Abstract
Background & Aims
Little is known regarding the impact of hospital-acquired infection (HAI) in acute pancreatitis (AP). We conducted a population-based assessment of the impact of HAI on outcome in AP.
Methods
Patient data was obtained from the Cardinal Health Clinical Outcomes Research Database, a large population-based dataset. Cases with principal diagnosis ICD9-CM 577.0 (AP) between Jan 2004 and Jan 2005 were identified. These cases were linked with recently reported HAI data collected by the Pennsylvania Health Care Cost Containment Council. Identification of HAI was based upon definitions set forth by the National Nosocomial Infection Surveillance System. We conducted a 5:1 multivariate propensity matched cohort study in order to determine the independent contribution of HAI to in-hospital mortality, length-of-stay and hospital charges.
Results
From 177 participating hospitals, there were 11,046 AP cases identified. Eighty-two (0.7%) patients developed an HAI. Mortality in the overall AP population was 1.2% vs. 11.4% among 405 matched non-HAI controls vs. 28.4% among patients that developed HAI (X2 p<0.0001). Fifteen percent of all deaths were associated with an HAI. Both average LOS and hospital charges were significantly increased among patients with HAI compared to matched non-HAI controls.
Conclusions
We determined that HAI had a major impact on mortality in AP. Patients that developed HAI also had significantly increased LOS and hospital charges. These differences were not explained by increased disease severity alone. Reducing HAI is an important step to improving outcome in AP.
INTRODUCTION
The natural history of acute pancreatitis (AP) is highly variable. In the majority of patients, it is a self-limited disease requiring only supportive measures. However, in some cases, AP develops into a life-threatening illness. Pancreatic infection is believed to play a major role in outcome. Specifically, infected pancreatic necrosis has been implicated as a major risk factor for mortality1–10.
The study of the impact of hospital-acquired infection (HAI) has become an area of active investigation 11–13. In 2004, Pennsylvania became the first state to issue mandatory hospital-wide public reporting on HAI. This created an opportunity for a population-based assessment of the contribution of HAI to outcome in AP.
In the present study we examine the impact of HAI on in-hospital mortality, length-of-stay (LOS) and healthcare cost.
METHODS
Data Collection
The Cardinal Health Clinical Outcomes Research Database (Cardinal Health, Marlborough, MA) is a large population-based dataset that has supported publicly reported hospital performance in Pennsylvania and elsewhere for 20 years. The largest database of its kind, it contains information on patient demographics, vital signs, laboratory values, key history and physical exam findings as well as all documented procedure and diagnosis codes. In addition, hospital disposition information as well as LOS and total billed hospital charges are tracked within this database. Details of this database have been published previously13.
Cases with a principal diagnosis of AP by International Classification of Diseases, ninth revision, clinical modification ICD9-CM 577.0 (AP) from the Cardinal database between Jan 2004 and Jan 2005 were identified. We collected patient information from the adult population (age>18) from 177 U.S. acute care hospitals. In order to restrict our analysis to patients admitted for acute pancreatitis, we excluded patients with primary procedure codes indicating pancreatic resection (Whipple procedure, proximal, distal or total pancreatectomy). Patients transferred to subsequent acute care facilities were not included in this study. All remaining cases were linked with recently reported HAI data collected by the Pennsylvania Health Care Cost Containment Council (PHC4). This Council consists of the following: infection control professionals, physicians, medical records specialists and quality assurance representatives who provide guidance throughout the data-collection process.
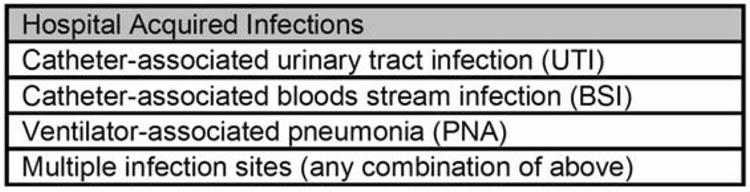
Methods of identification of HAI were previously published in a review of Pennsylvania’s experience with mandatory HAI reporting13–16. Identification of HAI was based upon definitions set forth by the National Nosocomial Infection Surveillance System (NNIS). Specifically, HAI was defined as a localized or systemic infection resulting from an adverse reaction to the presence of an infectious agent(s) or its toxin(s) that was not present or incubating at the time of hospital admission. Categories of HAI collected in the study are shown in figure 1. Neurosurgical, cardiovascular and orthopedic surgical-site infections were also tracked by PHC4 but not included in the present analysis.
Figure 1. Categories of HAI in the present study.
Details available at http://www.cdc.gov/ncidod/dhqp/nnis_pubs.html
Multivariate Propensity-Matched Cohort Study
In the present study, we sought to determine the independent contribution of HAI to in-hospital mortality, LOS and hospital charges. The development of HAI may be associated with additional factors which by themselves could influence mortality. These factors include both initial disease severity as well as specific interventions performed during the course of hospitalization. In order to evaluate the impact of HAI while simultaneously controlling for these factors, we performed a multivariate propensity matched cohort study.
Multivariate propensity matching has emerged as a means to achieve a more balanced cohort study design17–22. We matched patients according to their risk of infection through use of propensity scores that were in turn generated from a multivariate logistic regression equation.
In order to perform a propensity-matched study, we first needed to determine factors associated with HAI. We therefore developed a multivariate logistic regression model for prediction of HAI. This model was developed on the 11,036 cases in the overall AP population. We included in the model factors that we felt were most likely to be associated with development of HAI. These factors included initial disease severity (defined as the APACHE II score calculated within the first 24-hours of hospitalization), organ failure (either mechanical ventilation or hemodialysis) and additional invasive procedures associated with severe acute pancreatitis (central venous catheters, total parenteral nutrition (TPN), surgical necrosectomy and percutaneous abdominal catheter drainage). We were unable to include a term accounting for use of an indwelling urinary catheter in the model because this procedure was not reliably recorded in the database. We used the final logistic regression model to generate propensity scores for each patient based on their probability of developing HAI.
Each case of HAI was matched to five non-HAI cases based upon their risk of developing HAI (propensity score). The Parson’s greedy matching algorithm was used for identifying case-matches. This algorithm begins with 5-digit precision followed by subsequent relaxation steps by single-digit increments. We then studied the independent contribution of HAI to outcome in AP by comparing mortality, LOS and hospital charges between the matched groups of patients.
Statistical Analysis
Either the chi-square (χ2) method or Fisher exact test was used where appropriate for comparing proportions between the overall AP population and matched groups of patients. Subsequently pairwise testing was performed where appropriate. ANOVA was used for comparison of mean LOS and charges between the overall AP population and matched groups of patients. Pairwise Student’s t-test was then performed where appropriate. All reported p-values are two-sided. All statistical analyses were performed in SAS statistical software version 9.1 (SAS Institute, Cary NC).
RESULTS
Propensity Matching
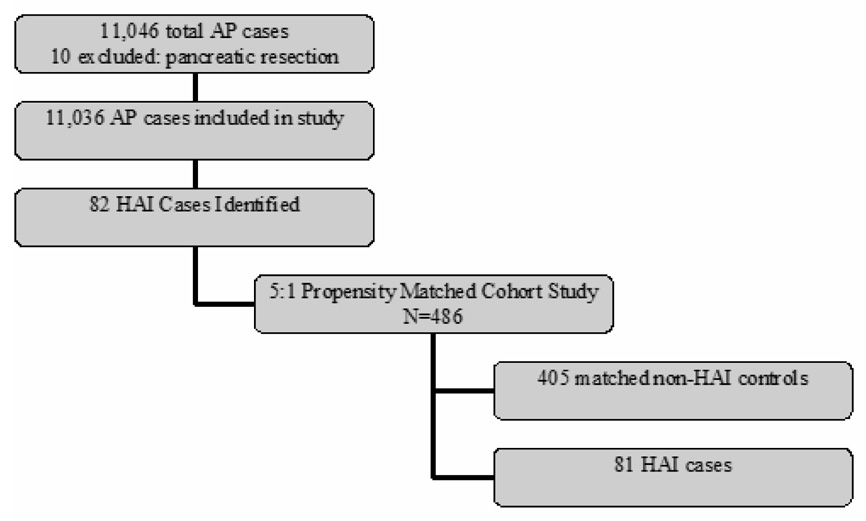
Figure 2 depicts the distribution of patients in the overall study design. From the 177 participating hospitals, there were 11,046 cases of acute pancreatitis reported during the study period. Ten cases were associated with pancreatic resection and excluded. Of the remaining patients, 82 (0.7%) developed an HAI.
Figure 2. Study Design.
The initial study group of 11,046 patients consisted of all AP patients in the 2004–2005 Cardinal Health Clinical Outcomes Research Database with data linked to the PHC4 infection surveillance program.
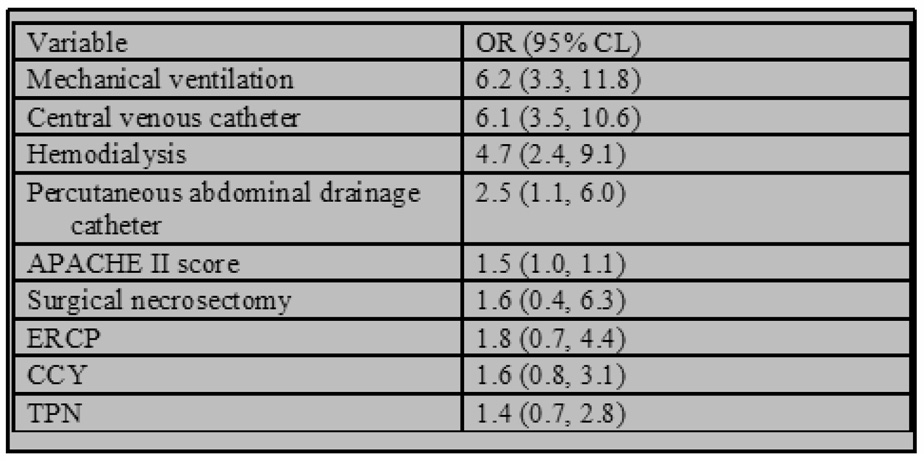
We included the following 9 factors in the logistic regression model used for propensity score development: 1) APACHE II score within the first 24 hours 2) mechanical ventilation 3) hemodialysis 4) central venous catheter 5) TPN 6) surgical necrosectomy 7) endoscopic retrograde cholangiopancreatography (ERCP) 8) cholecystectomy and 9) percutaneously placed abdominal catheter drainage. The model c-statistic for prediction of HAI was 0.883 (figure 2).
After 5-to-1 matching, the study population of HAI cases and matched controls consisted of 486 patients drawn from the AP population. Eighty-one of the 82 HAI cases were successfully matched, representing a 98.8% matching rate. These 81 cases were used in subsequent analyses. Clinical characteristics in the overall AP population and matched cohort of patients are depicted in table 1.
Table 1.
Clinical characteristics in overall AP population, propensity matched non-HAI controls and HAI patients.
| Overall AP N=11,036 |
Non HAI n=405 |
HAI n=81 |
|
|---|---|---|---|
| Age (median) | 53 | 60 | 63 |
| Men | 50.0% | 53% | 48% |
| Gallstones | 23.0% | 33.1% | 27.2%** |
| Alcohol history | 20.1% | 17.5% | 18.5%** |
| APACHE II (mean) | 7.5 | 10.7 | 10.5* |
| Mechanical ventilation | 1.7% | 27.0% | 37.0%* |
| Central venous catheter | 7.8% | 63.5% | 55.6%* |
| Hemodialysis | 1.9% | 20.0% | 25.9%* |
| Total parenteral nutrition | 2.6% | 16.0% | 21.0%* |
| ERCP | 3.5% | 5.9% | 7.4%** |
| necrosectomy | 0.2% | 3.0% | 4.9%* |
| Chol ecystectomy | 11.9% | 21.0% | 14A%** |
| Percutaneous drainage catheter | 1.1% | 12.3% | 12.3%* |
HAI vs. Overall AP population (p<0.0001, Wilcoxon, Fisher exact).
No significant difference between HAI vs. overall AP population at p<0.05 level.
No significant differences existed between matched-non HAI control vs. HAI patients for listed parameters at p<0.05 level.
ERCP= endoscopic retrograde cholangiopancreatography
HAI patients had significantly higher initial APACHE II score (p<0.0001, Wilcoxon) as well as frequency of mechanical ventilation, placement of central venous catheters, hemodialysis, TPN, necrosectomy and percutaneous abdominal catheter drainage than the overall population (all p<0.0001, Fisher exact). No significant differences existed in the rate of ERCP or cholecystectomy. By contrast, after propensity matching there were no significant differences in any of the aforementioned parameters between matched non-HAI controls and patients who developed HAI (table 1).
Mortality
Mortality in the overall AP population was 1.2% vs. 11.1% among the 405 matched non-HAI controls vs. 28.4% among the 81 patients that developed HAI (p<0.0001, χ2). Pairwise testing indicated that HAI patients had significantly increased mortality when compared to matched non-HAI controls (p<0.0001, χ2). Of the 150 total deaths in the entire AP population, 23 (15%) were HAI-associated. The distribution of HAI sites included 36% catheter associated-urinary tract infection, 35% catheter-associated blood-stream infections, 11% ventilator-associated pneumonia and 15.5% multiple sites. Site-specific mortality rates are presented in table 3. The mortality rate remained fairly consistent between various infection sites (range 27–33%, p=0.96, χ2).
Table 3.
Mortality in HAI by Site of Infection
| Site | Survived | Died | Mortality% |
|---|---|---|---|
| UTI | 22 | 8 | 26.7% |
| BSI | 21 | 8 | 27.6% |
| PNA | 6 | 3 | 33.3% |
| MULT | 9 | 4 | 30.8% |
Overall χ2 p=0.96
UTI= catheter-associated urinary tract infection
BSI= catheter-associated blood stream infection
PNA= ventilator-associated pneumonia
MULT= multiple infection sites
LOS and Charges
Mean LOS for the overall AP population was 5.6 days vs. 13.1 days for matched non-HAI controls vs. 34.5 days for patients with HAI (p<0.0001, ANOVA). Pairwise testing indicated significantly increased LOS for patients with HAI compared to matched non-HAI controls (p<0.0001, t-test). Mean per patient hospital charges were $28,749 in the overall AP population vs. $102,607 for matched non-HAI controls vs. $275,580 for patients with HAI (p<0.0001, ANOVA). Pairwise testing indicated significantly increased average hospital charges among patients with HAI compared to matched non-HAI controls (p<0.0001, t-test).
DISCUSSION
This represents the first population-based assessment of the impact of hospital-acquired infection on outcome in acute pancreatitis. Our finding that HAI is associated with increased mortality, LOS and hospital charges has several important implications.
First, the present study calls attention to the fact that HAI has a major impact on mortality in AP. Previous data regarding infection in AP is available primarily from clinical trials examining the efficacy of prophylactic antibiotics in necrotizing pancreatitis. Rates of extra-pancreatic infection among the control groups in these randomized-controlled studies ranged from 23%–48%23–27. It must be noted that these trials focused exclusively on patients with severe and/or necrotizing disease. The specific impact of extra-pancreatic infections and in particular HAI was unable to be examined in these studies primarily due to sample size constraints. .
Second, the development of HAI was not an unavoidable consequence of severe disease. Through propensity matching we identified a subgroup of patients with similar initial disease severity that had undergone a similar number of invasive procedures but did not develop HAI. These matched non-HAI controls had a mortality of 11.1%, which was significantly higher than the overall population (1.2%) but still well below the 28% mortality observed among patients that developed HAI.
A critical feature of this analysis was the ability to control for disease severity. Given the importance of accurately identifying a control group of patients at equal risk of developing HAI, we matched patients according to multiple risk factors. These included disease severity measured both at 24-hours (APACHE II) as well as throughout the hospital course (development of end-organ failure requiring either mechanical ventilation or hemodialysis). In addition, we controlled for the use of central venous catheters, TPN, ERCP, surgical necrosectomy, cholecystectomy and percutaneous abdominal catheter drainage. The final logistic regression model incorporating these parameters was very accurate for prediction of HAI with a c-statistic of 0.883.
Our study relied upon data collected jointly by PHC4 and the Cardinal Health Research data management staff. Given the importance of accurate reporting, the reliability of this data has been previously evaluated. Published internal evaluation of the methods used in collecting and abstracting data for the Pennsylvania hospital infection surveillance study found high levels of overall agreement between PHC4 Infection Control staff with results from chart abstraction15.
There were several potential limitations to the present study. First, all HAI were defined as either catheter or ventilator-associated. As a result, our study examined a select group of HAI. The impact of additional forms of HAI requires further investigation. In subsequent years, Pennsylvania has begun to collect additional data on additional forms of HAI. This may help to further define the scope of extra-pancreatic infection in AP.
One important remaining issue is the possibility for residual confounding. Two possible residual confounders that we did not account for were extent of pancreatic necrosis and presence of infected necrosis. Previous reports have suggested that patients with >50% necrosis have higher mortality than patients with <50% necrosis28 and that patients with infected necrosis have higher mortality than patients with sterile necrosis29. However, more recent studies have concluded that the presence of organ failure is the primary determinant of mortality in AP9,10,30,31,32. In our study, we were careful to control for severe organ failure (requirement for either mechanical ventilation or dialysis) when examining the impact of HAI.
Our study also does not address the possible role of antibiotics in preventing HAI. In the only two double-blind randomized controlled trials performed to date, there was no reduction in either extra-pancreatic infection or mortality with prophylactic antibiotics24,27. An important area for future study will be to determine effective methods for reducing HAI in AP.
In summary, patients with AP that developed an HAI had markedly increased in-hospital mortality, LOS and hospital charges. These differences persisted after controlling for initial disease severity and severe organ failure. Fifteen percent of all deaths were HAI-associated. Our findings suggest that aggressive efforts to reduce HAI may lead to significantly improved outcomes for patients with acute pancreatitis.
Figure 3. Multivariate Logistic Regression Model for Prediction of HAI.
C-statistic for regression model used for development of propensity scores was 0.883.
ERCP=endoscopic retrograde cholangiopacreatography
CCY=cholecystectomy
TPN=total parenteral nutrition
Table 2.
Mortality in AP
| Overall AP N=11,036 |
Matched Control n=405 |
HAI n=81 |
|
|---|---|---|---|
| Survived | 10,886 | 360 | 58 |
| Died | 150 | 45 | 23 |
| Mortality % | 1.4% | 11.1% | 28.4% |
Overall p<0.0001 χ2, pairwise HAI vs. non-HAI matched control p<0.0001 χ2
ACKNOWLEDGEMENTS
The authors would like to thank Karen Derby, Edward Cox and Michael Peng of Cardinal Health for their assistance with data management. In addition, we would like to thank Dr. Earl F. Cook of the Harvard School of Public Health for assistance with data analysis. We would also like to acknowledge the efforts of the Pennsylvania Health Care Cost Containment Council in tracking hospital-acquired infection.
Grant support: none
Abbreviations
- AP
acute pancreatitis
- CCY
cholecystectomy
- ERCP
endoscopic retrograde pancreatography
- HAI
hospital acquired infection
- LOS
length-of-stay
- NNISS
National Nosocomial Infection Surveillance System
- PHC4
Pennsylvannia Health Care Cost Containment Council
- TPN
total parenteral nutrition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: none
Writing assistance: none
Transcript profiling: not applicable
REFERENCES
- 1.Renner IG, Savage WT, 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 3.Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890–893. doi: 10.1002/bjs.1800810632. [DOI] [PubMed] [Google Scholar]
- 4.Uhl W, Isenmann R, Buchler MW. Infections complicating pancreatitis: diagnosing, treating, preventing. New Horiz. 1998;6:S72–S79. [PubMed] [Google Scholar]
- 5.Lowham A, Lavelle J, Leese T. Mortality from acute pancreatitis. Late septic deaths can be avoided but some early deaths still occur. Int J Pancreatol. 1999;25:103–106. doi: 10.1385/IJGC:25:2:103. [DOI] [PubMed] [Google Scholar]
- 6.Le Mee J, Paye F, Sauvanet A, O'Toole D, Hammel P, Marty J, Ruszniewski P, Belghiti J. Incidence and reversibility of organ failure in the course of sterile or infected necrotizing pancreatitis. Arch Surg. 2001;136:1386–1390. doi: 10.1001/archsurg.136.12.1386. [DOI] [PubMed] [Google Scholar]
- 7.Bhansali SK, Shah SC, Desai SB, Sunawala JD. Infected necrosis complicating acute pancreatitis: experience with 131 cases. Indian J Gastroenterol. 2003;22:7–10. [PubMed] [Google Scholar]
- 8.De Waele J, Blot S, Colardyn F. Bloodstream infections after surgery for severe acute pancreatitis. Pancreas. 2004;28:391–394. doi: 10.1097/00006676-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol. 2006;4:1053–1061. doi: 10.1016/j.cgh.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Perez A, Whang EE, Brooks DC, Moore FD, Jr, Hughes MD, Sica GT, Zinner MJ, Ashley SW, Banks PA. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229–233. doi: 10.1097/00006676-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal VD, Guzman S, Migone O, Crnich CJ. The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina: A prospective, matched analysis. Am J Infect Control. 2003;31:475–480. doi: 10.1016/j.ajic.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line-associated bloodstream infections. Am J Med Qual. 2006;21:7S–16S. doi: 10.1177/1062860606294631. [DOI] [PubMed] [Google Scholar]
- 13.Peng MM, Kurtz S, Johannes RS. Adverse outcomes from hospital-acquired infection in pennsylvania cannot be attributed to increased risk on admission. Am J Med Qual. 2006;21:17S–28S. doi: 10.1177/1062860606294632. [DOI] [PubMed] [Google Scholar]
- 14.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 15.Julian KG, Brumbach AM, Chicora MK, Houlihan C, Riddle AM, Umberger T, Whitener CJ. First year of mandatory reporting of healthcare-associated infections, Pennsylvania: an infection control-chart abstractor collaboration. Infect Control Hosp Epidemiol. 2006;27:926–930. doi: 10.1086/507281. [DOI] [PubMed] [Google Scholar]
- 16.Miller PR, Johnson JC, 3rd, Karchmer T, Hoth JJ, Meredith JW, Chang MC. National nosocomial infection surveillance system: from benchmark to bedside in trauma patients. J Trauma. 2006;60:98–103. doi: 10.1097/01.ta.0000196379.74305.e4. [DOI] [PubMed] [Google Scholar]
- 17.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61:721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 18.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465–476. doi: 10.1002/pds.1062. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2006 doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 20.Baser O. Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health. 2006;9:377–385. doi: 10.1111/j.1524-4733.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 21.Haro JM, Kontodimas S, Negrin MA, Ratcliffe M, Suarez D, Windmeijer F. Methodological aspects in the assessment of treatment effects in observational health outcomes studies. Appl Health Econ Health Policy. 2006;5:11–25. doi: 10.2165/00148365-200605010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rokke O, Harbitz TB, Liljedal J, Pettersen T, Fetvedt T, Heen LO, Skreden K, Viste A. Early treatment of severe pancreatitis with imipenem: a prospective randomized clinical trial. Scand J Gastroenterol. 2007;42:771–776. doi: 10.1080/00365520601173855. [DOI] [PubMed] [Google Scholar]
- 24.Isenmann R, Runzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997–1004. doi: 10.1053/j.gastro.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 25.Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480–483. [PubMed] [Google Scholar]
- 26.Delcenserie R, Yzet T, Ducroix JP. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas. 1996;13:198–201. [PubMed] [Google Scholar]
- 27.Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF, Maravi-Poma E, Kissler JJ, Sanchez-Garcia M, Utzolino S. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674–683. doi: 10.1097/01.sla.0000250414.09255.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotzinger P, Sautner T, Kriwanek S, Beckerhinn P, Barlan M, Armbruster C, Wamser P, Fugger R. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26:474–478. doi: 10.1007/s00268-001-0252-8. [DOI] [PubMed] [Google Scholar]
- 29.Isenmann R, Beger HG. Natural history of acute pancreatitis and the role of infection. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:291–301. doi: 10.1053/bega.1999.0025. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez JR, Razo AO, Targarona J, Thayer SP, Rattner DW, Warshaw AL. Fernandez-del Castillo C. Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg. 2008;247:294–299. doi: 10.1097/SLA.0b013e31815b6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malangoni MA, Martin AS. Outcome of severe acute pancreatitis. Am J Surg. 2005;189:273–277. doi: 10.1016/j.amjsurg.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]