Abstract
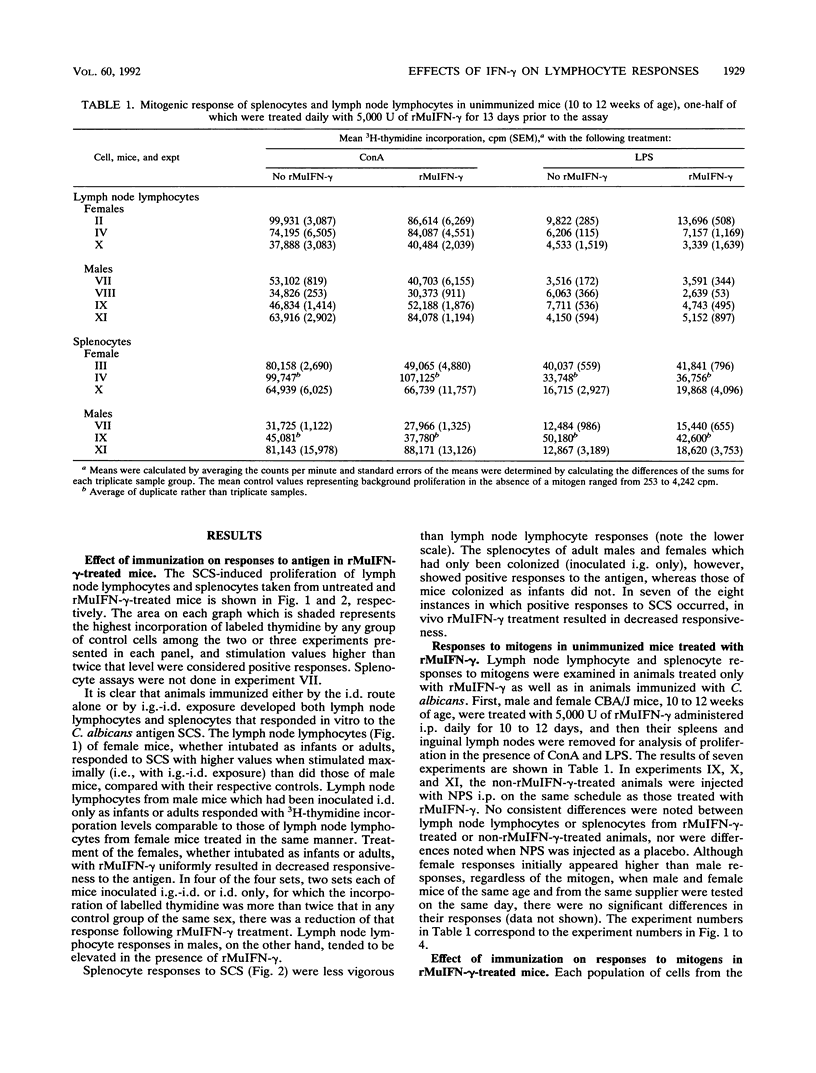
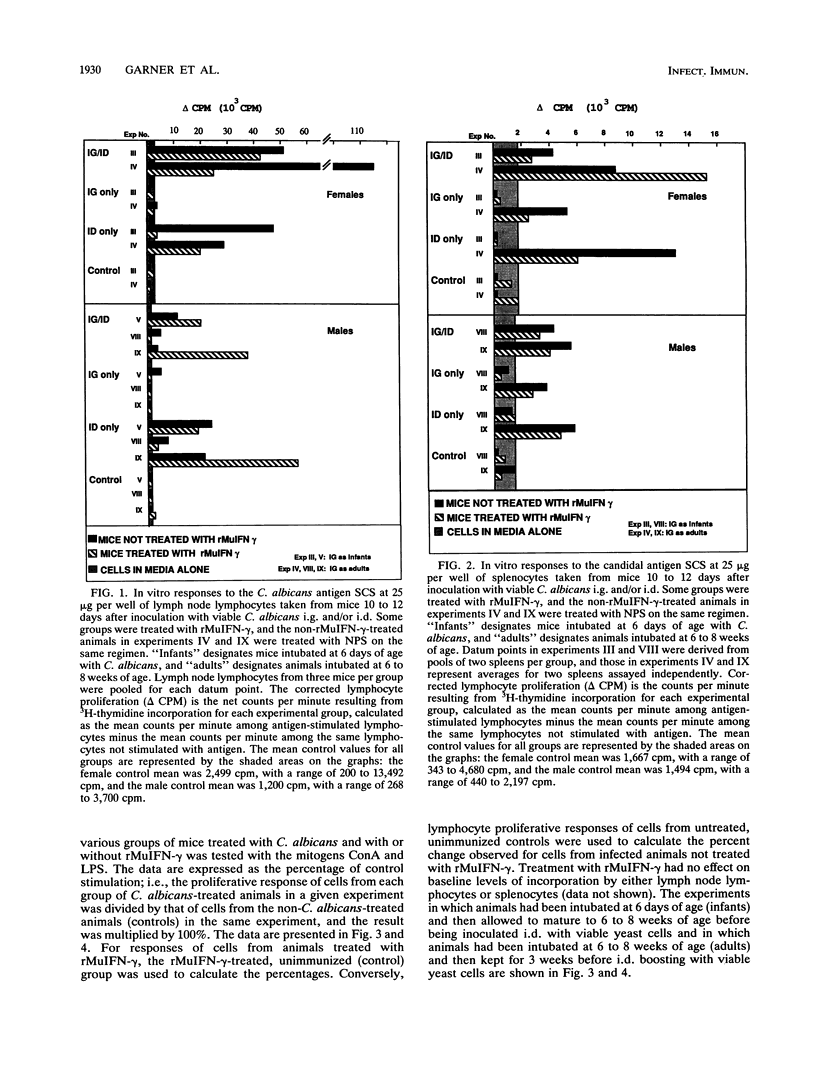
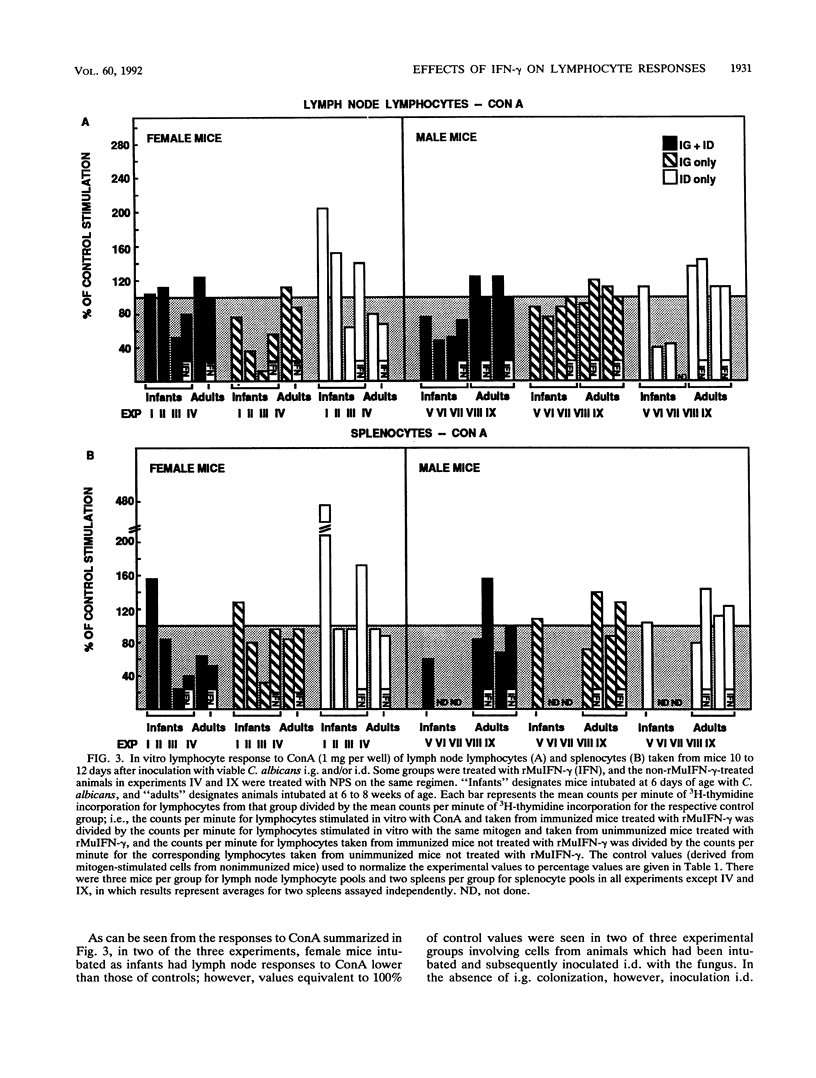
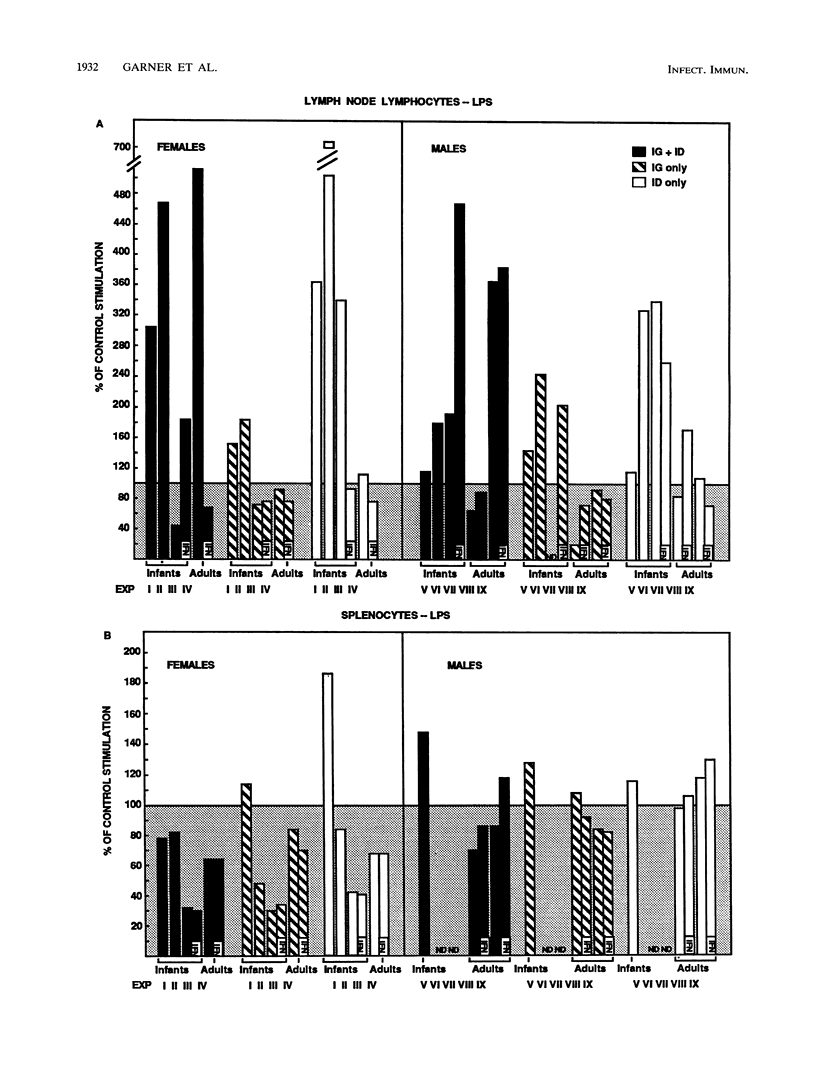
The effect of in vivo administration of recombinant murine gamma interferon (rMuIFN-gamma) on in vitro proliferation of lymphocytes to Candida antigens and lectins was examined in naive CBA/J mice and in similar mice colonized with Candida albicans by intragastric (i.g.) intubation and/or inoculated intradermally (i.d.) with the fungus. Lymph node lymphocyte and splenic lymphocyte (splenocyte) responses to soluble cytoplasmic substances derived from C. albicans varied with the route of inoculation of the fungus, the sex of the animal, and the presence or absence of rMuIFN-gamma treatment. In the absence of rMuIFN-gamma treatment, lymphoid cells from lymph nodes draining the site of the i.d. lesion responded well to soluble cytoplasmic substances. Colonization of the gut of female mice with C. albicans either had no effect or promoted better lymph node responses when such animals were also challenged i.d., whereas gut colonization of males followed by i.d. challenge appeared to have a suppressive influence on the level of proliferation in response to antigens in vitro. Antigen-specific splenocyte responses could be detected as well, and they were best in animals inoculated i.g.-i.d. or i.d. only. With the exception of lymph node lymphocytes from male mice, treatment of infected animals, regardless of the route of infection, with rMuIFN-gamma frequently resulted in lowered responses to antigens when comparable treatment groups were examined. With respect to mitogen stimulation, infection with C. albicans, especially i.g. or i.g.-i.d., resulted in a population of lymph node lymphocytes with lower-than-normal responses to concanavalin A but higher-than-normal responses to lipopolysaccharide (LPS). Splenocyte responses to mitogens were not altered as dramatically as the responses of lymph node lymphocytes, but splenocytes from female mice had a suppressed response regardless of the route of exposure to C. albicans, and those from mice which were maximally stimulated with C. albicans, i.e., inoculated i.g.-i.d., also had a suppressed response to concanavalin A. Treatment with rMuIFN-gamma either had no effect on the subsequent splenocyte responses or boosted subnormal mitogen responses toward the normal range. Collectively, these data illustrate that exposure to both C. albicans and rMuIFN-gamma influenced the responses to mitogen and C. albicans antigen of lymph node lymphocyte and splenocyte populations, as detected in vitro by lymphoproliferation. Treatment with rMuIFN-gamma often resulted in increased responsiveness to a B cell mitogen, LPS, and decreased responsiveness to a C. albicans antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect Immun. 1987 Dec;55(12):2951–2955. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhisel-Broadbent J., Camargo E. E., Jaffe H. S., Lederman H. M. Recombinant human interferon-gamma as adjunct therapy for Aspergillus infection in a patient with chronic granulomatous disease. J Infect Dis. 1991 Apr;163(4):908–911. doi: 10.1093/infdis/163.4.908. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Tedder T. F., Griffin J. D., Freedman A. S., Fisher D. C., Daley J., Nadler L. M. Preexposure of resting B cells to interferon-gamma enhances their proliferative response to subsequent activation signals. Cell Immunol. 1987 May;106(2):355–365. doi: 10.1016/0008-8749(87)90178-x. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Browning J. L., Ribolini A. Interferon blocks interleukin 1-induced prostaglandin release from human peripheral monocytes. J Immunol. 1987 May 1;138(9):2857–2863. [PubMed] [Google Scholar]
- Brummer E., Hanson L. H., Restrepo A., Stevens D. A. In vivo and in vitro activation of pulmonary macrophages by IFN-gamma for enhanced killing of Paracoccidioides brasiliensis or Blastomyces dermatitidis. J Immunol. 1988 Apr 15;140(8):2786–2789. [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Fungicidal mechanisms of activated macrophages: evidence for nonoxidative mechanisms for killing of Blastomyces dermatitidis. Infect Immun. 1987 Dec;55(12):3221–3224. doi: 10.1128/iai.55.12.3221-3224.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester T. J., Paucker K., Merigan T. C. Suppression of mouse antibody producing spleen cells by various interferon preparations. Nature. 1973 Nov 9;246(5428):92–94. doi: 10.1038/246092a0. [DOI] [PubMed] [Google Scholar]
- De Maeyer-Guignard J., Cachard A., De Maeyer E. Delayed-type hypersensitivity to sheep red blood cells: inhibition of sensitization by interferon. Science. 1975 Nov 7;190(4214):574–576. doi: 10.1126/science.1188355. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Domer J. E., Hector R. F. Enhanced immune responses in mice treated with penicillin-tetracycline or trimethoprim-sulfamethoxazole when colonized intragastrically with Candida albicans. Antimicrob Agents Chemother. 1987 May;31(5):691–697. doi: 10.1128/aac.31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Fleischmann J., Wu-Hsieh B., Howard D. H. The intracellular fate of Histoplasma capsulatum in human macrophages is unaffected by recombinant human interferon-gamma. J Infect Dis. 1990 Jan;161(1):143–145. doi: 10.1093/infdis/161.1.143. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Garner R. E., Kuruganti U., Czarniecki C. W., Chiu H. H., Domer J. E. In vivo immune responses to Candida albicans modified by treatment with recombinant murine gamma interferon. Infect Immun. 1989 Jun;57(6):1800–1808. doi: 10.1128/iai.57.6.1800-1808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms G., Zwingenberger K., Chéhadé A. K., Talhari S., Racz P., Mouakeh A., Douba M., Näkel L., Naiff R. D., Kremsner P. G. Effects of intradermal gamma-interferon in cutaneous leishmaniasis. Lancet. 1989 Jun 10;1(8650):1287–1292. doi: 10.1016/s0140-6736(89)92686-x. [DOI] [PubMed] [Google Scholar]
- Heron I., Berg K., Cantell K. Regulatory effect of interferon on T cells in vitro. J Immunol. 1976 Oct;117(4):1370–1373. [PubMed] [Google Scholar]
- Hershman M. J., Polk H. C., Jr, Pietsch J. D., Kuftinec D., Sonnenfeld G. Modulation of Klebsiella pneumoniae infection of mice by interferon-gamma. Clin Exp Immunol. 1988 Jun;72(3):406–409. [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Ellis D. A., Black P. H., Monaco A. P., Wood M. L. Immunosuppressive effects of an interferon preparation in vivo. Transplantation. 1974 Feb;17(2):234–236. doi: 10.1097/00007890-197402000-00014. [DOI] [PubMed] [Google Scholar]
- Kadish A. S., Tansey F. A., Yu G. S., Doyle A. T., Bloom B. R. Interferon as a mediator of human lymphocyte suppression. J Exp Med. 1980 Mar 1;151(3):637–650. doi: 10.1084/jem.151.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Fiers W., Strom T. B. Cloned human interferon-gamma, but not interferon-beta or -alpha, induces expression of HLA-DR determinants by fetal monocytes and myeloid leukemic cell lines. J Immunol. 1984 Jan;132(1):240–245. [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Mann E. A., Markovic S. N., Murasko D. M. Inhibition of lymphocyte recirculation by murine interferon: effects of various interferon preparations and timing of administration. J Interferon Res. 1989 Feb;9(1):35–51. doi: 10.1089/jir.1989.9.35. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Miörner H., Landström L. E., Larner E., Larsson I., Lundgren E., Strannegård O. Regulation of mitogen-induced lymphocyte DNA synthesis by human interferon of different origins. Cell Immunol. 1978 Jan;35(1):15–24. doi: 10.1016/0008-8749(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Morikawa K., Kubagawa H., Suzuki T., Cooper M. D. Recombinant interferon-alpha, -beta, and -gamma enhance the proliferative response of human B cells. J Immunol. 1987 Aug 1;139(3):761–766. [PubMed] [Google Scholar]
- Morrison C. J., Brummer E., Isenberg R. A., Stevens D. A. Activation of murine polymorphonuclear neutrophils for fungicidal activity by recombinant gamma interferon. J Leukoc Biol. 1987 May;41(5):434–440. doi: 10.1002/jlb.41.5.434. [DOI] [PubMed] [Google Scholar]
- Morrison C. J., Brummer E., Stevens D. A. In vivo activation of peripheral blood polymorphonuclear neutrophils by gamma interferon results in enhanced fungal killing. Infect Immun. 1989 Oct;57(10):2953–2958. doi: 10.1128/iai.57.10.2953-2958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E., Mather F. J. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1980 Jan;27(1):140–149. doi: 10.1128/iai.27.1.140-149.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Berman J. D., Wright S. D. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. J Infect Dis. 1988 May;157(5):973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Yasuda I., Yu C., Kato K. Prevention by gamma interferon of fatal infection with Listeria monocytogenes in mice treated with cyclosporin A. Infect Immun. 1988 Aug;56(8):2011–2015. doi: 10.1128/iai.56.8.2011-2015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischedda F., Kirchner H., Leanderson T. The role of gamma interferon in B-cell activation. Scand J Immunol. 1987 Mar;25(3):315–318. doi: 10.1111/j.1365-3083.1987.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Suppression of lymphocyte blastogenesis by Candida albicans. Clin Immunol Immunopathol. 1978 Jul;10(3):298–305. doi: 10.1016/0090-1229(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Rozes K. R., Lee S. H., Ngan J. Effect of priming on interferon inhibition of con A induced spleen cell blastogenesis. Nat New Biol. 1973 Sep 5;245(140):16–18. doi: 10.1038/newbio245016a0. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Peschel C., Paul W. E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988 Apr 1;140(7):2121–2127. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. Time and dosage dependence of immunoenhancement by murine type II interferon preparations. Cell Immunol. 1978 Oct;40(2):285–293. doi: 10.1016/0008-8749(78)90336-2. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Tweardy D. J., Fujiwara H., Scillian J. J., Ellner J. J. Concurrent enhancement of monocyte immunoregulatory properties and effector functions by recombinant interferon-gamma. Cell Immunol. 1986 Jun;100(1):34–46. doi: 10.1016/0008-8749(86)90004-3. [DOI] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]


