Abstract
Experiments were performed to investigate the potential role of Src family kinase(s) in the rat afferent arteriolar contractile response to ANG II. The in vitro blood perfused juxtamedullary nephron technique was employed to monitor afferent arteriolar lumen diameter responses to 1–100 nM ANG II before and during Src family kinase inhibition (10 μM PP2). PP2 did not alter baseline diameter, but attenuated ANG II-induced contractile responses by 33 ± 6%. An inactive analog of PP2 (PP3) had no effect on ANG II-induced afferent arteriolar contraction. The effect of Src kinase inhibition on ANG II-induced [Ca2+]i responses was probed in fura 2-loaded preglomerular microvascular smooth muscle cells (PVSMCs) obtained from explants and studied after 3–5 days in culture. In untreated PVSMCs, ANG II evoked peak (Δ = 295 ± 61 nM) and plateau (Δ = 29 ± 9 nM) increases in [Ca2+]i. In PVSMCs pre-treated with PP2, baseline [Ca2+]i was unaltered but both the peak (Δ = 140 ± 22 nM) and plateau (Δ = 5 ± 3 nM) phases of the ANG II response were significantly reduced compared with untreated cells. PP3 did not alter [Ca2+]i responses to ANG II. Immunoprecipitation and Western blot analysis confirmed that 100 nM ANG II increased phosphorylation of c-Src (at Y416) in PVSMCs. The phosphorylation response was maximal 1 min after ANG II exposure and was prevented by PP2. We conclude that the preglomerular vasoconstriction evoked by ANG II involves rapid c-Src activation with subsequent effects that contribute to the [Ca2+]i response to the peptide.
Keywords: tyrosine kinase, vascular smooth muscle, afferent arteriole
INTRODUCTION
ANG II elicits diverse cellular responses in the vasculature, including contraction, proliferation, migration, and inflammation (42). The vasoconstrictor response to ANG II requires engagement of the AT1 receptor (AT1R) (39), which activates a signaling network that ultimately leads to an increase in intracellular free Ca2+ concentration ([Ca2+]i) and contraction. Protein tyrosine kinases modulate ANG II-stimulated [Ca2+]i, intracellular pH, contraction and growth responses of vascular smooth muscle cells (VSMCs), and tyrosine kinase inhibitors attenuate ANG II-induced effects in large arteries or cultured aortic myocytes (41). In accord with such observations, our previous studies revealed that renal afferent arteriolar contractile responses to ANG II involve tyrosine kinase(s), including the EGF receptor (EGFR) tyrosine kinase (5;10). Although the mechanism linking the AT1R and the EGFR has not been established, it may involve activation of intervening tyrosine kinase(s) (4;12;13;48). The candidate tyrosine kinases include Src kinases (13;19;24;50), a family of widely expressed nonreceptor tyrosine kinases. There exist at least 14 Src-related kinases, of which the 60 kDa c-Src is the prototype (38). c-Src is highly expressed in VSMCs (30) and has been implicated as a candidate tyrosine kinase that mediates regulation of vascular smooth muscle tone. Src family kinases have been implicated in stretch-induced contraction of bovine coronary artery (31), the canine basilar artery contractile responses to low extracellular Mg2+ concentration (47) and endothelin-1 (49), α2-adrenergic contraction of the porcine palmar lateral vein (34) (but not the rat thoracic aorta (8)), the contraction of rat aorta evoked by 5-hydroxytryptamine (3), and ANG II-induced contraction in the mesenteric vasculature (43). However, no data are available concerning the potential role of Src in regulating renal microvascular function. The present study was performed to evaluate the hypothesis that Src family kinase activation contributes to afferent arteriolar contractile and intracellular [Ca2+]i responses to ANG II.
MATERIALS AND METHODS
Animals
The procedures used in this study were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (SAS:VAF strain) weighing 250 to 300 g were purchased from Charles River Laboratories (Wilmington, MA) and provided free access to food and water.
In vitro blood-perfused juxtamedullary nephron technique
Arteriolar contractile function was assessed using the rat in vitro blood-perfused juxtamedullary nephron technique (9). After anesthetization with sodium pentobarbital (50 mg/kg ip), a cannula was inserted into the left carotid artery and enalaprilat was administered (2 mg in 1 ml isotonic saline) to reduce endogenous ANG II formation. The left renal artery and vein were ligated and the right renal artery was cannulated through the superior mesenteric artery, thereby initiating renal perfusion with Tyrode’s solution containing 52 g/l dialyzed BSA and a mixture of L-amino acids (32). Blood was collected through the carotid cannula before harvesting the kidney. Renal perfusion was maintained throughout the ensuing dissection procedure needed to reveal the tubules, glomeruli and related vasculature of juxtamedullary nephrons. Tight ligatures were placed around the most distal accessible segments of the large arterial branches that supply the exposed microvasculature. Acute surgical papillectomy was performed to avoid the indirect, tubuloglomerular feedback-dependent influence of ANG II on the afferent arteriole (22). The Tyrode’s perfusate was then replaced with reconstituted blood, prepared as described previously (32). Renal arterial perfusion pressure was maintained at 110 mmHg throughout the experiment. The perfusion chamber was warmed, and the tissue surface was bathed continuously with Tyrode’s solution containing 10 g/l BSA at 37°C. The tissue was transilluminated on the fixed stage of a compound microscope equipped with a water-immersion objective (×40). A single afferent arteriole was selected for study based on visibility and acceptable blood flow (the inability to discern the passage of individual erythrocytes), and the ensuing protocol assessed arteriolar diameter at a single measurement site (>100 μm upstream of the glomerulus) under several experimental conditions. Video images of the microvessels were stored on DVD for later analysis, during which lumen diameter was measured at 12 s intervals using a digital image-shearing monitor. This device was calibrated using a stage micrometer (smallest division = 2 μm) and yielded diameter measurements reproducible to within <1 μm. The average diameter during the final minute of each treatment period was utilized for statistical analysis.
Culture of preglomerular microvascular smooth muscle cells (PVSMCs)
PVSMCs were cultured from the rat kidney by the explant method. Briefly, animals were anesthetized with pentobarbital sodium (50 mg/kg ip) and the abdominal aorta was cannulated to allow renal perfusion with physiological saline solution (PSS), followed by 400 U/ml collagenase in GIBCO™ 0.05% Trypsin-EDTA for 5 min, then 1% Fe3O4 in PSS. The kidneys were removed and the cortex was minced, transferred to 1100 U/ml collagenase and 400 U/ml hyaluronidase in PSS and incubated at 37°C with gentle shaking for 30 min. Iron oxide-laden tissue was isolated from the suspension using a magnet, washed 4–5 times with cold PSS and incubated in 270 U/ml collagenase at 37°C with gentle shaking for 10 min. The iron-containing tissue was again collected, washed with cold PSS and inspected under the microscope to confirm that it consisted of microvessels devoid of glomeruli. The microvessels were transferred to DMEM containing 20% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin (14). Cultures were maintained in 5% CO2 (balance air) at 37°C and 85% humidity. All studies using cultured PVSMCs were performed after growth arrest under serum-free conditions for 1 day.
Immunostaining of PVSMCs
Cells obtained from the preglomerular microvasculature by the explant method and seeded onto glass coverslips were washed with cold PBS and fixed with 1:1 methanol:acetone at −20°C for 20 min. The coverslips were washed with cold PBS, blocked by 10% goat serum in PBS, and incubated overnight at 4°C with either mouse monoclonal anti-myosin heavy chain (1:100) or mouse monoclonal anti-CD34 (1:100). After four washes with cold PBS, the coverslips were incubated in the dark at room temperature for 30 min with Texas red-conjugated goat anti-mouse antibody (1:200). Unbound antibodies were removed by four washes with 3% BSA in PBS. The coverslips were incubated at room temperature in 2 μg/ml bisbenzimide H 33258 (nuclear dye) and FITC-conjugated mouse monoclonal anti-α-smooth muscle actin (1:500). After a final wash (3% BSA in PBS, repeated three times), the cells were dehydrated in ethanol, fixed with mounting media to glass slides, viewed under epifluorescence using a Leica DMR research microscope and photographed with an Optronics Magnafire digital camera.
[Ca2+]i measurement
PVSMCs were allowed to migrate from the primary explants onto glass coverslips and studied after 3–5 days in culture (with the final day under serum-free conditions). [Ca2+]i was monitored in fura-2-loaded cells using dual excitation wavelength fluorescence microscopy, as described previously (6;17). Briefly, cells were loaded with fura-2 by 1 h exposure at room temperature to Ringer’s solution containing 7 μM fura-2AM, 0.09 g/dl DMSO and 0.018 g/dl Pluronic F-127. An adjustable optical sampling window was positioned over a single PVSMC, which was illuminated alternately at 340 and 380 nm excitation wavelengths while emission fluorescence (510 nm) was collected using a photometer assembly at a rate of 10 points/s. [Ca2+]i was calculated from background-corrected data with the use of the FeliX software package (version 1.42; Photon Technology International, Monmouth Junction, NJ) according to the standard equation with a 0.85 viscosity correction factor (16;33). Calibration of the fura-2 signal was performed daily using established methods previously described (6;16). The protocol was as follows: after a stabilization period, baseline afferent arteriolar [Ca2+]i was determined during exposure to normal Ringer’s bath. The vessel was then subjected to one of the following 10 min treatments: (a) untreated (no drugs), (b) exposure to 10 μM 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP2; Src family kinase inhibitor) or (c) exposure to 10 μM 4-amino-7-phenylpyrazolo[3,4-d]pyrimidine (PP3; the inactive analog of PP2). Finally, 100 nM ANG II was applied in the continued presence of the pretreatment drug. None of these compounds exhibited autofluorescence at the excitation and emission wavelengths used for fura-2.
Immunoprecipitation and immunoblotting
PVSMCs were grown to confluence in flasks, passaged 3–5 times and harvested using GIBCO™ 0.05% Trypsin-EDTA. Quiescent VSMCs were pretreated with or without 10 μM PP2 for 30 min, followed by exposure to 100 nM ANG II for different durations (0, 0.5, 1, 2 and 5 min). Cells were washed with ice-cold PBS and excess solution was tapped off. Cells were lysed in 0.5 ml Lysis Buffer (50 mM Na4P2O7, 50 mM NaF, 50 mM NaCl, 5 mM EDTA, 5 mM EGTA, 2 mM Na3VO4, 10 mM HEPES, 0.1% Triton X-100) and 0.1 ml Protease Inhibitor Cocktail for mammalian tissue (Sigma). The samples were quickly put on dry ice for 15 min, thawed on ice for 15 min, sonicated and spun at 12,000 g for 20 min at 4°C. The supernatants were quantitated using a detergent-compatible protein assay (Bio-Rad, Hercules, CA). Normalized protein lysates were immunoprecipitated with mouse monoclonal anti-c-Src at 4ºC for 1 h, and then protein G Sepharose was added and incubated overnight at 4°C. Immune complexes were washed 3 times in PBS containing 1% Nonidet P-40, 0.5% Na deoxycholate, and 0.1% SDS, and resuspended in SDS Sample Buffer (1.25% β-mercaptoethanol, 0.75% SDS, 3.125% 4X upper Tris, 2.5 mg-% Pyronin Y, 10 mg-% bromophenol blue, 6.25% glycerol). Proteins were separated by 7.5% SDS-PAGE and transferred onto nitrocellulose membranes. After blocking with 5% milk, the membrane was treated overnight (4°C) with rabbit polyclonal phospho-Src family Tyr416 antibody (anti-pY416Src; 1:1000), followed by 1 h at room temperature in the presence of the secondary antibody (goat anti-rabbit, 1:2000) conjugated with horseradish peroxidase (HRP). The signal was generated using SuperSignal West Femto chemiluminescence substrate, and the light emitted from the bands was directly captured using the EpiChemi II Darkroom gel documentation system (UVP, Upland, CA). For repeated immunoblotting, membranes were stripped and reprobed with mouse monoclonal antibody against total c-Src (1:67; recognizes v-Src and c-Src) using the appropriate secondary antibody (goat anti-mouse, 1:100,000).
Reagents
Rabbit polyclonal anti-pY416Src, HRP-conjugated goat anti-rabbit IgG and prestained protein marker were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti-myosin heavy chain, mouse anti-CD34 and Texas red-conjugated goat anti-mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). GIBCO™ 0.05% Trypsin-EDTA solution was from Invitrogen (Carlsbad, CA). HRP-conjugated goat anti-mouse IgG, Restore Western Stripping Buffer and SuperSignal West Femto chemiluminescence substrate were from Pierce Biotechnology (Rockford, IL). Fura-2, fura-2AM and pluronic F-127 were purchased from Molecular Probes (Eugene, OR). PP2, PP3, bisbenzimide H 33258 fluorochrome 3HCl and mouse monoclonal anti-c-Src were from Calbiochem (San Diego, CA). All other chemicals were purchased from Sigma Chemical (St. Louis, MO). The Tyrode’s solution used in the perfused juxtamedullary nephron studies was composed of (in mM) 1.8 CaCl2·2H2O, 1 MgCl2·6H2O, 2.7 KCl, 138 NaCl, 0.35 Na2HPO4 and 5.5 D-glucose. The PSS used in preparing preglomerular microvascular explants was composed of (in mM) 145 NaCl, 4 KCl, 1.5 CaCl2, 5.5 D-glucose and 10 HEPES, with 100 U/ml penicillin and 100 μg/ml streptomycin. For measurement of arteriolar [Ca2+]i responses, all agents were administered via the normal Ringer’s bath (in mM: 148 NaCl, 5 KCl, 1 MgSO4, 1.6 Na2HPO4, 0.4 NaH2PO4, 1.5 CaCl2, and 5 D-glucose).
Statistical analyses
Statistical analysis was performed by ANOVA, repeated-measures ANOVA or Friedman’s repeated-measures ANOVA on ranks, followed by the Newman-Keuls multiple range test, as appropriate. Statistical computations were performed with the SigmaStat 3.01 software package (SPSS Inc), with statistical significance defined as P < 0.05. All data are reported as means ± SE.
RESULTS
Effect of Src Family Kinase Inhibition on Afferent Arteriolar Constrictor Responses to ANG II
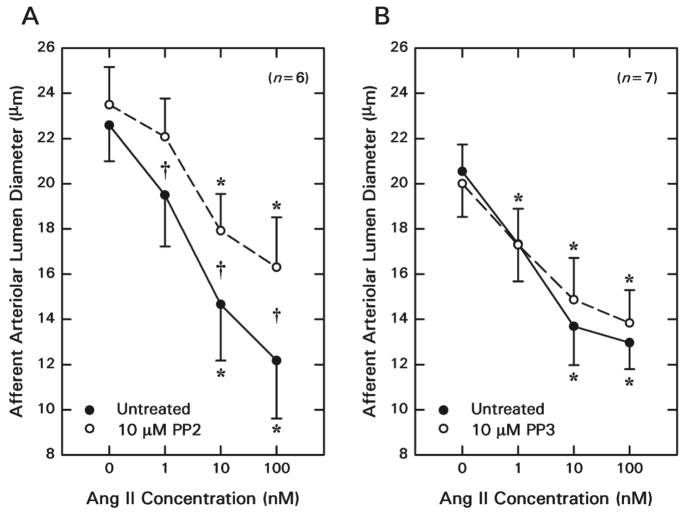
Afferent lumen diameter averaged 22.6 ± 1.6 μm (n = 6) under baseline conditions (Figure 1A), and 1, 10, and 100 nM ANG II reduced afferent diameter by 3.1 ± 1.4, 7.9 ± 2.1 and 10.4 ± 1.9 μm, respectively. Removal of ANG II from the bath allowed restoration of arteriolar diameter to 22.8 ± 1.9 μm. The Src family kinase inhibitor PP2 (10 rM) did not alter baseline afferent arteriolar diameter (23.5 ± 1.7 μm; P = 0.417) but suppressed ANG II-induced vasoconstriction (2 way repeated measures ANOVA, treatment × ANG II concentration: P = 0.017). During exposure to PP2, 1, 10 and 100 nM ANG II reduced afferent diameter by 1.4 ± 0.4, 5.6 ± 1.1 and 7.2 ± 1.9 μm (P < 0.05 versus untreated), respectively. Inhibition of Src family protein tyrosine kinase activity attenuated the afferent arteriolar response to 100 nM ANG II by 33 ± 6%.
Figure 1.
Effect of Src family kinase inhibition on juxtamedullary afferent arteriolar contractile responses to ANG II. A: Responses to ANG II before (Untreated) and during Src family kinase inhibition (PP2). B: Responses to ANG II before (Untreated) and during treatment with the inactive compound (PP3). *P < 0.05 vs. Baseline (0 nM ANG II); †P < 0.05 vs. Untreated vs. PP2.
The effect of the PP3 (inactive analog of PP2) on afferent arteriolar diameter responses to exogenous ANG II is depicted in Figure 1B. Afferent arteriolar diameter averaged 20.5 ± 1.2 μm (n = 7) under baseline conditions and decreased by 3.2 ± 0.7, 6.9 ± 1.2 and 7.6 ± 1.3 μm during exposure to 1, 10 and 100 nM ANG II, respectively. During the recovery period, afferent lumen diameter returned to 20.2 ± 1.2 μm (P > 0.05 vs. untreated baseline). Subsequent exposure to 10 μM PP3 did not significantly alter baseline diameter (20.0 ± 1.5 μm) or vasoconstrictor responsiveness to ANG II (1 nM, Δ = −2.7 ± 0.8 μm; 10 nM, Δ = −5.2 ± 0.7 μm; 100 nM, Δ = −6.2 ± 1.2 μm).
Characterization of Cultured PVSMCs
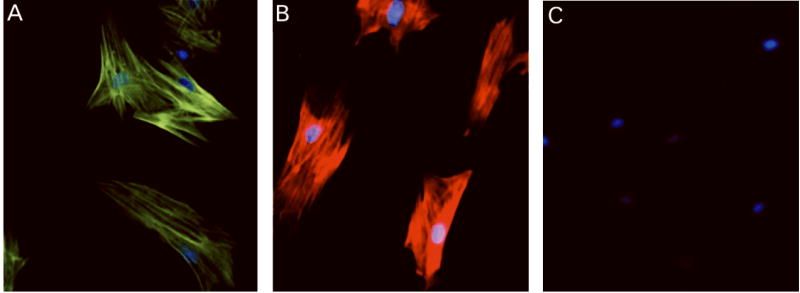
Cells cultured from preglomerular microvessel explants were characterized as vascular smooth muscle by morphological criteria (spindle-like or stellate shape) and immunostaining criteria. As shown in Figure 2, the cultured cells utilized in the present study stained positive for α-smooth muscle actin and myosin heavy chain, with no staining evident for CD34.
Figure 2.
Representative PVSMC immunostaining for α-smooth muscle actin (green; A), myosin heavy chain (red; B). The cells stained negative for the endothelial marker, CD34 (red; C). Blue, nuclear staining (bisbenzamide H 33258).
Effect of Src Family Kinase Inhibition on [Ca2+]i Responses to 100 nM ANG II
Figure 3 illustrates the effects of PP2 and PP3 on [Ca2+]i responses to ANG II in unpassaged PVSMCs studied after 3–5 days in culture. Basal [Ca2+]i averaged 45 ± 3 nM (n = 37) and was not significantly altered by treatment with either 10 μM PP2 (Δ = −13 ± 5 nM, n = 13) or 10 μM PP3 (Δ = −3 ± 2 nM, n = 11). Although Src family tyrosine kinase inhibition failed to alter basal [Ca2+]i under these experimental conditions, responses to ANG II were significantly affected. In the untreated group (n = 13), 100 nM ANG II caused a typical rapid [Ca2+]i increase that peaked at 293 ± 66 nM above baseline (peak response) followed by decline in [Ca2+]i until it achieved a plateau 23 ± 8 nM above baseline (P < 0.05; measured 3 min after the peak based on preliminary experiments). Compared with responses of untreated PVSMCs, both the peak and plateau phases of the [Ca2+]i response to ANG II were blunted significantly in cells treated with the Src family kinase inhibitor PP2. The magnitude of the peak response was reduced by 52% in PP2-treated cells, with no plateau phase evident (Δ = 3 ± 2 nM; P < 0.05 vs. untreated plateau response). The inactive compound PP3 did not significantly change the amplitude of the peak (Δ = 263 ± 59 nM) or the plateau (Δ = 16 ± 6 nM) of the [Ca2+]i response to ANG II. Thus, Src family kinase inhibition attenuated the peak ANG II-induced increase in [Ca2+]i and abrogated the plateau phase of the response in renal PVSMCs.
Figure 3.
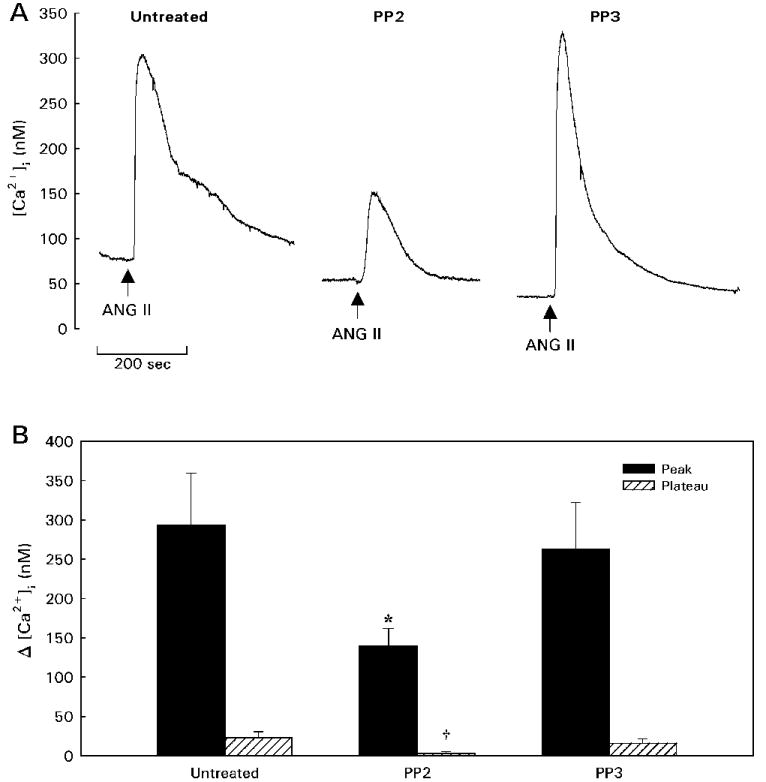
Effect of Src family kinase inhibition on [Ca2+]i responses to ANG II in unpassaged PVSMCs. A: Typical responses to 100 nM ANG II are shown for Untreated cells and cells pretreated with either the Src family tyrosine kinase inhibitor PP2 or its inactive analog PP3. B: Summary data showing mean peak (solid bars) and plateau (hatched bars) responses to 100 nM ANG II in each treatment group. *P < 0.05 vs. Untreated peak; †P < 0.05 vs. Untreated plateau.
Effect of ANG II on c-Src Phosphorylation in PVSMCs
Figure 4 illustrates the effect of ANG II on Src phosphorylation as assessed in cell lysates immunoprecipitated with anti-c-Src. ANG II did not alter c-Src protein levels at any time point within the 5 min treatment regimen. Phosphorylation of Src at Y416 was increased transiently by exposure of PVSMCs to 100 nM ANG II, averaging approximately 2 times baseline when measured 1 min after ANG II exposure. The Src family tyrosine kinase inhibitor PP2 prevented this phosphorylation response. The effect of ANG II to stimulate Src phosphorylation waned by the 2 min time point, with phosphorylated Src levels numerically similar to baseline evident after 5 min.
Figure 4.
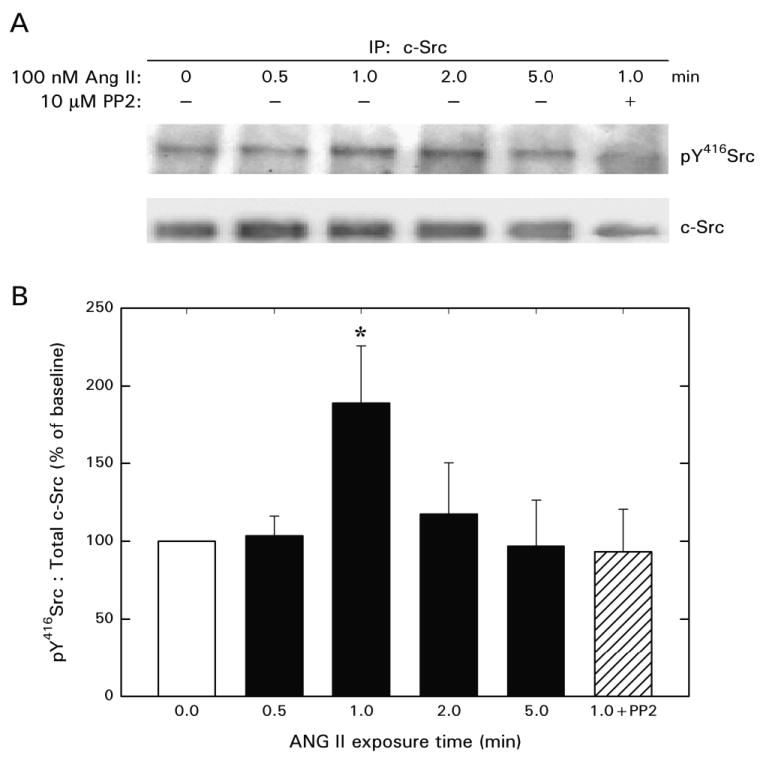
c-Src phosphorylation in cultured PVSMCs exposed to ANG II in the absence (n = 4) or presence (n = 3) of PP2. After immunoprecipitation with c-Src, Western blot analysis was performed using an antibody against phospho-Y416Src. The membrane was stripped and re-probed for total c-Src. A: Representative blots. B: Summary of densitometric analysis.
DISCUSSION
Accumulating evidence suggests that GPCRs, such as the AT1R, can take advantage of signaling pathways involving receptor- and nonreceptor-tyrosine kinases to exert their effects on cells. We recently reported that afferent and efferent arteriolar contractile responses to ANG II involve tyrosine kinase(s), including the EGFR tyrosine kinase (5). Moreover, EGFR tyrosine kinase activity contributes to the afferent arteriolar intracellular [Ca2+]i response to ANG II though a process that promotes Ca2+ influx (10). The present study extends these observations to implicate the Src family of nonreceptor tyrosine kinases in the afferent arteriolar contractile response to ANG II, including effects on ANG II-induced [Ca2+]i responses in PVSMCs.
The pyrazolopyrimidine PP2 was utilized to assess the role of Src activity in functional responses to ANG II. PP2 interacts with the ATP-binding site within the tyrosine kinase domain of Src, presumably preventing enzyme activation by interfering with autophosphorylation at Y416. PP2 does not discriminate between Src family members because the kinase domain is highly conserved within the family (45). PP2 inhibits Src family kinases with IC50 values in the low nanomolar range (2;18); a few other protein kinases (Csk, p38) are inhibited an order of magnitude less potently (2). At the concentration employed in the present study (10 μM), PP2 virtually abolishes Src family kinase activity, but may also reduce p38 and MAPK activities by 50–75% as indicated by in vitro kinase assays (2). Thus, we cannot rule out a direct or indirect (via Src) involvement of p38 and MAPK in the effects of PP2 in the present study. However, a primary involvement of Src family members in the preglomerular microvascular effects of PP2 is highly likely, especially in light of the demonstration that this agent prevents ANG II-induced c-Src phosphorylation. The ability of PP2 to prevent Src phosphorylation is in accord with previous reports (35).
When studied using the in vitro blood-perfused juxtamedullary nephron technique, the afferent arteriole displays basal tone that includes a myogenic component (but not a tubuloglomerular feedback component due to the acute papillectomy), as well as the likely influence of myriad vasoactive substances delivered via the perfusate blood or through paracrine processes (7). The lack of an effect of PP2 on basal diameter suggests minimal involvement of Src in establishing myogenic tone and/or other tonic vasoactive influences on the afferent arteriole under our experimental conditions, in accord with our previous reports that broad-spectrum tyrosine kinase inhibition (AG9) has no effect on afferent arteriolar tone (5). However, the ability of PP2 to attenuate afferent arteriolar contractile responses to ANG II indicates involvement of Src family kinase activity in the signaling network activated by engagement of the AT1R, presumably in the resident PVSMCs.
Although studies using the in vitro blood-perfused juxtamedullary nephron technique provide useful insight into afferent arteriolar function, the vasculature studied in this setting is subject to NO-dependent regulation of basal tone and ANG II responsiveness (23). As Src is a known regulator of endothelial NO synthase (20), further investigation of Src involvement in ANG II activation of PVSMCs was pursued using cells cultured from preglomerular microvascular explants (primarily interlobular arterial and afferent arteriolar segments). These cells stained positive for α-smooth muscle actin and myosin heavy chain (using an antibody that recognizes SM1 and SM2 isoforms), with no immunostaining evident for CD34 (a glycoprotein expressed by hematopoietic progenitor cells, endothelial cells and fibroblasts (15)). Although sometimes detected during glomerular disease, normal mesangial cells do not express SM1, SM2, α-smooth muscle actin or CD34 (25;27;29;37). Based on these characteristics, as well as morphological criteria, the cells cultured from preglomerular explants were considered authentic PVSMCs. We cannot rule out the possibility that the PVSMCs undergo transformation in culture. To minimize the likelihood of this occurrence, [Ca2+]i responses were studied in PVSMCs maintained only 3–5 days in culture; however, 3–5 passages were necessary to obtain sufficient PVSMCs for immunoblotting studies. Although phenotypic transformation may have occurred in PVSMCs maintained in culture for 3–5 passages, we note that the responses of these cells (ANG II activation of c-Src) are consistent with those obtained after 3–5 days in culture and in intact afferent arterioles studied using the in vitro blood-perfused juxtamedullary nephron technique (Src kinase inhibition blunts ANG II-induced responses).
ANG II evokes a rapid increase in vascular smooth muscle [Ca2+]i, which represents a primary determinant of contraction. However, contractile responses can also be influenced by phosphorylation-dependent changes in the Ca2+-sensitivity of the contractile apparatus. Indeed, Src family protein tyrosine kinases appear to be involved in Rho-kinase-mediated Ca2+ sensitization of coronary arterial contraction (28). ANG II-induced Ca2+ signaling events may also lead to Src activation via Pyk2 (a Ca2+-sensitive tyrosine kinase), with consequent effects on Ca2+ sensitivity. In these two scenarios, Src activity promotes the contractile response to ANG II but is not a determinant of the [Ca2+]i response. On the other hand, Src kinase activity has been implicated in tracheal smooth muscle [Ca2+]i responses to serotonin (40) and the [Ca2+]i response of human VSMCs to ANG II (43), situations in which Src activity is required for full expression of the ANG II-induced [Ca2+]i response. In order to distinguish the between these two potential mechanisms through which Src might contribute to ANG II-induced contraction in the preglomerular microvasculature, we assessed the effects of PP2 on [Ca2+]i responses of individual fura-2-loaded PVSMCs. Neither PP2 nor PP3 altered basal [Ca2+]i, in accord with the failure of the agents to alter basal tone in perfused juxtamedullary nephrons. Moreover, Src family kinase inhibition blunted the peak and virtually abolished the plateau [Ca2+]i responses to ANG II, while PP3 (the inactive analog) had no effect. Thus, Src family kinase activity and/or its downstream effectors ultimately influence Ca2+ influx or Ca2+ release events in the renal preglomerular microvasculature. Both Ca2+ influx and Ca2+ mobilization from intracellular stores contribute to the transient peak phase of the afferent arteriolar [Ca2+]i response to ANG II, while the sustained plateau phase of the [Ca2+]i response reflects only Ca2+ influx (10;26). Thus, the effect of PP2 on the PVSMC [Ca2+]i response to ANG II is suggestive of an effect on Ca2+ influx. Tyrosine kinases, including Src, have been suggested to regulate various ion channels (1;21;46), including the L-type Ca2+ channels that play a predominant role in ANG II-induced Ca2+ influx in the afferent arteriole. Thus, it is possible that Src promotes the [Ca2+]i response to ANG II in PVSMCs via effects on voltage-gated Ca2+ influx. In contrast, Src activity contributes to the AngII-induced contractile responses of human VSMCs isolated from resistance arteries in adipose tissue via effects on Ca2+ mobilization (43). The apparent role for Src in promoting ANG II-induced Ca2+ influx in PVSMCs, rather than the effect on Ca2+ mobilization that occurs in other VSMC populations, may reflect a species difference or the general prominence of Ca2+ influx mechanisms in the preglomerular microvasculature relative to other vascular beds.
As Src kinase inhibition did not alter basal afferent arteriolar diameter or PVSMC [Ca2+]I, tonic levels of Src activity appear insufficient to contribute to ANG II-induced responses. Thus, the ability of ANG II to provoke Src kinase activation was assessed in ANG II-treated PVSMCs by Western blot analysis of Src phosphorylation. Src activity is regulated by phosphorylation at two sites: Y416, located in the activation loop of the kinase domain (phosphorylation increases enzyme activity) and Y527, located in the carboxy-terminal tail (phosphorylation decreases enzyme activity). The antibody employed in these studies detects Src only when phosphorylated at Y416, thereby indicting enzyme activation. Although this antibody can react with other Src family members when phosphorylated at the corresponding sites, the use of c-Src immunoprecipitates for the phosphoanalysis makes it highly likely that our observations reflect phosphorylation of c-Src at Y416. However, other Src family members may also be activated in response to ANG II and participate in the PP2-sensitive component of the contractile response. ANG II provoked c-Src phosphorylation within the time frame (≤1 min) required for a potential contribution of this event to the contractile response. The c-Src phosphorylation response was transient, in accord with previous observations in cultured (1 to 4 passages) VSMCs from human small arteries (43) and rat mesenteric arteries (44).
The signaling cascade through which Src is involved in ANG II-induced [Ca2+]i and contractile responses of the preglomerular microvasculature is not known. In rat aortic VSMCs, Src plays a critical role in rapid ANG II activation of Ras (36) and ERK (12), although a contribution of these processes to contraction has not been established. The effects of Src inhibition unveiled by the present study are reminiscent of the effects of EGFR tyrosine kinase inhibition, which attenuates the juxtamedullary afferent arteriolar contractile response to ANG II and reduces the peak and plateau phases of the [Ca2+]i response of isolated afferent arterioles (5;10). As ANG II has been shown to induce association of Src with the EGFR in rat aortic myocytes (13), it is possible that these two tyrosine kinases operate in the same branch of the ANG II-induced contractile signaling network. Src has been suggested to act upstream or downstream of the EGFR in various cell systems (11;50); thus, further studies are required to clarify the relationship between Src and the EGFR in the signaling events that contribute to ANG II-induced contractile responses in the preglomerular microvasculature.
In summary, studies using the rat in vitro blood-perfused juxtamedullary nephron technique revealed that Src family kinase inhibition attenuated afferent arteriolar contractile responses to ANG II. In rat PVSMCs maintained 3–5 days in culture, Src family kinase inhibition blunted the peak [Ca2+]i response to ANG II and abrogated the plateau phase of the response. Immunoprecipitation and immunoblotting studies confirmed the effect of ANG II to rapidly activate c-Src in cultured PVSMCs. We conclude that ANG II acts on PVSMCs to provoke rapid activation of Src family kinase(s), including c-Src, which contributes to the [Ca2+]i response and, ultimately, to vasoconstriction. These observations indicate that the physiological context within which the Src family of tyrosine kinases is viewed to influence vascular function must include not only proliferative responses, but also agonist-induced contractile function in the renal microvasculature. Additional studies are required to determine if Src is involved in afferent arteriolar contractile responses to other agonists, efferent arteriolar contractile responses and/or vasoconstrictor responses in other microvascular beds. Further investigation in this arena may unveil mechanisms through which chronic pro-proliferative states (such as ANG II-dependent hypertension) could influence arteriolar tone via tyrosine kinase-mediated events.
Acknowledgments
The authors thank Rachel W. Fallet for excellent technical support.
GRANTS
These studies were supported by funding from the National Institutes of Health (R01 DK39202) and a Predoctoral Fellowship from the Heartland Affiliate of the American Heart Association.
References
- 1.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage-and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Nat Acad Sci USA. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banes A, Florian JA, Watts SW. Mechanisms of 5-hydroxytryptamine2A receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J Pharmacol Exp Ther. 1999;291:1179–1187. [PubMed] [Google Scholar]
- 4.Brinson AE, Harding T, Diliberto PA, He YQ, Li X, Hunter D, Herman B, Earp HS, Graves LM. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor: Dependence on calcium and the actin cytoskeleton. J Biol Chem. 1998;273:1711–1718. doi: 10.1074/jbc.273.3.1711. [DOI] [PubMed] [Google Scholar]
- 5.Carmines PK, Fallet RW, Che Q, Fujiwara K. Tyrosine kinase involvement in renal arteriolar constrictor responses to angiotensin II. Hypertension. 2001;37:569–573. doi: 10.1161/01.hyp.37.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmines PK, Fowler BC, Bell PD. Segmentally distinct effects of depolarization on intracellular [Ca2+] in renal arterioles. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;265:F677–F685. doi: 10.1152/ajprenal.1993.265.5.F677. [DOI] [PubMed] [Google Scholar]
- 7.Carmines PK, Inscho EW. Perfusate composition modulates in vitro renal microvascular pressure responsiveness in a segment-specific manner. Microvasc Res. 1992;43:347–351. doi: 10.1016/0026-2862(92)90031-j. [DOI] [PubMed] [Google Scholar]
- 8.Carter RW, Kanagy NL. Tyrosine kinases regulate intracellular calcium during α2-adrenergic contraction in rat aorta. Am J Physiol Heart Circ Physiol. 2002;283:H1673–H1680. doi: 10.1152/ajpheart.01034.2001. [DOI] [PubMed] [Google Scholar]
- 9.Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;246:F349–F358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- 10.Che Q, Carmines PK. Angiotensin II triggers EGFR tyrosine kinase-dependent Ca2+ influx in afferent arterioles. Hypertension. 2002;40:700–706. doi: 10.1161/01.hyp.0000035524.10948.93. [DOI] [PubMed] [Google Scholar]
- 11.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. The EMBO Journal. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley ED, Owada KM, Marumo F, Hirata Y. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension. 1999;33:201–206. doi: 10.1161/01.hyp.33.1.201. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 14.Endlich N, Endlich K, Taesch N, Helwig JJ. Culture of vascular smooth muscle cells from small arteries of the rat kidney. Kidney Int. 2000;57:2468–2475. doi: 10.1046/j.1523-1755.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- 15.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaver MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 17.Hall DA, Carmines PK, Sansom SC. Dihydropyridine-sensitive Ca2+ channels in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2000;278:F97–F103. doi: 10.1152/ajprenal.2000.278.1.F97. [DOI] [PubMed] [Google Scholar]
- 18.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 19.Harris RC. EGF receptor activation by G-protein coupled receptors. Kidney Int. 2000;58:898–899. doi: 10.1046/j.1523-1755.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- 20.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 21.Hu XQ, Singh N, Mukhopadhyay D, Akbarali HI. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem. 1998;273:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- 22.Ikenaga H, Fallet RW, Carmines PK. Contribution of tubuloglomerular feedback to renal arteriolar angiotensin II responsiveness. Kidney Int. 1996;49:34–39. doi: 10.1038/ki.1996.5. [DOI] [PubMed] [Google Scholar]
- 23.Ikenaga H, Fallet RW, Carmines PK. Basal nitric oxide production curtails arteriolar vasoconstrictor responses to ANG II in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F365–F373. doi: 10.1152/ajprenal.1996.271.2.F365. [DOI] [PubMed] [Google Scholar]
- 24.Ishida M, Ishida T, Thomas SM, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ Res. 1998;82:7–12. doi: 10.1161/01.res.82.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Nagai R, Sakai T, Aikawa M, Kuro-o M, Kobayashi N, Shirato I, Inagami T, Oshi M, Suzuki N, Oba S, Mise N, Tojo A, Hirata Y, Goto A, Yazaki Y, Omata M. Diversity and variability of smooth muscle phenotypes of renal arterioles as revealed by myosin isoform expression. Kidney Int. 1995;48:372–382. doi: 10.1038/ki.1995.305. [DOI] [PubMed] [Google Scholar]
- 26.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: Differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res. 2000;87:551–557. doi: 10.1161/01.res.87.7.551. [DOI] [PubMed] [Google Scholar]
- 27.Makino H, Kashihara N, Sugiyama H, Kanao K, Sekikawa T, Okamoto K, Maeshima Y, Ota Z, Nagai R. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes. 1996;45:488–495. doi: 10.2337/diab.45.4.488. [DOI] [PubMed] [Google Scholar]
- 28.Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, Todoroki-Ikeda N, Ito M, Matsuzaki M. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- 29.Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Kuroda N, Nakayama H, Kiyoku H, Hiroi M, Kurashige T, Enzan H. CD34 expression as a novel marker of transformed mesangial cells in biopsied glomerular diseases. J Pathol. 1999;189:105–111. doi: 10.1002/(SICI)1096-9896(199909)189:1<105::AID-PATH388>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol. 1999;77:606–617. [PubMed] [Google Scholar]
- 31.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- 32.Ohishi K, Carmines PK, Inscho EW, Navar LG. EDRF-angiotensin II interactions in rat juxtamedullary afferent and efferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol. 1992;263:F900–F906. doi: 10.1152/ajprenal.1992.263.5.F900. [DOI] [PubMed] [Google Scholar]
- 33.Poenie M. Alteration of intracellular fura-2 fluorescence by viscosity: A simple correction. Cell Calcium. 1990;11:85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RE. Role of the extracellular signal-regulated kinase (Erk) signal transduction cascade in α2 adrenoceptor-mediated vasoconstriction in porcine palmar lateral vein. Br J Pharmacol. 2001;133:859–866. doi: 10.1038/sj.bjp.0704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenone S, Bruno O, Ranise A, Bondavalli F, Brullo C, Fossa P, Mosti L, Menozzi G, Carraro F, Naldini A, Bernini C, Manetti F, Botta M. New pyrazolo[3,4-d]pyrimidines endowed with A431 antiproliferative activity and inhibitory properties of Src phosphorylation. Bioorg Med Chem Lett. 2004;14:2511–2517. doi: 10.1016/j.bmcl.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Schieffer B, Paxton WG, Chai Q, Marrero MB, Bernstein KE. Angiotensin II controls p21ras activity via pp60c-src. J Biol Chem. 1996;271:10329–10333. doi: 10.1074/jbc.271.17.10329. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF. Regulation of smooth muscle α-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998;54:1175–1187. doi: 10.1046/j.1523-1755.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 39.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 40.Tolloczko B, Turkewitsch P, Choudry S, Bisotto S, Fixman ED, Martin JG. Src modulates serotonin-induced calcium signaling by regulating phosphatidylinositol 4,5-bisphosphate. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1305–L1313. doi: 10.1152/ajplung.00304.2001. [DOI] [PubMed] [Google Scholar]
- 41.Touyz RM, Schiffrin EL. Angiotensin II regulates vascular smooth muscle cell pH, contraction, and growth via tyrosine kinase-dependent signaling pathways. Hypertension. 1997;30:222–229. doi: 10.1161/01.hyp.30.2.222. [DOI] [PubMed] [Google Scholar]
- 42.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 43.Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19:441–449. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Touyz RM, Wu XH, He G, Salomon S, Schiffrin EL. Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2002;39:479–485. doi: 10.1161/hy02t2.102909. [DOI] [PubMed] [Google Scholar]
- 45.Vojtechová M, Tuhácková Z, Hlavácek J, Velek J, Sovová V. The v-Src and c-Src tyrosine kinases immunoprecipitated from Rous sarcoma virus-transformed cells display different peptide substrate specificities. Arch Biochem Biophys. 2004;421:277–282. doi: 10.1016/j.abb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Wijetunge S, Lymn JS, Hughes AD. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60c-src kinase activity. Br J Pharmacol. 2000;129:1347–1354. doi: 10.1038/sj.bjp.0703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z-W, Wang J, Zheng T, Altura BT, Altura BM. Low [Mg2+]o induces contraction of cerebral arteries: roles of tyrosine and mitogen-activated protein kinases. Am J Physiol Heart Circ Physiol. 2000;279:H152–H194. doi: 10.1152/ajpheart.2000.279.1.H185. [DOI] [PubMed] [Google Scholar]
- 48.Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase: Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 49.Zubkov AY, Rollins S, Parent AD, Zhang J. Mechanism of endothelin-1-induced contraction in rabbit basilar artery. Stroke. 2000;31:526–533. doi: 10.1161/01.str.31.2.526. [DOI] [PubMed] [Google Scholar]
- 50.Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]