Abstract
Lipopolysaccharide (LPS), a major component of the outer membranes of Gram-negative bacteria, is comprised of a polysaccharide chain attached to a lipid A base, which contains a disaccharide headgroup with two negative phosphate groups and at least four acyl chains. Lipid A is an essential component of the membranes of a large number of bacteria and also a substrate for a wide variety of proteins. Here we report the synthesis of a nitroxide spin-labeled lipid A, characterize its localization at the membrane bilayer surface, and demonstrate that it remains a viable substrate for the E. coli lipid flippase, MsbA.
Lipopolysaccharide (LPS) is the major component of the outer leaflet of the outer membranes of Gram-negative bacteria such as E. coli and S. typhimurium and is essential to membrane stability and impermeability. This large lipid is comprised of a polysaccharide chain attached to a lipid A base, which contains two phosphate groups and thus an overall negative charge. Lipid A is synthesized in the cytoplasm of the cell before being inserted into the inner leaflet of the inner membrane, where it is flipped across the membrane by the ATP-binding cassette protein MsbA [1]. Modifications of lipid A are made while in the inner membrane, such as the addition of acyl chains by PagP and the addition of polysaccharide to make LPS [2]. And in some bacteria, the phosphate groups of lipid A are modified by the arabinose transferase protein ArnT before leaving the inner membrane [3]. It has been proposed that LptA, LptB, Imp/RlpB and other accessory proteins are responsible for its transport across the periplasm and targeting to the outer membrane [4;5]. As a result, not only is lipid A an essential component of the outer membranes of many bacteria, but it is also a substrate for a wide variety of proteins. Therefore, we have synthesized a spin-labeled version of E. coli lipid A for use in a variety of biophysical approaches to greatly expand the study of lipid-protein and lipid-peptide interactions.
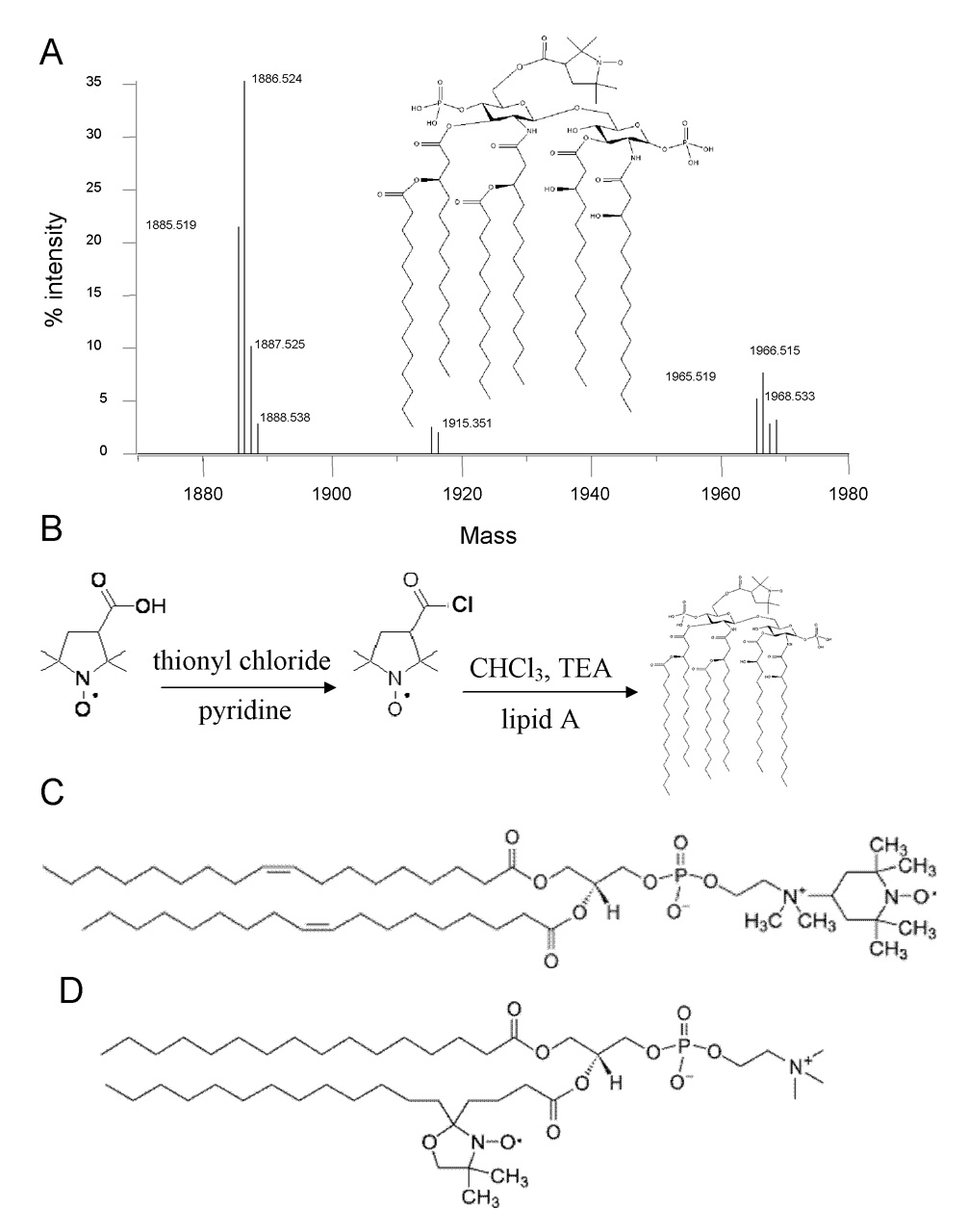
To create the spin-labeled lipid A molecule (lipid A-SL), a proxyl (2,2,5,5-tetramethyl-1-pyrrolidinyloxy) group was modified to react with the hydroxyl group on LPS-free lipid A (Figure 1B). First, 3-carboxy-proxyl (Molecular Probes) was dissolved in benzene followed by the addition of pyridine and one equivalent of thionyl chloride and stirred at room temperature for 1 h. The solvent was evaporated using a stream of nitrogen and the remaining solute was dissolved in 1 mL CHCl3 (Aldrich) to produce the proxyl-acid chloride [6], which was then used to covalently spin-label lipid A. To accomplish the lipid A labeling reaction, 1 mL of CHCl3 was added to 1 mg of diphosphoryl lipid A from E. coli K12 lacking LPS (List Biological Laboratories), followed by the addition of 0.2 µL of triethylamine and 3 µL (1.3 molar excess) of the proxyl-acid chloride solution generated immediately prior to this step. The mixture was stirred at 4°C for 19 h and resulted in the formation of the final lipid A-SL product, which is spin-labeled at the primary alcohol site where LPS ordinarily attaches (Figure 1A inset). This position was chosen not only for the faster reactivity of its primary alcohol in the presence of secondary alcohols [7], but also for its nonperturbing location since the lipid typically accommodates a considerably larger moiety at this position.
Figure 1.
A) ESI-FTMS analysis of lipid A-SL. One µL (1 mg/mL) lipid A-SL was flow-injected at 10 µL/min through a high-performance liquid chromatograph (Agilent) using water:methanol (10:90) containing 0.1% formic acid. The compound was detected by a 7.0T Fourier transform ion cyclotron resonance mass spectrometer (Varian/IonSpec) with a Z-spray ESI source (Waters Corporation) using Omega software for data collection and analysis. Ion guides were optimized for m/z of 1000 and detection was made in the positive mode for the m/z range of 900–1000. The detected spectrum was deconvoluted to get the molecular weights plotted above. Inset) Structure of lipid A-SL. Lipid A generally contains 4–7 acyl chains. The site of the spin label attachment is a hydroxyl moiety in unmodified lipid A and is the site of polysaccharide attachment in LPS. B) Basic reaction scheme for synthesis of lipid A-SL from lipid A (1967 Da with 6 acyl chains and two phosphates) and 3-carboxy proxyl. C) Structures of TEMPO-PC and D) 5-doxyl-PC.
To characterize the successful formation of the lipid A-SL, the final product was first analyzed by mass spectrometry (MS). Lipid A-SL was observable in the positive ion mode on a Varian electrospray ionization-Fourier transform mass spectrometer (ESI-FTMS) where masses of 1886.5 and 1966.5 Da were detected, corresponding to a single spin label (169 Da) attached to lipid A containing six acyl chains and one or two phosphate groups, respectively (Figure 1A). Higher masses were not observed, confirming that each lipid A contains a single spin label.
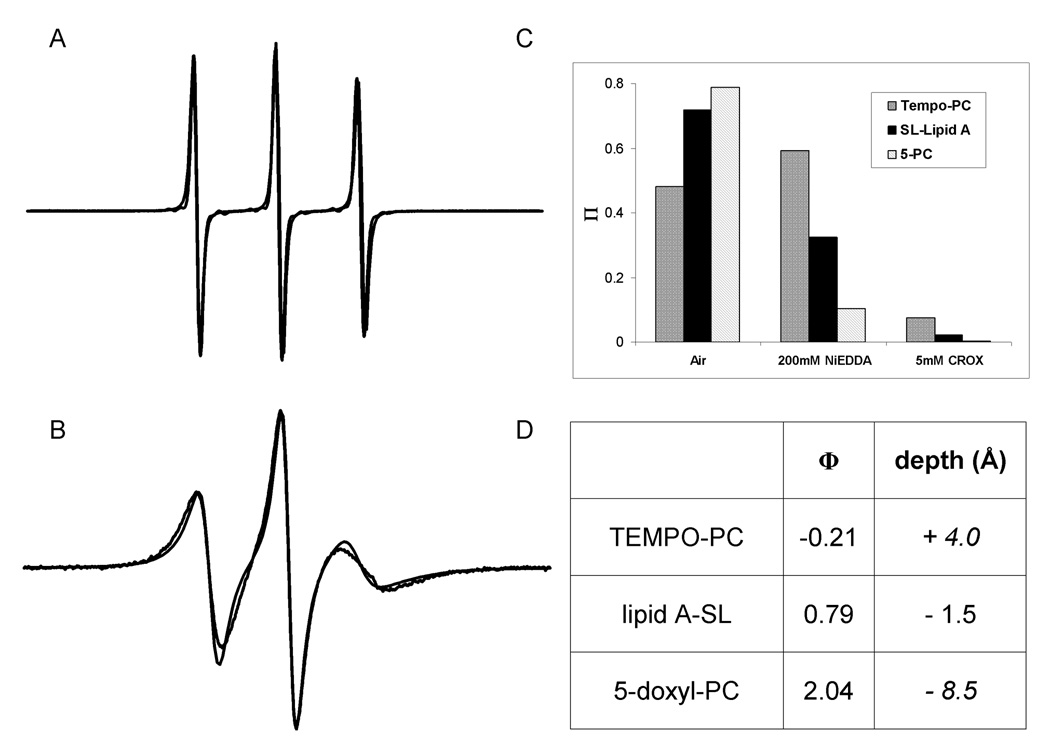
Lipid A-SL was also subjected to analysis by electron paramagnetic resonance (EPR) spectroscopy to detect and quantify the attached spin label. The proxyl group contains a stable free radical that is EPR-detectable. Figure 2A shows the EPR spectrum of lipid A-SL in 50 mM NaPO4, pH 7.0, 10% glycerol, 0.01% dodecylmaltoside buffer, and Figure 2B shows lipid A-SL incorporated at 1 mol% into washed 20:40:40 mol% DOPE:DOPG:DOPC (Avanti Polar Lipids) lipid bilayers. The spectrum of lipid A-SL in buffer is nearly identical to that of 3-carboxy-proxyl in the same buffer due to the rapid tumbling of both small molecules in the absence of membranes. Upon incorporation into lipid membranes, the lipid A-SL spectrum shows significantly slower motion (Figure 2B), due to its inability to freely tumble once restricted within the bilayer. In contrast, the spectrum of 3-carboxy-proxyl in the presence of lipid bilayers is identical to its spectrum in buffer (spectrum not shown).
Figure 2.
EPR characterization of lipid A-SL. A) X-band EPR spectrum recorded over 100 Gauss of lipid A-SL in aqueous buffer and B) incorporated into bilayers. Black lines represent the experimental spectra and gray lines correspond to the simulated spectra for the reported τc values. C) Power saturation data. Air represents the paramagnetic broadening agent oxygen, and nickel ethylenediaminediacetate (NiEDDA) and chromium oxalate (CROX) are neutral and negatively charged water-soluble agents, respectively. ∏ is a dimensionless accessibility parameter calculated from EPR power saturation data. D) Known depths from the phosphate groups for TEMPO-PC and 5-doxyl-PC [12] were used to calculate an approximate depth for lipid A-SL: depth (Å) = −5.56Φ + 2.8 and Φ = ln[∏ (air)/∏(Ni)].
Quantitation of the number of spins in the reconstituted sample prior to washing away excess proxyl-acid was carried out using well-established double integration and spectral subtraction methods (e.g. [8,9]). Analysis indicated that there was approximately 22% excess proxyl-acid for each lipid A-SL, which nearly matches the amount of excess proxyl-acid chloride during synthesis and verifies a complete spin-labeling reaction. The acid chloride reactivity is rapidly quenched by exposure to air and therefore the small amount of excess proxyl-acid is readily removed without modifying other lipids during the reconstitution procedure. The resulting spectrum contains a single motional component with slightly slowed rotational motion (the rotational correlation time, τc, of the spin label slows from 0.8 ns to 16 ns; calculated using the simulation program described in [10] with fits shown in rid=Figure 2AB). This is consistent with its incorporation into the bilayer and with a single labeling site.
To further characterize the position of the spin label on lipid A-SL and to verify its correct orientation in the bilayer, accessibility measurements to paramagnetic reagents were carried out using EPR spectroscopy. The collision rate of the spin label with reagents such as oxygen and metal complexes (e.g., NiEDDA, CROX) can be readily detected by EPR power saturation methods, and is a proven method of determining the local environment of a spin label [7;9;11]. The EPR accessibility data shown in Figure 2C indicate that the spin label on lipid A-SL is not deeply buried within the bilayer because it shows less accessibility to oxygen and more accessibility to the metal complexes than the 5-doxyl-PC (Avanti Polar Lipids) control, which is located within the lipid bilayer at approximately 8.5 Å below the phosphate groups (structure shown in Figure 1C). The spin label is also not freely accessible to the solution phase because the data indicate that it comes into contact with more oxygen and less of the water-soluble metal complexes than TEMPO-PC (Avanti Polar Lipids), where the spin label is attached to the headgroup of the lipid, above the phosphate groups (structure shown in Figure 1D). Rather, the data suggest that the spin label on lipid A-SL is located approximately 1.5 Å below the lipid phosphates at the bilayer surface, as calculated using a calibration equation based on the positions of the two spin label controls (Figure 2D). This result is consistent with the spin label moiety being somewhat hydrophobic and tethered to lipid A by a short 4-bond flexible linker, allowing the spin label to insert slightly into the lipid bilayer.
Finally, to verify its use as a functional substrate for just one of its interaction partners, lipid A-SL was shown to stimulate the ATPase activity of E. coli MsbA. The ATPase activity of MsbA is known to be stimulated by the presence of its native substrate lipid A [12]. MsbA has also been shown to transport other hydrophobic compounds, such as Hoescht33342 and ethidium bromide in the absence of lipid A, however the presence the lipid A inhibits transport of these other compounds [13;14]. Therefore, ATPase activity of cysteine-free (C88S/C315S) MsbA was tested in the presence of 25-fold molar excess lipid A-SL and compared to 25x lipid A and 250x E. coli inner membrane lipids (60:30:10 phosphotidylethanolamine, phosphotidylglycerol, and cardiolipin; Avanti Polar Lipids) after addition of 3 mM Mg[γ-32P]ATP. The hydrolysis reaction was stopped at five time points between 0 and 2 min by addition of perchloric acid and placement on ice. γ-32Pi release was quantitated after organic extraction in a liquid scintillation counter (Perkin Elmer). The initial slope (v0) of the reaction in the presence of lipid A-SL was 175 nmol/mg/min, which is slightly faster than unlabeled lipid A (148 nmol/mg/min), and is nearly 50% more than observed for the lipid control (119 nmol/min/mg), confirming that lipid A-SL remains a functional substrate.
In summary, the methods used to synthesize the lipid A-SL allow us to selectively attach the spin label to the primary alcohol of lipid A, and the MS and EPR characterizations allow us to confirm that the lipid A is only labeled once and at the correct position. Proposed uses of this new spin-labeled lipid A include a variety of biophysical characterization methods. Applications include EPR studies on the binding of this substrate by the proteins involved in its synthesis, modification, and transport. Additionally, it may prove useful in NMR studies as a paramagnetic relaxation agent or in fluorescence quenching experiments. Once incorporated into the bilayer, it may also prove useful for reporting on the membrane surface binding of peptides or proteins. It is anticipated that specific applications utilizing lipid A-SL will facilitate studies of its movement from the cytoplasm to the outer membrane by identifying its exact location within each of its carrier and transport proteins as well as a reporter for its environment at the surface of the membrane.
Supplementary Material
Acknowledgements
We thank Dr. Jimmy Feix for helpful discussions and critical reading of the manuscript, and Kathryn Westfahl is gratefully acknowledged for providing the activity data. The use of the ESI-FTMS was made possible by the generosity of the Kern Family Foundation, the Walter Schroeder Foundation, the Stackner Family Foundation, Associated Bank, the Raymond and Bernice Eschenburg Fund of the Greater Milwaukee Foundation, and an anonymous donor. This work was supported by the NIH (GM70642 and AI58024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doerrler WT, Reedy MC, Raetz CR. An Escherichia coli Mutant Defective in Lipid Export. J.Biol.Chem. 2001;276:11461–11464. doi: 10.1074/jbc.C100091200. [DOI] [PubMed] [Google Scholar]
- 2.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A Modification Systems in Gram-Negative Bacteria. Ann.Rev.Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J.Biol.Chem. 2001;276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- 4.Sperandeo P, Cescutti R, Villa R, Di Benedetto C, Candia D, Deho G, Polissi A. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J.Bacteriol. 2007;189:244–253. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, McCandlish AC, Gronenberg LS, Chng SS, Silhavy TJ, Kahne D. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. PNAS. 2006;103:11754–11759. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu D, Hall E, Ober CK, Moscicki JK, Freed JH. Translational Diffusion in Polydisperse Polymer Samples Studied by Dynamic Imaging of Diffusion ESR. J.Phys.Chem. 1996;100:15856–15866. [Google Scholar]
- 7.Morrison RT, Boyd RN. Organic Chemistry. Boston: Allyn and Bacon, Inc.; 1973. p. 603. [Google Scholar]
- 8.Klug CS, Feix JB. Guanidine hydrochloride unfolding of a transmembrane beta-strand in FepA using site-directed spin labeling. Protein Sci. 1998;7:1469–1476. doi: 10.1002/pro.5560070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klug CS, Feix JB. Methods and Applications of Site-Directed Spin Labeling EPR Spectroscopy. In: Correia JJ, Detrich HW, editors. Methods in Cell Biology Biophysical Tools for Biologists, Volume One: In Vitro Techniques. Academic Press; 2008. pp. 617–658. [DOI] [PubMed] [Google Scholar]
- 10.Budil DE, Lee S, Saxena S, Freed JH. Nonlinear-least-squares Analysis of Slow-motion EPR Spectra in one and two dimensions using a Modified Levenberg-Marquardt Algorithm. J.Magn.Reson. 1996;120:155–189. [Google Scholar]
- 11.Fanucci GE, Cafiso DS. Recent advances and applications of site-directed spin labeling. Curr.Opin.Struct.Biol. 2006;16:644–653. doi: 10.1016/j.sbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Doerrler WT, Raetz CR. ATPase activity of the MsbA lipid flippase of Escherichia coli. J.Biol.Chem. 2002;277:36697–36705. doi: 10.1074/jbc.M205857200. [DOI] [PubMed] [Google Scholar]
- 13.Reuter G, Janvilisri T, Venter H, Shahi S, Balakrishnan L, van Veen HW. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J.Biol.Chem. 2003;278:35193–35198. doi: 10.1074/jbc.M306226200. [DOI] [PubMed] [Google Scholar]
- 14.Woebking B, Reuter G, Shilling RA, Velamakanni S, Shahi S, Venter H, Balakrishnan L, van Veen HW. Drug-lipid A interactions on the Escherichia coli ABC transporter MsbA. J.Bacteriol. 2005;187:6363–6369. doi: 10.1128/JB.187.18.6363-6369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton LA, McIntyre JO, Flewelling RF. Distance estimate of the active center of D-β-hydroxybutyrate dehydrogenase from the membrane surface. Biochemistry. 1987;26:2117–2130. doi: 10.1021/bi00382a009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.