6-carboxy-X-rhodamine dye (GeneScan-500 Tamra, Applied Biosystems) was run on an ABI Prism 310 Genetic Analyzer (Applied Biosystems). Sizes of the allele and PCR fragments were determined with GenScan 3.0 (Applied Biosystems, Weiterstadt, Germany). To assign a specific length to a PCR fragment, all electromorphs were aligned with that of the type strain (CBS 94). Each allele was named after the length of PCR fragments. Isolates for which 1 signal was observed for a given locus on the electromorph were considered homozygous for this locus by analogy with what is reported for another diploid yeast, C. albicans (8).
Multilocus Sequence Typing Analysis
Three of the 6 MLST loci recently described were analyzed as reported in Tavanti et al. (9). These loci were selected because, according to these authors, they were associated with more polymorphism (XYR1 and SAPT4) and with antifungal resistance (MDR1). Both strands of purified amplified fragments were sequenced, and sequences were edited as described above. Heterozygosity was defined by the presence of 2 coincident peaks of similar height in the forward and the reverse sequence chromatograms. The 1-letter code for nucleotides from the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC, www.bioinformatics.org/sms/iupac.html) was used. Sequences were compared with the allele sequences of the C. tropicalis MLST database (www.pubmlst.org/ctropicalis). For each gene, distinct alleles were identified and numbered by using the Internet-based MLST program (www.mlst.net). New alleles were submitted to the MLST C. tropicalis database.
Statistical Analysis
In accordance with French regulations, the clinical database was approved by the Commission Nationale de l’Informatique et des Libertés. Information concerning demographic data, risk factors for candidiasis, and outcome 30 days after the diagnosis of fungemia were recorded. We considered 3 groups of patients according to the infecting isolate: S5FC, R5FC that belong to the clone (R5FC clone, see below), and R5FC that do not belong to the clone (termed “other R5FC”). The sociodemographic and clinical characteristics were compared between the 3 groups of isolates by using the Fisher exact test. The χ2 Armitage trend test (10) was used to assess a trend in the evolution of the R5FC clone’s proportion among resistant strains across years of study. Multinomial logistic regression (11) adjusted on clinical center was used to investigate the factors associated with infection by the R5FC clone or other R5FC isolates compared to S5FC isolates according to sociodemographic and clinical characteristics. A logistic regression model adjusted on clinical center was also performed to identify the factors associated with the acquisition of the R5FC clone compared with other R5FC isolates. Regression models were constructed by using the backward procedure. First, all covariates with a p value <0.25 in univariate models were simultaneously entered into the regression model. The set of covariates with the largest p value was iteratively removed from the model until all of the covariates (or blocks of covariates) remaining in the reduced model had a p value <0.05. Statistical analyses were performed with Stata software, version 9.0 (StataCorp, College Station, TX, USA).
Results
Phenotypic Characterization of R5FC Isolates
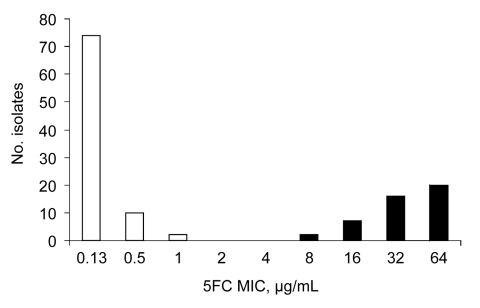
We analyzed the episodes of fungemia caused by C. tropicalis and recorded during the first 4 years of the YEASTS study; 130 episodes were recorded in 24 of the 27 participating centers. Distribution of flucytosine MICs showed 2 populations, 1 with MICs <2 μg/mL and 1 with MICs >8 μg/mL (Figure). In light of these results, susceptibility to 5FC (S5FC) was defined by an MIC <8 μg/mL and resistance (R5FC) by an MIC >8 μg/mL.
Figure.
Distribution of 130 Candida tropicalis isolates recovered from blood cultures during the first 4 years of an active surveillance program (YEASTS study) on yeasts fungemia in the Paris area, France (October 2002 through September 2006), according to the MICs of flucytosine determined with the EUCAST microdilution method (4).
The proportion of R5FC isolates (45 [35%]) of the 130 isolates) was uneven, ranging from 0% to 67% of the isolates, depending on the center of isolation. However, the proportion of R5FC isolates did not differ over the study period (data not shown). We first studied the characteristics of a subset of 16 S5FC and 14 R5FC C. tropicalis isolates (strain CBS 94 had all the characteristics of S5FC clinical isolates described below but is not included in the analysis). There was no difference in terms of growth in hyperosmolar media between R5FC and S5FC clinical isolates. By contrast, R5FC and S5FC isolates differed in the proportion of isolates growing at 45°C (40% vs. 100%, p<0.001) and assimilating starch (12.5% vs. 50%, p = 0.054) and xylitol (62.5% vs. 12.5%, p = 0.009). No difference in the MIC of azoles or caspofungin was noted. All 29 strains of C. tropicalis synonyms stored at the CBS exhibited 5FC MICs <0.5 μg/mL.
Genotypic Characterization of R5FC Isolates
This subset of isolates (16 S5FC and 14 R5FC) was further analyzed. The deletion of 1 nucleotide (A) in position 106 (according to the type strain sequence, GenBank accession no. AY939810) of the ITS2 region was observed in 14 (100%) of the 14 R5FC isolates compared to 5 (32%) of the 16 S5FC isolates (p<0.001) (GenBank accession no. EU288196). No difference in nucleotide sequences was found for the D1/D2 region of the 26S rDNA or in the portion of the 14-α demethylase (490 bp) and actin (550 bp) genes analyzed. PMM results are summarized in Table 2. The URA3 and CT14 PMM led to 6 and 7 different allelic associations, respectively. The association of both markers led to 13 PMM profiles. The 14 R5FC isolates had the same URA3/CT14 PMM profile; however, among the 16 S5FC, 15 had a PMM profile different from that of the R5FC, and 1 (ODL6–560) had the R5FC PMM profile. All but 1 (ODL2–237) of the R5FC isolates had the same MLST profile, whereas none of the S5FC isolates exhibited the same combination of the 3 MLST studied. The entire URA3 nucleotidic sequence of the translated region was identical except in 1 position (GenBank accession no. EU288194 for the type strain CBS 94 and EU288195 for 1 of the R5FC isolates). S5FC isolates were either homozygous or heterozygous at position 529 (A-A or A-G), whereas all the R5FC isolates were homozygous G-G. This produced a change of K177E (lysine → glutamate) for the R5FC isolates.
Table 2. Comprehensive analysis of 30 Candida tropicalis isolates*.
| Strain no. | 5FC MIC (μg/mL) | Month of isolation | SNP ITS2 | PMM alleles |
MLST |
URA3 base 529 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| URA3 | CT14 | MDR1 | XYR1 | SAPT4 | ||||||
| ODL1–18 | >64 | 2002 Nov | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL1–40 | >64 | 2002 Nov | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL1–41 | >64 | 2002 Nov | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL1–53 | 16 | 2002 Dec | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL2–105 | >64 | 2003 Mar | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL2–198 | 32 | 2003 Jul | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL2–199 | >64 | 2003 Jul | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL3–237 | >64 | 2003 Sep | – | 178/178 | 148/151 | 18 | 26 | 10 | G | |
| ODL3–293 | >64 | 2003 Oct | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL4–311 | >64 | 2003 Sep | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL4–328 | >64 | 2003 Nov | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL4–341 | >64 | 2003 Nov | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL5–426 | >64 | 2003 Dec | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL6–558 | 32 | 2004 Apr | – | 178/178 | 148/151 | 20 | 26 | 10 | G | |
| ODL1–58 | <0.125 | 2003 Jan | + | 176/176 | 148/148 | 24 | 30 | 7 | A | |
| ODL3–211 | 0.25 | 2003 Jul | + | 174/178 | 148/148 | 1 | 79 | 1 | A-G† | |
| ODL3–231 | <0.125 | 2003 Sep | – | 176/176 | 151/151 | 66 | 9 | 18 | A | |
| ODL4–302 | 0.25 | 2003 Sep | – | 176/178 | 148/151 | 4 | 36 | 23 | A | |
| ODL4–347 | 0.25 | 2003 Nov | + | 174/174 | 151/154 | 7 | 52 | 6 | A | |
| ODL4–384 | 0.5 | 2003 Dec | – | 174/176 | 151/151 | 67 | 4 | 19 | A | |
| ODL5–460 | <0.125 | 2004 Feb | + | 174/178 | 148/148 | 68 | 76 | 36 | A-G† | |
| ODL5–474 | <0.125 | 2004 Mar | + | 174/174 | 154/154 | 7 | 52 | 6 | A | |
| ODL5–476 | <0.125 | 2004 Mar | + | 178/178 | 151/151 | 27 | 4 | 11 | A | |
| ODL5–485 | <0.125 | 2004 Mar | – | 176/178 | 148/157 | 22 | 41 | 7 | A | |
| ODL5–488 | <0.125 | 2004 Apr | + | 176/178 | 148/151 | 25 | 24 | 7 | A | |
| ODL6–504 | <0.125 | 2004 Feb | + | 174/178 | 148/151 | 22 | 9 | 38 | A | |
| ODL6–511 | <0.125 | 2004 Apr | + | 174/178 | 148/148 | 1 | 80 | 1 | A-G† | |
| ODL6–521 | <0.125 | 2004 May | + | 176/178 | 148/151 | 69 | 77 | 41 | A | |
| ODL6–539 | <0.125 | 2004 Mar | + | 174/174 | 148/154 | 58 | 48 | 13 | A | |
| ODL6–560 | <0.125 | 2004 Jul | – | 178/178 | 148/151 | 4 | 36 | 23 | A | |
| CBS94 | <0.125 | – | + | 176/176 | 148/148 | 70 | 78 | 5 | A | |
*5FC, flucytosine; SNP, single nucleotide polymorphism; ITS2, internal transcribed spacer 2; PMM, polymorphic microsatellites marker; MLST, multilocus sequence typing (boldface corresponds to new alleles). †A-G heterozygous.
Thus, results obtained with nucleotide changes (deletion of A in position 106 in the ITS2 region, mutation in position 529 of the URA3 gene) and polymorphisms in 3 MLST and 2 PMMs suggested that the 14 R5FC studied were clonal. We thus decided to use the 2 PMMs (URA3 PMM, CT14 PMM) to genotype all the C. tropicalis isolates recovered during the study period in the YEASTS program. Of the 130 C. tropicalis isolates (including the 30 isolates studied above), 45 were R5FC (Table 3). Thirty-three different profiles were observed when both PMMs were combined for the 130 isolates. Among the 45 R5FC, a total of 29 isolates exhibited the profile associated with the R5FC clone; 16 were different, with 11 different profiles, 6 of which were shared with S5FC isolates. Among the S5FC isolates, 4 had the PMM profile associated with the R5FC clone. The URA3 gene was sequenced for these 4 isolates, and none had a G in position 529.
Table 3. Distribution of the polymorphic microsatellites markers (PMM) profiles among 130 Candida tropicalis isolates, according to their susceptibility to flucytosine (5FC)*.
| Allelic association |
Total no. isolates (N = 130) | No. isolate types |
|||
|---|---|---|---|---|---|
| URA3 PMM | CT14 PMM | S5FC (n = 85) | R5FC clone (n = 29) | Other R5FC (n = 16) | |
| 172/172 | 142/148 | 3 | 3 | – | – |
| 172/172 | 148/154 | 2 | 2 | – | – |
| 172/174 | 142/148 | 3 | 2 | – | 1 |
| 172/174 | 148/148 | 1 | 1 | – | – |
| 172/174 | 148/154 | 1 | 1 | – | – |
| 172/176 | 142/148 | 5 | 4 | – | 1 |
| 174/174 | 142/148 | 5 | 3 | – | 2 |
| 174/174 | 148/148 | 1 | – | – | 1 |
| 174/174 | 148/151 | 1 | 1 | – | – |
| 174/174 | 148/154 | 6 | 6 | – | – |
| 174/174 | 151/154 | 1 | 1 | – | – |
| 174/174 | 154/154 | 1 | 1 | – | – |
| 174/176 | 142/148 | 6 | 6 | – | – |
| 174/176 | 148/151 | 1 | 1 | – | – |
| 174/176 | 151/151 | 3 | 3 | – | – |
| 174/178 | 142/148 | 8 | 5 | – | 3 |
| 174/178 | 145/151 | 1 | – | – | 1 |
| 174/178 | 148/148 | 5 | 5 | – | – |
| 174/178 | 148/151 | 2 | 1 | – | 1 |
| 176/176 | 142/148 | 11 | 10 | – | 1 |
| 176/176 | 148/148 | 3 | 3 | – | – |
| 176/176 | 148/151 | 4 | 4 | – | – |
| 176/176 | 151/151 | 1 | 1 | – | – |
| 176/178 | 142/148 | 3 | 3 | – | – |
| 176/178 | 145/151 | 1 | 1 | – | – |
| 176/178 | 148/148 | 1 | – | – | 1 |
| 176/178 | 148/151 | 5 | 5 | – | – |
| 176/178 | 148/157 | 1 | 1 | – | – |
| 176/180 | 151/151 | 1 | 1 | – | – |
| 178/178 | 142/148 | 3 | 2 | – | 1 |
| 178/178 | 145/151 | 6 | 3 | – | 3 |
| 178/178 | 148/151 | 33 | 4 | 29 | – |
| 178/178 | 151/151 | 1 | 1 | – | – |
*S subscript, susceptible; R subscript, resistant.
We then studied genes potentially involved in the mechanisms of 5FC resistance. For the FUR1 sequences, no missense mutation was observed, and the complete coding sequence of the type strain CBS 94 (GenBank accession no. EU327978) was similar to the sequence of the R5FC clone; however, a few silent mutations were observed in a few S5FC isolates (GenBank accession nos. EU327979, EU327980, and EU327981). Concerning the cytosine deaminase sequences (FCY1), only 1 silent mutation, C21T, occurred for the R5FC clone (GenBank accession no. EU327982). Finally, for the purine cytosine permease (FCY2), the sequences of the type strain (GenBank accession no. EU327983) and of the R5FC clone were similar. A few heterozygosities were observed for the S5FC isolates (GenBank accession nos. EU327984 and EU327985), but all these mutations were silent.
Factors Associated with Fungemia Caused by the C. tropicalis R5FC Clone
All 130 isolates corresponded to incident fungemia in different persons. The R5FC clone was recovered during the 4 years of study with a trend toward a decreased proportion over time (11/13 [85%], 6/10 [60%], 8/13 [61.5%], and 4/9 [44%]) during the first, second, third, and fourth year of the study, respectively; p = 0.06). The proportion of the clone also varied across clinical centers (data not shown). Factors associated with fungemia caused by the R5FC clone of C. tropicalis were analyzed (Table 4). The proportion of patients infected by the R5FC clone was significantly higher among patients with malignancies but their death rate was significantly lower than for patients infection with other R5FC or S5FC isolates. Multinomial logistic regression was adjusted by clinical centers to investigate the factors associated with infection by the R5FC clone or others R5FC isolates compared with S5FC isolates. The risk of being infected by the R5FC clone compared with a S5FC isolate significantly increased in case of malignancy (odds ratio [OR] 3.7, 95% confidence interval [CI] 1.4–10.1, p = 0.009), and the risk for death at day 30 after fungemia was significantly decreased in patients infected by the R5FC clone compared with the S5FC isolates (OR 0.3, CI 0.1–0.9, p = 0.04), while no independent factor accounted for infection by other R5FC isolates versus an S5FC isolate. The only independent factor associated with infection by the R5FC clone compared with other R5FC isolates was the death rate at day 30 (OR 0.1, CI 0.03–0.6, p = 0.006).
Table 4. Patient characteristics according to the 3 categories delineated by the susceptibility of the Candida tropicalis isolates to flucytosine (5FC) and their belonging to the R5FC clone*.
| Characteristic | S5FC (n = 85) | R5FC clone (n = 29) | Other R5FC (n = 16) | p value† |
|---|---|---|---|---|
| >60 y of age | 39 (46) | 17 (59) | 9 (56) | 0.452 |
| Male | 55 (65) | 18 (62) | 10 (63) | 0.962 |
| Had malignancies | 41 (48) | 22 (76) | 9 (56) | 0.033 |
| Cancerous | 15 (18) | 7 (24) | 5 (31) | 0.374 |
| Hematologic | 26 (31) | 15 (52) | 4 (25) | 0.082 |
| In intensive care unit | 44 (52) | 12 (41) | 4 (25) | 0.116 |
| Had central venous catheter | 68 (80) | 24 (83) | 12 (75) | 0.894 |
| Had recent surgery | 27 (32) | 8 (30) | 4 (25) | 0.918 |
| Had prior antifungal therapy | 11 (13) | 0 | 2 (13) | 0.093 |
| Died before day 30 | 34/81 (42) | 6/28 (21) | 10/15 (67) | 0.014 |
*S subscript, susceptible; R subscript, resistant. Values are no. (%). †Fisher exact test.
Discussion
The bimodal distribution of 5FC MICs against C. tropicalis isolates prospectively collected from 27 different clinical centers in the Paris area (YEASTS program, Figure) suggested that the C. tropicalis population was heterogenous. On the basis of physiologic characteristics and molecular analysis (nucleotide sequences of the ITS regions, D1/D2 region of the large subunit, and large portions of the actin and 14-α-demethylase genes showing >99% similarity), we first assessed a subset of isolates (the first consecutive 14 R5FC and 16 S5FC isolates) and determined that both populations belong to the same species.
All 14 R5FC isolates had a single nucleotide deletion in position 106 of the ITS2 region, although 5 of the 16 S5FC isolates harbored it. When additional genotypic markers were used, all 14 R5FC isolates had the same allelic combination for 2 PMMs selected (URA3 and CT14), the same missense mutation in the URA3 gene, and the same diploid sequences for the 3 MLST loci studied. By contrast, only 1 of 16 S5FC isolates had the same PMM profiles as the R5FC isolates, but this isolate differed in its MLST profile and the lack of mutation in the URA3 gene. In addition, the 16 S5FC isolates exhibited 16 different MLST and 12 different PMM profiles. This finding suggested the existence of a R5FC clone, but the rest of the population was genetically diverse. We thus analyzed the 130 isolates of C. tropicalis collected over 4 years in the YEASTS program by using the 2 PMMs and sequenced the URA3 gene when the PMM profile was identical to that of the 14 R5FC isolates previously studied. We discovered that 29 (64%) of 45 R5FC isolates had an identical PMM profile (R5FC clone), while 11 and 30 different PMM profiles were found among the other 16 R5FC isolates and the 85 S5FC isolates, respectively. According to these data, we assumed that these 29 R5FC isolates were clonal or at least highly genetically related.
The proportion of 35% of C. tropicalis isolates resistant to 5FC is unusual (3). Other studies report between 0 (12) and 15% (13) with intermediate values (14–16), and all the isolates stored as C. tropicalis in the CBS collection since 1912 exhibited 5FC MIC <0.5 μg/mL. When we started the YEASTS program in 2002, the proportion of R5FC was already at 46% and the clone accounted for 85% of the R5FC isolates. The trend test suggested that the dispersal of the clone is declining in the Paris area. Whether this decline is specific for blood isolates or is a geographically and temporally restricted phenomenon deserves evaluation by using isolates collected over time from various body sites and geographic areas. An old report on isolates collected from various regions of France established with a nonstandardized technique that as many as 70% of the 63 isolates tested had an MIC >32 μg/mL in the 1980s (17), and a recent study from Germany on clinical isolates recovered from various body sites including blood reported that 58.3% of isolates were resistant (18). Whether any of these isolates belong to the R5FC clone would be of interest. Of note, a recent study of 104 C. tropicalis clinical isolates recovered from various countries (9) showed that none of the 5 R5FC isolates collected in the United Kingdom has the MLST profile of the R5FC clone.
In the univariate analysis, patients infected by the S5FC or R5FC isolates or by the R5FC clone differed significantly in terms of proportion of underlying malignancies (higher in patients with the clone) and death rate 30 days after fungemia (lower for patients infected by the clone). The R5FC isolates as a whole, and the R5FC clone specifically, were unevenly distributed around the Paris area. When adjusted for clinical center, logistic regression analysis showed that, compared to infection by S5FC isolates, no factor was independently associated with infection by R5FC isolates other than the clone, whereas 2 parameters were associated with infection by the clone. Indeed, malignancies multiplied the risk of being infected by the clone by almost 4 and the risk for death was divided by 3 in case of infection with the clone. C. tropicalis fungemia, independent of susceptibility to flucytosine, has already been associated with hematologic malignancies (19,20) (unpub. data from the YEASTS group). Whether the R5FC clone is less virulent, as established for C. albicans isolates with decreased susceptibility to 5FC, remains to be determined (21).
The resistance to 5FC was associated with the K177E mutation in the URA3 gene in the clone. The mechanism of 5FC action is a consequence of intrafungal formation of 2 metabolites, 5-fluorodeoxyuridine monophosphate and 5-fluorouridine triphosphate, which alter DNA and protein synthesis (22). The URA3 enzyme (orotidine 5′-phosphate decarboxylase, ODCase) is involved in the metabolic pathway of uridyl-monophosphate (UMP), which is a substrate of thymidylate synthetase and UMP kinase, both involved in nucleic acid synthesis. This mutation involves an amino acid already known to be variable among reference strains (e.g., ATCC 20336), but it has not been associated with modification of the URA3 properties thus far (T. Noël, pers. comm.). Nevertheless, this mutation could, for example, modify the tridimensional structure of the protein, thereby affecting the binding affinity of the substrate for the catalytic site and thus modifying the ODCase efficacy. The ODCase is not known to interfere directly with 5FC activity. However, one of the resistance mechanisms against 5FC consists in increasing the transcription of all the genes involved in the de novo pyrimidine biosynthetic pathway (including URA3) to overproduce UMP (23).
The K177E mutation is associated with a specific PMM upstream the gene, with a possible role in the level of transcription. The fact that this PMM is homozygous may be due to a loss of heterozygosity. This phenomenon has been recently reported for C. albicans and the resistance to azoles (24) and for a specific C. albicans isolate and the resistance to caspofungin (25).
The mutations described in the 5FC resistance of C. albicans (26) or C. lusitaniae (27) involved 3 major genes: FCY2 coding for the purine cytosine permease, which enables 5FC to enter the fungal cell; FCY1 coding for the cytosine deaminase, which transforms 5FC into 5FU; and FUR1 coding for the uracil phosphoribosyl transferase, which transforms 5FU into 5FUMP. The fact that the clone was susceptible to 5FU suggests that the 5FC resistance could result from a mutation in the cytosine deaminase, the cytosine permease, or both (23). However, the sequences of the R5FC clone, some S5FC isolates, and the type strain CBS94 did not show any mutation in coding sequences of FCY1, FCY2, or FUR1 susceptible to explain the resistance of the clone to 5FC. The mechanism explaining the possible relationship between the specific PMM (URA3 178/178 and CT14 148/151), the K177E mutation, and the resistance to 5FC remains to be determined.
Our results suggest that a clone of R5FC isolates responsible for fungemia is widespread among patients hospitalized with malignancies in the Paris area and is associated with a lower mortality than that of other C. tropicalis isolates. Despite a trend toward a decreased proportion over time, further studies are needed to assess this clone’s geographic and temporal distribution. Analysis of the 2 PMMs described in this study, coupled with determination of nucleotide at position 529 in the URA3 gene, should provide reliable means to track this clone.
Biography
Ms Desnos-Ollivier is an engineer at the National Reference Center of Mycoses and Antifungal at the Pasteur Institute in Paris, France. Her research interests include studying genotyping, sensibility to antifungal agents, and physiology of the yeasts responsible for human fongemia and underlining the relationship between genomic and phenotypic data.
Footnotes
Suggested citation for this article: Desnos-Ollivier M, Bretagne S, Bernède C, Robert V, Raoux D, Chachaty E, et al. Clonal population of flucytosine-resistant Candida tropicalis from blood cultures, Paris, France. Emerg Infect Dis [serial on the Internet]. 2008 Apr [date cited]. Available from http://www.cdc.gov/EID/content/14/4/557.htm
The YEASTS group is composed of (in alphabetical order by city): Claire Bouges-Michel (Hôpital Avicenne, Bobigny), Isabelle Poilane (Hôpital Jean Verdier, Bondy), Marie-Elisabeth Bougnoux, Jean Dunand (Hôpital Ambroise Paré, Boulogne), Guy Galeazzi (Hôpital Louis Mourier, Colombes), Stéphane Bretagne, Françoise Botterel (Hôpital Henri Mondor, Créteil), Nathalie Fauchet (Centre Hospitalier Intercommunal de Créteil, Créteil), Elisabeth Forget (Hôpital Beaujon, Clichy), Françoise Botterel, Christine Bonnal (Hôpital du Kremlin Bicêtre), Odile Eloy (Hôpital Mignot, Le Chesnay), Christine Lawrence (Hôpital Raymond Poincaré, Garches), Marie-Françoise David, Liliana Mihaila (Hôpital Paul Brousse, Villejuif), Elisabeth Chachaty, Olivier Adam (Institut Gustave Roussy, Villejuif), and in Paris: Christian Chochillon (Hôpital Bichat), André Paugam, Marie-Thérèse Baixench (Hôpital Cochin), Muriel Cornet (Hôpital de l’Hôtel Dieu), Marie-Christine Escande (Institut Curie), Svetlana Challier, Marie-Elisabeth Bougnoux (Hôpital Necker), Eric Dannaoui (Hôpital Européen Georges Pompidou), Annick Datry, Houria Laklache, Bader Lmimouni, Sophie Brun (Hôpital de la Pitié-Salpétrière), Jean-Louis Poirot (Hôpital Saint Antoine), Claire Lacroix (Hôpital Saint Louis), Didier Moissenet (Hôpital Trousseau), Michel Develoux (Hôpital Tenon), and Stéphane Bonacorsi (Hôpital Robert Debré).
References
- 1.Odds FC. Ecology of Candida and epidemiology of candidosis. Candida and candidosis: a review and bibliography. 2nd ed. London: Bailliere Tindall; 1988. pp. 68–82. [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Messer SA, Boyken L, Huynh H, Hollis RJ, Diekema DJ. In vitro activities of 5-fluorocytosine against 8,803 clinical isolates of Candida spp.: global assessment of primary resistance using National Committee for Clinical Laboratory Standards susceptibility testing methods. Antimicrob Agents Chemother. 2002;46:3518–21. 10.1128/AAC.46.11.3518-3521.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuenca-Estrella M, Moore CB, Barchiesi F, Bille J, Chryssanthou E, Denning DW, et al. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9:467–74. 10.1046/j.1469-0691.2003.00592.x [DOI] [PubMed] [Google Scholar]
- 5.de Hoog GS, van den Ende GAH. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;41:183–9. [DOI] [PubMed] [Google Scholar]
- 6.Masclaux F, Gueho E, de Hoog GS, Christen R. Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LS rRNA sequences. J Med Vet Mycol. 1995;33:327–38. 10.1080/02681219580000651 [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell K. Fusarium and its near relatives. Wallingford (UK): CAB International; 1993. [Google Scholar]
- 8.Foulet F, Nicolas N, Eloy O, Botterel F, Gantier JC, Costa JM, et al. Microsatellite marker analysis as a typing system for Candida glabrata. J Clin Microbiol. 2005;43:4574–9. 10.1128/JCM.43.9.4574-4579.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavanti A, Davidson AD, Johnson EM, Maiden MC, Shaw DJ, Gow NA, et al. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J Clin Microbiol. 2005;43:5593–600. 10.1128/JCM.43.11.5593-5600.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armitage P, Berry G. Statistical methods in medical research. 3rd ed. Oxford (UK): Blackwell Publishing; 1994. [Google Scholar]
- 11.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. Toronto; John Wiley & Sons Ltd.; 2000. [Google Scholar]
- 12.Cuenca-Estrella M, Diaz-Guerra TM, Mellado E, Rodriguez-Tudela JL. Flucytosine primary resistance in Candida species and Cryptococcus neoformans. Eur J Clin Microbiol Infect Dis. 2001;20:276–9. [DOI] [PubMed] [Google Scholar]
- 13.Moore CB, Walls CM, Denning DW. Comparison of three methods for in vitro susceptibility testing of Candida species with flucytosine. J Antimicrob Chemother. 2003;51:297–304. 10.1093/jac/dkg077 [DOI] [PubMed] [Google Scholar]
- 14.Alexander BD, Byrne TC, Smith KL, Hanson KE, Anstrom KJ, Perfect JR, et al. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27–A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol. 2006;45:698–706. 10.1128/JCM.01840-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quindos G, Ruesga MT, Martin-Mazuelos E, Salesa R, Alonso-Vargas R, Carrillo-Munoz AJ, et al. In-vitro activity of 5-fluorocytosine against 1,021 Spanish clinical isolates of Candida and other medically important yeasts. Rev Iberoam Micol. 2004;21:63–9. [PubMed] [Google Scholar]
- 16.Takakura S, Fujihara N, Saito T, Kudo T, Iinuma Y, Ichiyama S. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J Antimicrob Chemother. 2004;53:283–9. 10.1093/jac/dkh053 [DOI] [PubMed] [Google Scholar]
- 17.Nerson D, De Closets F, Dupouy-Camet J, Kures L, Marjollet M, Poirot JL, et al. Antifungal susceptibility of yeasts (and a few filamentous fungi) by a standardized micromethod [in French]. Bulletin de la Société Francaise de Mycologie Médicale. 1987;16:395–8. [Google Scholar]
- 18.Fleck R, Dietz A, Hof H. In vitro susceptibility of Candida species to five antifungal agents in a German university hospital assessed by the reference broth microdilution method and Etest. J Antimicrob Chemother. 2007;59:767–71. 10.1093/jac/dkl555 [DOI] [PubMed] [Google Scholar]
- 19.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43:1829–35. 10.1128/JCM.43.4.1829-1835.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, et al. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis. 2004;23:317–22. 10.1007/s10096-004-1103-y [DOI] [PubMed] [Google Scholar]
- 21.Fasoli MO, Kerridge D, Ryley JF. Pathogenicity of 5-fluorocytosine resistant strains of Candida albicans. J Med Vet Mycol. 1990;28:27–34. 10.1080/02681219080000041 [DOI] [PubMed] [Google Scholar]
- 22.Waldorf AR, Polak A. Mechanisms of action of 5-fluorocytosine. Antimicrob Agents Chemother. 1983;23:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope WW, Tabernero L, Denning DW, Anderson MJ. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob Agents Chemother. 2004;48:4377–86. 10.1128/AAC.48.11.4377-4386.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coste A, Turner V, Ischer F, Morschhauser J, Forche A, Selmecki A, et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2005;172:2139–56. 10.1534/genetics.105.054767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baixench MT, Aoun N, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S, Ramires S, et al. Acquired resistance to echinocandins in Candida albicans: case report and review. J Antimicrob Chemother. 2007;59:1076–83. 10.1093/jac/dkm095 [DOI] [PubMed] [Google Scholar]
- 26.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46:171–9. 10.1093/jac/46.2.171 [DOI] [PubMed] [Google Scholar]
- 27.Papon N, Noël T, Florent M, Gibot-Leclerc S, Jean D, Chastin C, et al. Molecular mechanism of flucytosine resistance in Candida lusitaniae: contribution of the FCY2, FCY1, and FUR1 genes to 5-fluorouracil and fluconazole cross-resistance. Antimicrob Agents Chemother. 2006;51:369–71. 10.1128/AAC.00824-06 [DOI] [PMC free article] [PubMed] [Google Scholar]