Abstract
Erythroid cells undergo enucleation and the removal of organelles during terminal differentiation1–3. Although autophagy has been suggested to mediate the elimination of organelles for erythroid maturation2–6, the molecular mechanisms underlying this process remain undefined. Here we report a role for a Bcl-2 family member, Nix (also called Bnip3L)7–9, in the regulation of erythroid maturation through mitochondrial autophagy. Nix−/− mice developed anaemia with reduced mature erythrocytes and compensatory expansion of erythroid precursors. Erythrocytes in the peripheral blood of Nix−/− mice exhibited mitochondrial retention and reduced lifespan in vivo. Although the clearance of ribosomes proceeded normally in the absence of Nix, the entry of mitochondria into autophagosomes for clearance was defective. Deficiency in Nix inhibited the loss of mitochondrial membrane potential (ΔΨm), and treatment with uncoupling chemicals or a BH3 mimetic induced the loss of ΔΨm and restored the sequestration of mitochondria into autophagosomes in Nix−/− erythroid cells. These results suggest that Nix-dependent loss of ΔΨm is important for targeting the mitochondria into autophagosomes for clearance during erythroid maturation, and interference with this function impairs erythroid maturation and results in anaemia. Our study may also provide insights into molecular mechanisms underlying mitochondrial quality control involving mitochondrial autophagy.
Nix, a BH3-only member of the Bcl-2 family, is upregulated in erythroid cells undergoing terminal differentiation10. To determine the potential function for Nix in erythroid maturation, we generated Nix−/− mice using embryonic stem (ES) cells with a gene trap insertion between exons 3 and 4 of Nix (Supplementary Fig. 2). We first examined red blood cells in the peripheral blood (RBCs), including reticulocytes and erythrocytes, in Nix−/− mice. Although RBC counts were decreased (Supplementary Table 1), polychromasia and increased reticulocytes were observed in Nix−/− mice (Fig. 1a and Supplementary Fig. 3a). We also examined RBCs for the expression of an erythroid cell marker, glycophorin-A-associated Ter119, and for transferrin receptor CD71, which is downregulated during terminal erythroid differentiation 11,12. Although Ter119lowCD71high and Ter119+ CD71high early erythroblasts13 were absent in the peripheral blood, a significant increase in Ter119+ CD71+ reticulocytes was observed in Nix−/− mice (Fig. 1b). Electron microscopy also showed more irregularly shaped cells characteristic of reticulocytes in the peripheral blood of Nix−/− mice (Supplementary Fig. 3c). In contrast, CD71− mature erythrocytes were reduced in Nix−/− mice (Fig. 1b). Together, these data suggest a potential defect in the development from CD71+ reticulocytes into mature CD71− erythrocytes in Nix−/− mice.
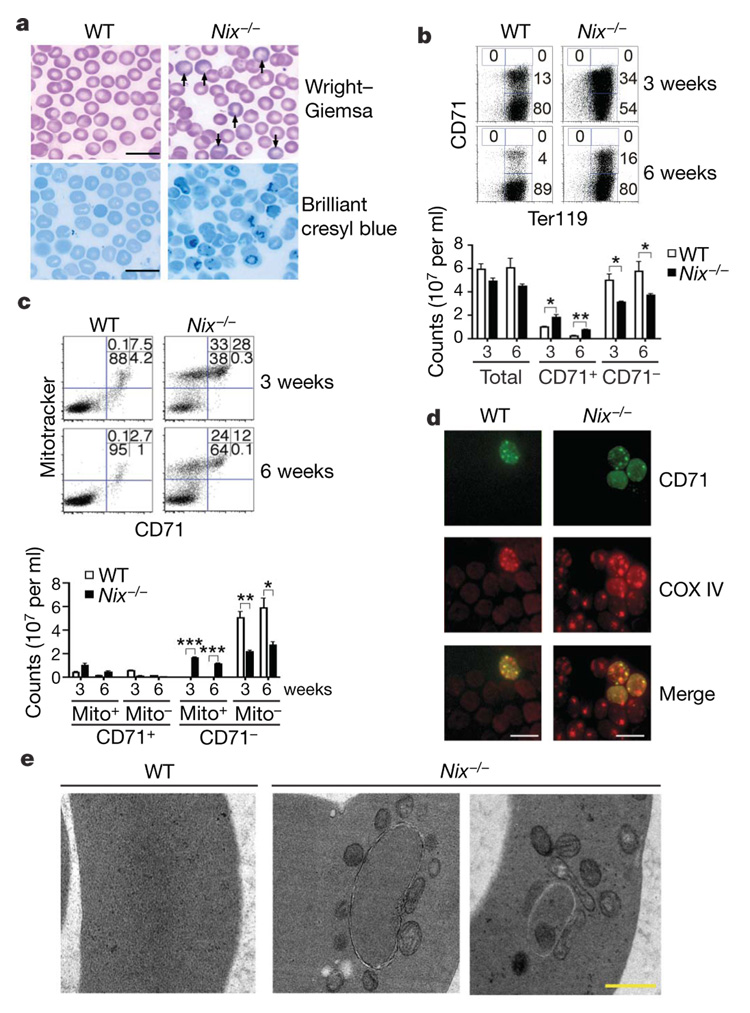
Figure 1. Reticulocytosis and retention of mitochondria in Nix−/− RBCs.
a, Polychromasia (Wright–Giemsa stain, indicated by arrows) and increased reticulocytes (brilliant cresyl blue stain) revealed by staining blood smears from 6-week-old wild-type (WT) or Nix−/− mice (scale bar, 20 µm). b, c, Ter119 (b) or Mitotracker deep red (c) versus CD71 staining of RBCs from 3- or 6-week-old mice. Cell counts were also calculated (mean ± s.e.m.; n = 3). *P <0.05; **P <0.01; ***P<0.001. d, e, Analyses of RBCs as in b by immunocytochemistry (d; scale bar, 10 µm) or transmission electron microscopy (e; scale bar, 0.5 µm).
Terminal differentiation of reticulocytes into erythrocytes involves the coordinated removal of organelles14. Because Nix localizes to the mitochondria10, we examined whether Nix−/− reticulocytes might be defective in eliminating the mitochondria. Consistent with increased reticulocytes, more RBCs (28% at 3 weeks and 12% at 6 weeks of age) were positive for CD71 and mitochondrial staining in Nix−/− mice than in wild-type controls (Fig. 1c). Notably, a significant portion of CD71− RBCs in Nix−/− mice retained mitochondria (33% and 24% of total RBCs in 3-week-old and 6-week-old mice, respectively), whereas mitochondria were virtually absent in wild-type CD71− RBCs (Fig. 1c). Immunocytochemistry staining for COX IV, a catalytic subunit of cytochrome c oxidase localized to the inner mitochondrial membrane, also confirmed the presence of mitochondria in both CD71+ and CD71− RBCs in Nix−/− mice (Fig. 1d). Electron microscopy revealed that very few RBCs in wild-type mice contained mitochondria or detectable autophagosomes (Fig. 1e). Autophagosomal structures were abundant in Nix−/− RBCs (Fig. 1e). However, all Nix−/− RBCs containing autophagosomes also displayed multiple mitochondria outside of autophagosomes, while approximately 20% of autophagosomes contained one mitochondrion inside (Fig. 1e). This suggests a potential defect in the inclusion of mitochondria by autophagosomes for clearance in Nix−/− RBCs.
The loading and unloading of oxygen by haemoglobin can induce oxidant stress in RBCs15. The mitochondrion is a major site for the production of reactive oxygen species (ROS) and can function as an apoptotic machinery16. The removal of mitochondria is therefore potentially beneficial for the survival of RBCs. We therefore examined whether Nix deficiency could affect RBC survival in vivo. Wild-type and Nix−/− mice were injected with N-hydroxysuccinimido (NHS)-biotin. Cell clearance was determined by the loss of biotinylated RBCs17. We observed that biotinylated RBCs disappeared more rapidly in Nix−/− mice than in wild-type controls (Fig. 2a, top panel), suggesting a faster clearance of RBCs in Nix−/− mice. We also labelled RBCs with 5-chloromethylfluorescein diacetate (CMFDA)18. We then measured the loss of CMFDA-labelled wild-type or Nix−/− RBCs after transfer into wild-type recipient mice. We observed accelerated clearance of transferred Nix−/− RBCs compared to wild-type controls (Fig. 2a, bottom panel). These data suggest that Nix−/− RBCs have a shorter lifespan in vivo.
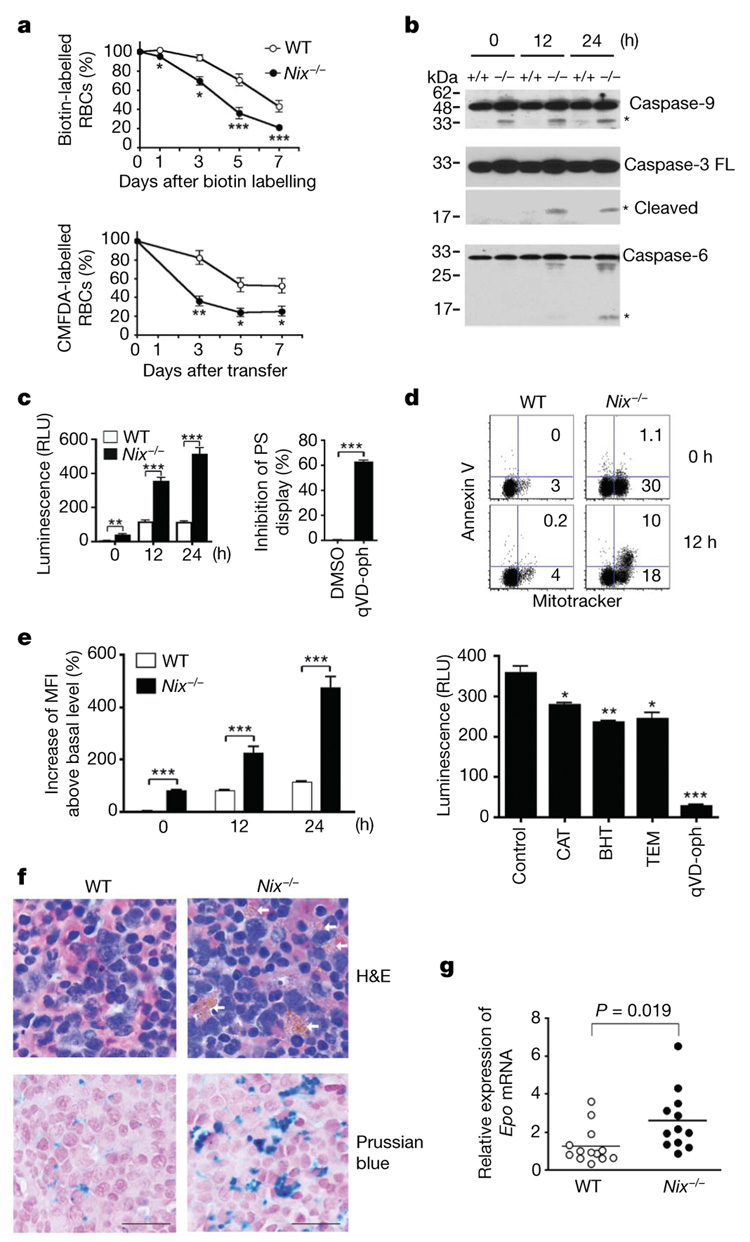
Figure 2. Decreased survival of RBCs in Nix−/− mice.
a, Quantification of NHS-biotin-labelled RBCs or transferred CMFDA-labelled RBCs (n = 3). b, Western blot analyses of caspases in RBCs after in vitro culture. Asterisks denote processed caspases. FL, full length. c, The relative luminescence units (RLU) of caspase activities in cultured RBCs and the suppression of annexin V staining in Nix−/− RBCs after 12-h culture in the presence of qVD-oph or solvent control (DMSO) (n = 3). d, Mitotracker versus annexin V staining of RBCs after in vitro culture. e, Mean fluorescent intensity (MFI) of ROS staining in RBCs after in vitro culture, and caspase activities in Nix−/− RBCs after 24 h culture with solvent control, ROS scavengers or qVD-oph (n = 3). BHT, butylated hydroxytoluene; CAT, catalase; TEM, tempol.
f, Haematoxylin and eosin (H&E) staining of spleen sections of 9-week-old wild-type and Nix−/− mice. Arrows denote iron deposits within macrophage cytoplasm. Iron deposits were also stained with Prussian blue, followed by counterstain with nuclear-fast red. Scale bar, 20 µm. g, Real-time RT–PCR of erythropoietin (Epo) (n = 12). For all relevant panels, statistical significance of the data (mean ± s.e.m.) is: *P<0.05; **P<0.01; ***P<0.001.
Consistent with decreased survival of Nix−/− RBCs in vivo, spontaneous caspase activation was observed in Nix−/− but not wild-type RBCs after in vitro culture (Fig. 2b, c). Moreover, mitochondrion-containing RBCs from Nix−/− mice displayed phosphatidylserine, a signal for phagocytosis19, before and after in vitro culture, as revealed by annexin V staining (Fig. 2d). A pan-caspase inhibitor, quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl ketone (qVD-oph)20, blocked caspase activation and suppressed annexin V staining on Nix−/− RBCs (Fig. 2c, e), indicating that caspase activation contributed to phosphatidylserine display. Increased ROS was detected in Nix−/− RBCs, and ROS scavengers inhibited caspase activation in Nix−/− RBCs (Fig. 2e). In agreement with accelerated clearance of RBCs, histochemistry showed increased iron-laden macrophages in the spleens of Nix−/− mice (Fig. 2f). Decreases in mature erythrocytes have been shown to induce compensatory erythropoiesis13. Consistently, reduced mature RBCs were correlated with increased erythropoietin mRNA and an expansion of Ter119+CD71+ erythroblasts in Nix−/− mice (Fig. 2g and Supplementary Fig. 8). The increased erythropoietin may contribute to the expansion of Ter119+ CD71+ erythroblasts, thus ameliorating the anaemia in Nix−/− mice. Together, our data suggest that mitochondrial retention in Nix−/− RBCs results in increased caspase activation and phosphatidylserine display, leading to faster clearance of RBCs and compensatory erythropoiesis. Removing mitochondria by autophagy would limit mitochondrial disruption in the cytosol and prevent the induction of apoptosis signalling in RBCs.
To induce the generation of sufficient numbers of reticulocytes for analyses, we treated mice with phenylhydrazine (PHZ), which induces reticulocytosis21. Ter119+ CD71+ reticulocytes in the peripheral blood were then sorted either on day 3 or on day 6 after PHZ treatment. Notably, a significant portion of the freshly sorted wild-type reticulocytes (18% at day 3 and 37% at day 6 after PHZ treatment) had already lost their mitochondria (Fig. 3a, top row). In contrast, fewer Nix−/− reticulocytes were negative for mitochondrial staining (Fig. 3a). This implies that Nix−/− reticulocytes were undergoing less efficient elimination of the mitochondria in vivo. After culture for in vitro maturation3, Nix−/− reticulocytes showed significant delays in the removal of the mitochondria compared to wild-type controls (Fig. 3a and Supplementary Fig. 4a). This suggests that Nix−/− reticulocytes are defective in clearing mitochondria during maturation.
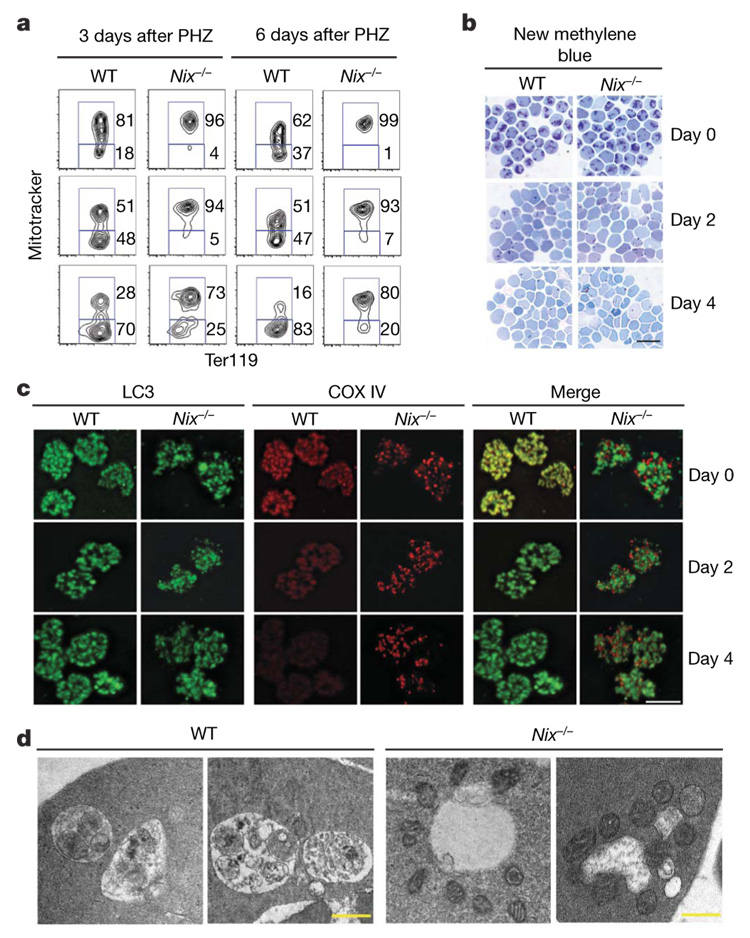
Figure 3. Defective clearance of mitochondria by autophagy in Nix−/− reticulocytes.
a, Ter119+ CD71+ reticulocytes sorted at day 3 or 6 after PHZ treatment were cultured for in vitro maturation for 0 (top row), 2 (middle row) or 4 (bottom row) days, followed by Mitotracker deep red and Ter119 staining. b, CD71+ Ter119+ reticulocytes sorted at day 3 after PHZ treatment were cultured and stained with new methylene blue. Scale bar, 20 µm. c, Cells as in b were stained for LC3 and COX IV and analysed by deconvolution microscopy. Scale bar, 5 µm. The Pearson coefficiency for LC3 and COX IV co-localization at day 0 (mean ± s.e.m.) is: wild type, 0.81 ± 0.001; Nix−/−, 0.44 ± 0.036 (n = 35, P = 0.0006). d, Cells sorted as in b were analysed by electron microscopy. Scale bar, 0.5 µm.
Inhibition of autophagy with 3-methyladenine, wortmannin or chloroquine22,23 suppressed the removal of mitochondria but not ribosomes in reticulocytes (Supplementary Fig. 5), indicating that the clearance of mitochondria, but not ribosomes, is dependent on autophagy in RBCs. Abnormal autophagosome formation in Nix−/− reticulocytes might lead to defective mitochondrial clearance. We therefore stained reticulocytes for the processed form of microtubule-associated protein light chain 3 (LC3) that is characteristic of autophagosome formation24. Wild-type and Nix−/− reticulocytes had similar levels of LC3 punctate staining at the initiation of culture (Fig. 3c; LC3 dots per cell at day 0: wild type, 19.7 ± 3.9; Nix−/−, 21.3 ± 4.5; P=0.19), indicating that the formation of autophagosomes was normal in the absence of Nix. Notably, LC3 co-localized with COX IV in wild-type but not Nix−/− reticulocytes (Fig. 3c). Moreover, wild-type but not Nix−/− reticulocytes had lost most COX IV staining by day 4 of in vitro culture (Fig. 3c). Electron microscopy showed that autophagic vacuoles in wild-type reticulocytes contained engulfed mitochondria; however, most mitochondria remained clustered outside of the autophagic vacuoles in Nix−/− reticulocytes (Fig. 3d and Supplementary Fig. 6). This suggests that Nix has a specific role in targeting the mitochondria into autophagosomes for clearance. In contrast to defective mitochondrial removal, Nix−/− RBCs showed no retention of the nucleus and underwent the loss of reticular staining similar to wild-type cells (Fig 1a, and Fig 3b and Supplementary Fig. 3c). Therefore, Nix seems to be required for the clearance of mitochondria, but not the nucleus or ribosomes, during erythroid maturation.
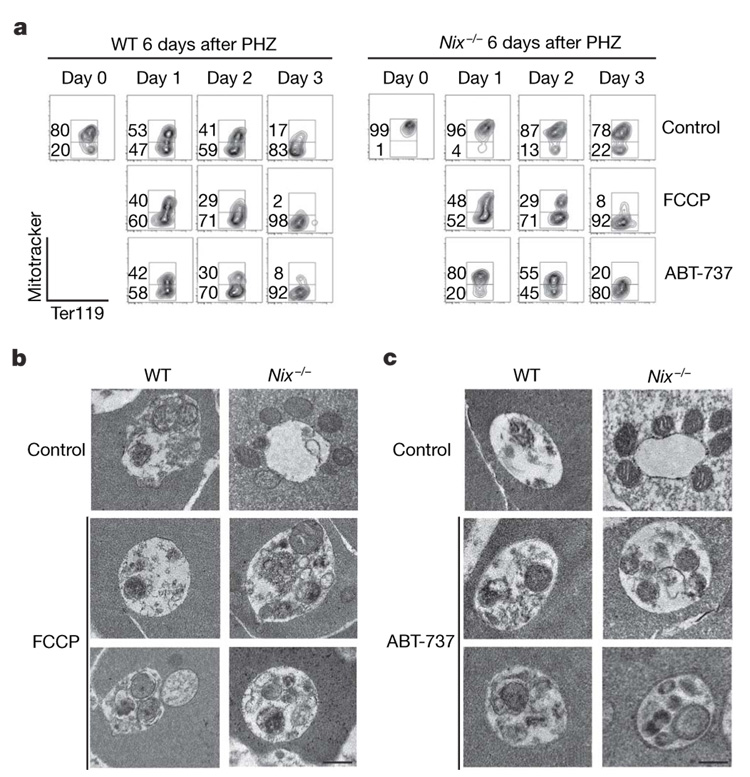
Incubation of Nix with purified mitochondria induced the loss of ΔΨm (ref. 9). Wild-type reticulocytes showed continued decreases in ΔΨm that preceded mitochondrial removal during in vitro maturation, whereas Nix−/− reticulocytes were defective in the loss of ΔΨm (Supplementary Fig. 7a). This supports a role for Nix in disrupting ΔΨm during erythroid maturation. Treatment with carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), an uncoupling agent that dissipates the proton gradient across mitochondrial inner membrane to abolish ΔΨm (ref. 25) (Supplementary Fig. 7c, d), promoted the clearance of mitochondria and restored the entry of the mitochondria into autophagic vacuoles in Nix−/− reticulocytes (Fig. 4a, b and Supplementary Fig. 4b). A BH3 mimetic, ABT-737 (ref. 26), which induced the loss of ΔΨm (Supplementary Fig. 7c), also restored the entry of mitochondria into autophagosomes and promoted mitochondrial removal in Nix−/− reticulocytes (Fig. 4a, c and Supplementary Fig. 4b). ABT-737 has been shown to promote the initiation of autophagy by releasing beclin 1 from the suppression mediated by Bcl-2 or Bcl-xL27. Because Nix is not required for autophagosome formation (Fig. 3c), ABT-737 potentially rescued mitochondrial autophagy in Nix−/− reticulocytes by inducing the loss of ΔΨm. Although a BH3 mimetic can rescue the defects in Nix−/− RBCs, deletion of another BH3-only molecule, Bim, did not affect erythroid differentiation (Supplementary Fig. 8). Therefore, Nix may be a specific sensor triggered by maturation signals in reticulocytes to promote mitochondrial autophagy.
Figure 4. Removal of mitochondria by autophagy in FCCP-treated or ABT-737-treated Nix−/− reticulocytes.
a, Ter119+ CD71+ reticulocytes were sorted from the peripheral blood of wild-type and Nix−/− mice at day 6 after PHZ treatment. The cells were cultured for in vitro maturation in the presence of 10 µM FCCP or 1 µM ABT-737, followed by staining with Mitotracker deep red and phycoerythrin-conjugated anti-Ter119. b, c, Reticulocytes were cultured with FCCP (b) or ABT-737 (c) for 24 h and analysed by transmission electron microscopy. Scale bar, 0.5 µm.
Our study identifies an unexpected function for Nix in mitochondrial autophagy during erythroid differentiation. Nix-dependent loss of ΔΨm may induce the display of molecules on the outer surface of mitochondria for recognition and sequestration by autophagosomes (Supplementary Fig. 1). Two yeast mitochondrial proteins, Uth1p (ref. 28) and Aup1p (ref. 29), have been shown to regulate mitochondrial autophagy in yeast. Here, we provide an example of specific regulation of mitochondrial autophagy in mammalian cells. Defective mitochondrial removal in Nix−/− RBCs was associated with spontaneous caspase activation, increased phosphatidylserine display and accelerated clearance by macrophages. Defective autophagy-dependent maturation and increased RBC clearance may contribute to anaemia in Nix−/− mice. In human K562 cells that were induced to undergo erythroid maturation, Nix-dependent removal of mitochondria was also observed (Supplementary Fig. 9). Defects in autophagic maturation of erythroid cells have been linked to anaemia in both humans and mice4,14. Understanding the mechanisms for mitochondrial autophagy in erythroid cells should facilitate the development of novel therapeutic approaches for treating haematological disorders involving defective erythroid maturation. It may also shed light on the mechanisms underlying mitochondrial quality control by autophagy in the protection against ageing, cancer and neurodegenerative diseases30.
METHODS SUMMARY
Mice
A mouse embryonic stem cell clone with a gene trap insertion targeting the Nix locus (The Sanger Institute Gene Trap Consortium) was used to generate Nix−/− mice (Supplementary Fig. 2). Mice were backcrossed to the C57BL/6 background for more than five generations.
Analyses of autophagosomes
RBCs were incubated with mouse anti-COX IV (Invitrogen) and a rabbit antibody to processed LC3 (Abgent), followed with Alexa Fluro-conjugated secondary antibodies (Molecular Probes). The slides were examined by deconvolution microscopy (Applied Precision). RBCs were also fixed and embedded in Spurr’s low-viscosity resin, and the sections were prepared and stained with uranyl acetate and lead citrate for analyses by transmission electron microscopy.
Analyses of apoptosis signalling
Cell lysates were used for SDS–PAGE and western blot analysis by probing with antibodies to different caspases, followed by incubation with horseradish peroxidase-conjugated secondary antibodies and developed by the chemiluminescent method (Pierce). Caspase activities were also measured using Ac-DEVD-pNA caspase-Glo reagent (Promega). To measure the display of phosphatidylserine on the cell surface, RBCs labelled with Mitotracker deep red were incubated with FITC–annexin V and analysed by flow cytometry.
Full Methodand any associated references are available in the online version of the paper at http://www.nature.com/nature.
METHODS
Mice
A mouse embryonic stem cell clone with a gene trap insertion targeting the Nix locus (The Sanger Institute Gene Trap Consortium) was used to generate Nix−/− mice (Supplementary Fig. 2). Mice were backcrossed to the C57BL/6 background for more than five generations.
Flow cytometry and immunocytochemistry
Erythroid cells from peripheral blood, bone marrow or spleen of wild-type and Nix−/− mice were stained with FITC–anti-CD71 and phycoerythrin–anti-Ter119 (BD Bioscience). Phosphatidylserine display was measured by staining with FITC–annexin V (BD Biosciences). To stain mitochondria, wild-type and Nix−/− RBCs were labelled with 200 nM Mitotracker deep red (Molecular Probes) at 37 °C for 30 min. The cells were then stained with FITC–anti-CD71 and phycoerythrin–anti-Ter119 and analysed by flow cytometry. For immunocytochemistry analyses, RBCs from wild-type and Nix−/− mice were stained with FITC–anti-CD71 and applied to slides by cytospin. The cells were fixed, incubated with mouse anti-COX IV (Invitrogen) and Alexa Fluor-conjugated secondary antibody. For mitochondria and LC3 co-staining, wild-type and Nix−/− RBCs were incubated with mouse anti-COX IV (Invitrogen) and a rabbit antibody to processed LC3 (Abgent), followed by staining with Alexa Fluor-conjugated secondary antibodies (Molecular Probes). The slides were examined using an AxioPlan2 fluorescent microscope (Zeiss) or a SoftWorx Image deconvolution microscope (Applied Precision). The Pearson coefficiency of correlation for LC3 and COX IV co-localization was determined by using the SoftWorx software (Applied Precision), with a value greater than 0.5 considered to have co-localization between the two signals.
Measurement of clearance of RBCs
Wild-type and Nix−/− mice were injected intravenously with (150 mg kg−1 body weight) NHS-biotin (Pierce), which covalently binds to free amino groups of cell surface proteins17. Cell clearance was determined by the loss of biotinylated RBCs17. RBCs were collected at indicated time points and the cells were incubated with phycoerythrin–Streptavidin (BD Bioscience). The percentage of labelled cells was analysed by flow cytometry. RBCs of wild-type or Nix−/− mice were also labelled with 4mM CMFDA (Molecular Probes) which emits green fluorescence after cleavage by intracellular esterases18. The labelled cells were injected into wild-type recipient mice intravenously. Blood was collected at indicated time points to quantify CMFDA-labelled cells by flow cytometry.
Induction of reticulocytosis in mice
Mice were injected intraperitoneally on day −1 and day 0 with phenylhydrazine (PHZ, Sigma; 60 mg kg−1 body weight). Ter119+CD71+ reticulocytes were then sorted on day 3 or day 6 after PHZ treatment using a FACSAria cell sorter (BD Bioscience).
Transmission electron microscopy
RBCs of wild-type and Nix−/− mice were fixed and embedded in Spurr’s low viscosity resin similar to described protocols31. Sections were prepared and stained with uranyl acetate and lead citrate, followed by analyses using a Hitachi H-7500 transmission electron microscope.
Quantitative real-time RT–PCR
RNA was isolated from kidneys of 6-week-old wild-type and Nix−/− mice using the MELT total nucleic acid isolation system (Ambion). Erythropoietin mRNA was quantified by real-time RT–PCR and normalized against 18S RNA as described32.
Analyses of apoptosis signalling
Cell lysates were used for SDS–PAGE and western blot analysis by probing with antibodies to Nix (Kamiya), caspase-6, caspase-9 (Cell Signaling), or actin (Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Southern Biotechnology). The blots were developed by the chemiluminescent method (Pierce). To detect caspase activities, cells were added to 96-well plates with caspase-Glo reagent containing Ac-DEVD-pNA (Promega) and incubated at room temperature for 2 h. The relative luminescence unit (RLU) was measured in a luminometer (Labsystems). In some experiments, Nix−/− RBCs were cultured with solvent control, 4,000 U ml−1 catalase, 100 µM butylated hydroxytoluene, 5 µM tempol or 200 µM qVD-oph for 24 h. Caspase activities were measured as above. To measure the display of phosphatidylserine on the cell surface, RBCs with or without Mitotracker deep red labelling were incubated with FITC–annexin V (BD Biosciences) and analysed by flow cytometry. To measure ROS, wild-type and Nix−/− RBCs were cultured in vitro and stained with 20 µM 2′,7′-dichlorofluorescin diacetate. Mean fluorescent intensity (MFI) was plotted.
Statistical analysis
Data were presented as the mean ± s.e.m. and P values were determined by two-tailed Student’s t-test using GraphPad Prism software version 4.0 for Macintosh.
Supplementary Material
Supplementary Information is linked to the online version of the paper at http://www.nature.com/nature.
Acknowledgements
We thank L. Huang, D. Yoon, A. Syed and D. Townley for technical assistance, and M. Andreeff for ABT-737. This work was supported by grants from the American Society of Hematology (M.C.), the American Heart Association (M.C.) and the NIH (J.W. and J.T.P.), a VA Merit grant (P.T.) and by a Ruth L. Kirschstein National Research Service Award (H.S.).
References
- 1.Yoshida H, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 2.Fader CM, Colombo MI. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy. 2006;2:122–125. doi: 10.4161/auto.2.2.2350. [DOI] [PubMed] [Google Scholar]
- 3.Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105:2168–2174. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- 4.Kent G, Minick OT, Volini FI, Orfei E. Autophagic vacuoles in human red cells. Am. J. Pathol. 1966;48:831–857. [PMC free article] [PubMed] [Google Scholar]
- 5.Heynen MJ, Verwilghen RL. A quantitative ultrastructural study of normal rat erythroblasts and reticulocytes. Cell Tissue Res. 1982;224:397–408. doi: 10.1007/BF00216882. [DOI] [PubMed] [Google Scholar]
- 6.Takano-Ohmuro H, Mukaida M, Kominami E, Morioka K. Autophagy in embryonic erythroid cells: its role in maturation. Eur. J. Cell Biol. 2000;79:759–764. doi: 10.1078/0171-9335-00096. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, et al. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J. Biol. Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Imazu T, et al. Bcl-2/E1B 19 kDa-interacting protein 3-like protein (Bnip3L) interacts with bcl-2/Bcl-xL and induces apoptosis by altering mitochondrial membrane permeability. Oncogene. 1999;18:4523–4529. doi: 10.1038/sj.onc.1202722. [DOI] [PubMed] [Google Scholar]
- 9.Diwan A, et al. Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc. Natl Acad. Sci. USA. 2007;104:6794–6799. doi: 10.1073/pnas.0610666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aerbajinai W, Giattina M, Lee YT, Raffeld M, Miller JL. The proapoptotic factor Nix is coexpressed with Bcl-xL during terminal erythroid differentiation. Blood. 2003;102:712–717. doi: 10.1182/blood-2002-11-3324. [DOI] [PubMed] [Google Scholar]
- 11.Kina T, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 12.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 13.Socolovsky M, et al. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 14.Holm TM, et al. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002;99:1817–1824. doi: 10.1182/blood.v99.5.1817. [DOI] [PubMed] [Google Scholar]
- 15.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol. Rev. 2004;23:169–188. doi: 10.2165/00139709-200423030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am. J. Med. Genet. 2001;106:62–70. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann-Fezer G, et al. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann. Hematol. 1993;67:81–87. doi: 10.1007/BF01788131. [DOI] [PubMed] [Google Scholar]
- 18.Levin J, et al. Pathophysiology of thrombocytopenia and anemia in mice lacking transcription factor NF-E2. Blood. 1999;94:3037–3047. [PubMed] [Google Scholar]
- 19.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 20.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 21.Vannucchi AM, et al. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1low mice) Blood. 2001;97:3040–3050. doi: 10.1182/blood.v97.10.3040. [DOI] [PubMed] [Google Scholar]
- 22.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 24.Kabeya Y, et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor α-induced apoptosis. Mol. Cell. Biol. 2000;20:5680–5689. doi: 10.1128/mcb.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 27.Maiuri MC, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissova I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J. Biol. Chem. 2004;279:39068–39074. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 29.Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J. Biol. Chem. 2007;282:5617–5624. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 30.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J. Cell Biol. 1989;109:3005–3013. doi: 10.1083/jcb.109.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mok H, Mendoza M, Prchal JT, Balogh P, Schumacher A. Dysregulation of ferroportin 1 interferes with spleen organogenesis in polycythaemia mice. Development. 2004;131:4871–4881. doi: 10.1242/dev.01342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at http://www.nature.com/nature.