Abstract
The Caenorhabditis elegans defecation motor program (DMP) is a highly coordinated rhythmic behavior that requires two GABAergic neurons that synapse onto the enteric muscles. One class of DMP mutants, called anterior body wall muscle contraction and expulsion defective (aex) mutants, exhibits similar defects to those caused by the loss of these two neurons. Here, we demonstrate that aex-2 encodes a G-protein–coupled receptor (GPCR) and aex-4 encodes an exocytic SNAP25 homologue. We found that aex-2 functions in the nervous system and activates a Gsα signaling pathway to regulate defecation. aex-4, on the other hand, functions in the intestinal epithelial cells. Furthermore, we show that aex-5, which encodes a pro-protein convertase, functions in the intestine to regulate the DMP and that its secretion from the intestine is impaired in aex-4 mutants. Activation of the Gsα GPCR pathway in GABAergic neurons can suppress the defecation defect of the intestinal mutants aex-4 and aex-5. Lastly, we demonstrate that activation of GABAergic neurons using the light-gated cation channel channelrhodopsin-2 is sufficient to suppress the behavioral defects of aex-2, aex-4, and aex-5. These results genetically place intestinal genes aex-4 and aex-5 upstream of GABAergic GPCR signaling. We propose a model whereby the intestinal genes aex-4 and aex-5 control the DMP by regulating the secretion of a signal, which activates the neuronal receptor aex-2.
Keywords: defecation, receptors, secretion, peptides, channelrhodopsin
The Caenorhabditis elegans defecation motor program (DMP) is a highly coordinated series of three muscle contractions that are executed every 45 sec [Fig. 1A and supporting information (SI) Movie S1]. The cycle is initiated by a posterior body wall muscle contraction (pBoc), followed 2–3 sec later by an anterior body wall muscle contraction (aBoc). About 1 sec after the aBoc, enteric muscles contract, thus causing the expulsion (Exp) of intestinal contents. The process repeats itself ≈45 sec later with little variability in the timing of contractions (1). A genetic screen for mutants that displayed defects in the DMP isolated mutants defective in each of the three muscle contractions, known as pbo, abo, and exp (1). The screen also recovered mutants defective in the last two muscle contractions (aBoc and Exp [aex]) and mutants defective in the cycle periodicity (i.e., longer or shorter than normal DMP cycling times) (1). Molecular studies of these mutants have suggested that the behavior is orchestrated through the communication between the intestine, GABAergic neurons, and muscle.
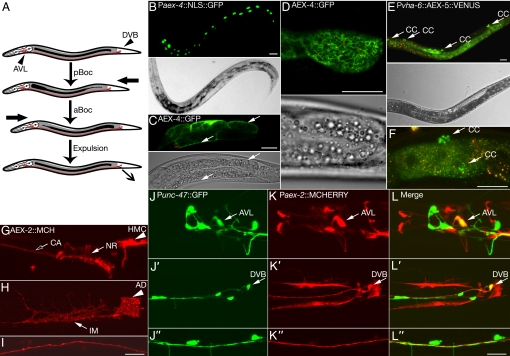
Fig. 1.
aex genes are expressed in neuronal and nonneuronal tissues of C. elegans. (A) Diagram of the C. elegans DMP. First, a posterior contraction (pBoc) forces the intestinal contents to the anterior end of the worm. About 3 sec later, an anterior contraction (aBoc) forces intestinal contents to the posterior end. Within about 1 sec of the aBoc, an enteric muscle contraction in the tail leads to excretion of intestinal contents (Exp). Arrowheads indicate the location of AVL and DVB GABAergic neurons. (B–D) aex-4 is expressed in the intestine. (B) GFP is detected solely in the intestinal nuclei in a transgenic animal that expresses nuclear localized GFP under the aex-4 promoter. (C, D) Functional AEX-4::GFP fusion is expressed in intestinal cells and enriched at the cell surface (arrows; red indicates autofluorescence). (C) Confocal slice through the middle of the intestine. (D) Cell surface view of the posterior intestinal cell. Bright field images are provided for orientation. (E, F) Intestinal AEX-5::VENUS (which is driven by the intestinal promoter Pvha-6) is secreted from the intestine and taken up by coelomocytes (CC, arrows; red indicates autofluorescence). (G″–L″) aex-2 is expressed in the GABAergic neurons AVL and DVB as well as in enteric muscles. (G and I) AEX-2::mCherry is detected in the nerve ring (NR, arrow), ciliary sensory processes (CA, open arrow), nerve cord (I), and head mesodermal cell (HMC, arrowhead). (H) AEX-2::mCherry is expressed in the intestinal muscle (IM, arrow) and anal depressor (AD, arrowhead). (J″–L″) mCherry expressed under the aex-2 promoter is detected in AVL (J–L, arrow) and DVB (J′–L′, arrow) GABAergic neurons. AEX-2::mCherry signal does not significantly overlap with GABAergic GFP in the ventral nerve cord (J″–L″). (Scale bar, 20 μm.) See SI Text for transgenes and clones.
The periodicity of the DMP is regulated by the C. elegans intestine, a single-cell layer tube of polarized epithelial cells joined by gap junctions (2, 3). Intestinal Ca2+ oscillations with ≈45-sec periodicity appear to play a central role in this timing. They consist of a posterior-to-anterior Ca2+ wave whose levels peak in the posterior and anterior intestinal cells just before the pBoc and aBoc contractions, respectively (3–5). Mutations in genes involved in the maintenance of Ca2+ oscillations or in the propagation of Ca2+ waves between cells affect the periodicity of the DMP (3–5). These studies suggest that the intestine may control the timing of the DMP via a Ca2+-dependent process, such as Ca2+-induced exocytosis.
Furthermore, recent work demonstrates that the intestine induces the pBoc by releasing protons (through a Na+/H+ exchanger) onto posterior body wall muscle cells (6). The posterior body wall muscle cells contract in response to the change in pH because they express a proton-gated cation channel (6). By contrast, the Exp step of the DMP is regulated by the GABAergic neurons AVL and DVB (7, 8). These neurons secrete GABA onto enteric muscles that express the excitatory GABA receptor EXP-1 and cause them to contract (9).
If the intestine is the cycle timer and initiates the pBoc step and neurons initiate the Exp step, then how are the intestinal- and neuronal-mediated behaviors synchronized? It seems likely that studies of aex genes will give some insight into how the AVL and DVB neurons are activated, because the behavioral defects of aex mutants are reminiscent of animals whose AVL and DVB GABAergic neurons are laser ablated (7, 8). aex-3 and aex-6 regulate synaptic transmission, probably by regulating exocytosis of neurotransmitter: aex-3 is a guanine nucleotide exchange factor that regulates Rab small guanosine triphosphatase function, and aex-6 (also known as rab-27) is a Rab small guanosine triphosphatase that regulates secretory vesicle exocytosis (10, 11). aex-5 encodes a pro-protein convertase, an enzyme that is copackaged with pro-peptides and processes them to make mature secretory molecules (12, 13). Lastly, Doi and Iwasaki (12) demonstrated that aex-1 is a distant homologue of the synaptic gene unc-13 (or Munc13), which acts in the intestine to regulate the DMP. Thus, prior molecular characterization of aex genes implicates that a secretory event is in control of aBoc and Exp.
Here, we uncover how the intestinal cells regulate the activity of GABAergic neuronal function during the DMP. We cloned aex-4, which encodes a SNAP25 soluble N-ethylmaleimide sensitive factor attachment receptor (SNARE) homologue, and aex-2, which encodes a G-protein–coupled receptor (GPCR). We demonstrate that whereas aex-4 and aex-5 act in the intestine to regulate defecation, aex-2 functions in GABAergic neurons to control this behavior. Disruption of aex-4 function blocks AEX-5 secretion from the intestine. Moreover, GABAergic expression of activated adenylyl cyclase or photoactivatable channelrhodopsin (ChR2) rescues the defecation defects of aex-2, aex-4, and aex-5. We propose a model in which intestinal aex genes, aex-4 and aex-5, regulate secretion of a signal that activates the GPCR aex-2 in AVL and DVB, which, in turn, activates these neurons to complete the DMP.
Results
aex Mutants Are Primarily Defective in Exp and Only Mildly Defective in aBoc.
To understand better how the DMP operates, we carefully characterized the defecation defects of each of the aex mutants. aex mutants are primarily defective in the Exp step (Figs. S1 and S2). Surprisingly, aex mutants have only slightly fewer aBoc contractions per defecation cycle than WT; however, those aBoc contractions are usually significantly later in the cycle than in a WT DMP (Figs. S1 and S2). Therefore, the aBoc defects of most aex mutants are relatively mild when compared with the Exp defects. Most aex mutants have a relatively normal cycle length (or period), although aex-5 did exhibit a slightly longer defecation cycle period (Fig. S1). The normal cycle periodicity suggests that these mutants do not have a defect in cycle time generation. Taken together, these results suggest that aex mutants are primarily defective in the Exp step.
aex-4 Encodes a SNARE Protein.
We predicted that aex-4 would encode a protein involved in exocytosis, neuropeptide production, or neuropeptide signaling, because all other aex strains have mutations in genes regulating these pathways (10, 12, 14). By searching the genomic interval in which aex-4 had been mapped (1), we identified a candidate gene for the aex-4 locus. All aex-4 alleles sequenced have mutations in the gene T14G12.2, and a 4-kb genomic clone of this gene is sufficient to rescue the defecation defect of aex-4 (Fig. S3 and S4A; SI Text, section V.B). Table S1 describes all transgenic strains used in this work. T14G12.2 encodes a homologue of the SNARE protein SNAP25 (Fig. S3) (15, 16). These findings implicate aex-4 in exocytosis.
AEX-4 Regulates the DMP from the Intestine.
To dissect how AEX-4 regulates defecation behavior, we first determined the expression pattern of aex-4 by building a transgenic animal that expresses GFP fused to a nuclear localization signal under the aex-4 promoter (SI Text, section V.1). Surprisingly, aex-4 expresses only in intestinal cells (Fig. 1B). To determine the subcellular localization of AEX-4, we built a transgene expressing a functional GFP-tagged AEX-4 fusion under its native promoter (SI Text, section V.C). GFP-tagged AEX-4 localizes along the plasma membrane of intestinal cells (Fig. 1 C and D). Therefore, AEX-4 likely acts at the surface of intestinal cells to regulate the DMP.
We expressed the aex-4 gene under an intestinal-specific promoter, a muscle-specific promoter, and a neuronal-specific promoter to determine in what tissue aex-4 regulates the DMP (SI Text, section V.D–F). Intestinal expression of aex-4 fully rescues the defecation defects of aex-4 mutant animals (Fig. 2A and Fig. S4 B and C), whereas muscle- and neuronal-specific promoters only partially rescue the defecation defect of aex-4 mutants (Fig. 2A).
Fig. 2.
aex-4 and aex-5 function in the intestine, whereas aex-2 acts in GABAergic neurons to regulate defecation. (A) Neuronal promoter (Prab-3), muscular promoter (Pmyo-3), and intestinal promoter (Pges-1 or Pvha-6) were used to drive the expression of individual aex genes. Intestinal aex-4 and aex-5 fully rescue the aex-4 and aex-5 Exp defect, respectively, and neuronal aex-2 rescues the aex-2 Exp defect. Muscular and neuronal expressions of aex-4 and aex-5 only partially rescue the aex-4 and aex-5 Exp defect. (B) Punc-47 (GABAergic neurons) but not Punc-17 (cholinergic neurons) or Pglr-1 (subset of interneurons) expression of aex-2 rescues the aex-2 Exp defect. DMP function was assayed by observation of 8–20 animals for 10 cycles and plotted as the ratio of Exps to pBocs. *P < 0.05; **P < 0.005; ***P < 0.0005 significantly different from the respective mutant. +P > 0.05 not significantly different from WT. (C) Intestinal RNAi of aex-4, aex-5, and aex-6 induces strong Exp defects. RNAi of aex-3 induces a moderate defecation defect, whereas the RNAi of aex-1 and aex-2 induces only mild defecation defects. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 significantly different from the vector control RNAi. Error bars represent SEM. See SI Text for transgenes and clones.
To verify our tissue-specific rescue experiments, we tested whether RNAi of aex-4 in a strain that is only sensitive to RNAi in the intestine would result in a defecation defect (17). Indeed, intestinal-specific RNAi of aex-4 results in a strong Exp defect consistent with aex-4 functioning in the intestine (Fig. 2C). In addition to aex-4, RNAi of aex-5 and aex-6 in the intestinal-specific RNAi strain causes a strong Exp defect. RNAi of aex-3 causes a moderate Exp defect similar to that of the aex-3 loss of function (Fig. S1). These results are consistent with aex-3, aex-4, aex-5, and aex-6 functioning in the intestine. RNAi of aex-1, in contrast, causes only a mild defect in Exp, although tissue-specific rescue experiments suggest that aex-1 functions in the intestine (12). We suspect that aex-1 levels were not reduced enough in the intestinal-specific RNAi strain, because RNAi of aex-1 in WT strains causes a strong Exp defect (data not shown). Combined together, these results strongly suggest that a cohort of exocytic aex genes, including aex-4, all function in the intestine to regulate the DMP.
AEX-5 Regulates the DMP from the Intestine.
aex-5 encodes a pro-protein convertase and was identified in the same screen that isolated aex-4 (1, 12, 13). We confirmed that the pro-protein convertase gene is mutated in aex-5(sa23) by sequencing (lesion is C443W) and by genomic fosmid rescue (Fig. S4A). Because aex-4 likely regulates secretion of a signal from the intestine and pro-protein convertases are typically packaged into secretory vesicles (14), we wished to determine whether AEX-5 is secreted from the intestine. A transgene in which intestinal-specific expression of AEX-5, fused to the VENUS variant of GFP (18), was driven fully rescues the defecation defects of aex-5 mutant animals, whereas muscle- and neuronal-specific expression only partially rescues the defecation defects (Fig. 2A; Fig. S4 B and C; and SI Text, section V.G–I). AEX-5::VENUS is secreted from the intestine and subsequently endocytosed by coelomocytes, specialized endocytic cells in C. elegans (Fig. 1 E–F). These findings suggest AEX-5 is secreted from the intestine, where it regulates the DMP.
AEX-4 Regulates the Secretion of AEX-5 from the Intestine.
We hypothesized that AEX-4 regulates the secretion of AEX-5 from the intestine. To test this, we determined if aex-4 mutants are defective in AEX-5::VENUS secretion. WT animals that express AEX-5::VENUS in the intestine (SI Text, section V.T) secrete AEX-5::VENUS, which accumulates in coelomocytes. In contrast, aex-4 mutants accumulate AEX-5::VENUS in intestinal cells and accumulate significantly less AEX-5::VENUS in coelomocytes (Fig. 3 and Fig. S5). There is no defect in the secretion of AEX-5::VENUS in WT or aex-2 mutants. Therefore, AEX-4 likely regulates the secretion of AEX-5 from the intestine during the DMP. These results lead us to the question of what receives this signal.
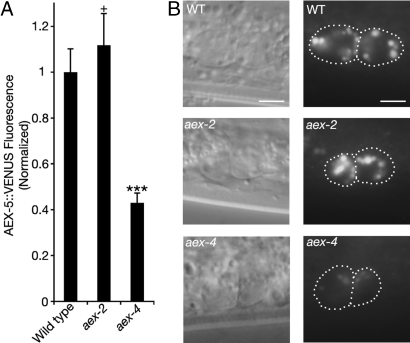
Fig. 3.
Intestinal aex-4 regulates the secretion of AEX-5. (A) In aex-4 mutants, the total fluorescence, normalized to WT, of intestinal AEX-5::VENUS in coelomocytes is significantly less than that of WT. There is no significant change in coelomocyte fluorescence in aex-2 mutants. ***, P < 0.0005 significantly different from the respective mutant. +, P > 0.05 not significantly different from WT. Error bars represent SEM. (B) Representative photographs of anterior coelomocytes in WT, aex-2, and aex-4. The left image is a Nomarski image. The right image is AEX-5::VENUS fluorescence. (Scale bars, 5 μm.)
aex-2 Encodes a GPCR.
We mapped aex-2 to a region on the X chromosome and revealed that the gene T14B1.2 encodes aex-2. All sequenced aex-2 alleles have mutations in T14B1.2, and a transgene that contains the T14B1.2 gene rescues the aex-2 mutant phenotype (SI Text, section V.10–11 and Figs. S4A and S6). T14B1.2 encodes a protein that shares homology with the A class of GPCRs, some of which mediate peptide signaling (Fig. S6) (19). When expressing an mCherry-tagged aex-2 genomic fusion construct (which fully rescues the aex-2 defecation defects), we detected AEX-2::mCherry signal in the nerve ring, ventral nerve cord, and enteric muscles (Fig. 1 G–I; SI Text, section V.J–K). So, unlike aex-4, which is exclusively expressed in the intestine, aex-2 is expressed in neuronal and nonneuronal tissues.
aex-2 Regulates the DMP from the Nervous System.
To determine what tissue aex-2 functions in, we expressed an aex-2 cDNA::GFP fusion under a neuronal promoter, a muscular promoter, and an intestinal promoter (SI Text, section V.L–N). Although neuronal expression of AEX-2::GFP fully rescues the defecation defects of aex-2 mutants, muscular and intestinal aex-2 does not (Fig. 2A). Consistent with this observation, intestinal-specific RNAi of aex-2 does not cause a robust defecation defect (Fig. 2C). Thus, unlike aex-4 and aex-5, aex-2 acts in neurons to regulate the DMP.
Because previous studies have indicated that the GABAergic neurons AVL and DVB are required for Exp (7), we analyzed the expression pattern of our aex-2 in various neuronal subtypes. We coexpressed mCherry protein under the aex-2 promoter with the GFP under a unc-17 (drives expression in cholinergic neurons), a glr-1 (drives expression in subsets of interneurons), or a unc-47 (drives expression in GABAergic neurons) promoter to assess if any of the aex-2–positive neurons are colabeled by these neuronal subtype-specific markers (SI Text, section V.O). We found that the aex-2 reporter is detected in all three neuronal cell types (Fig. 1 J–L and Fig. S7). Briefly, at least one glutamatergic interneuron (likely AVD), several head and pharyngeal cholinergic neurons, and two GABAergic neurons (AVL and DVB) express mCherry under the control of the aex-2 promoter (Fig. 1 J–L and Fig. S7). None of the ventral nerve cord motor neurons (neither cholinergic nor GABAergic) are labeled by Paex-2::mCherry. Therefore, aex-2 is expressed in the defecation regulating GABAergic neurons (AVL and DVB) as well as in other types of nonmotor neurons.
To determine which neuronal subtype aex-2 functions in, we expressed an aex-2::GFP cDNA fusion under neuronal subtype-specific promoters. GABAergic expression of aex-2 significantly rescues the Exp defect and partially rescues the aBoc defect of aex-2 mutants, whereas the other two promoters do not (Fig. 2B and Fig. S4 B and C and SI Text, section V.P–R). Taken together, these results suggest that aex-2 acts in GABAergic neurons (i.e., AVL and DVB) to control the Exp, and perhaps the aBoc.
aex-2 Functions Through a Gsα Signaling Pathway.
Because aex-2 encodes a GPCR, we examined which Gα subunit might act downstream of the aex-2 receptor signaling. To determine this, we genetically tested a variety of candidate Gα subunits for their ability to suppress aex-2 mutant phenotype. We built double mutants between an aex-2 loss-of-function mutant and a gsa-1 (Gsα) gain-of-function mutant (20) and an egl-30 (Gqα) gain-of-function mutant (21–23). We also built an aex-2 and dgk-1 (which encodes a diacylglycerol kinase) double loss-of-function mutant, because dgk-1 normally antagonizes egl-30 signaling and its loss-of-function phenocopies egl-30 gain of function (21–23). Gsα gain-of-function mutation causes ectopic Exp in WT and aex-2 mutant animals, and it suppresses the Exp defect in aex-2 mutants (Fig. 4A). On the other hand, the Gqα pathway mutants do not have a strong effect on the aex-2 phenotype (Fig. 4A). Interestingly, gain of function in gsa-1 does not rescue the aBoc defect (Fig. S4 B and C). The irregular timing of Exps in gsa-1 mutants (i.e., some Exps do not occur at the proper time, which is 3 sec after pBoc) may account for the lack of rescue of aBoc in aex-2 mutants. To assess if the Gsα rescue is specifically mediated by GABAergic neurons, we tested whether the GABAergic expression of a gain-of-function adenylyl cyclase (acy-1) (24), a downstream effector of Gsα, would suppress the defecation defects in aex-2 mutants (SI Text, section V.S). Similar to the gsa-1 gain-of-function mutant, overexpression of a gain-of-function acy-1 gene in aex-2 mutants causes ectopic Exps, and this expression significantly rescues the Exp defects in aex-2 mutants (Fig. 4A). Taken together, these results support a model in which the aex-2 GPCR acts in GABAergic neurons to regulate the DMP via a downstream Gsα and adenylyl cyclase pathway.
Fig. 4.
aex genes likely regulate the defecation through downstream Gsα and adenylyl cyclase signaling and GABAergic neuron activation. (A) Gain-of-function allele of Gsα [gsa-1(ce81)] completely suppresses the Exp defects in aex-2 mutants. In contrast, a gain-of-function in Gqα [egl-30(js126)] and a loss-of-function [dgk-1(sy428)] that phenocopies egl-30 gain-of-function have only mild effects on the aex-2 Exp defects. When an activated adenylyl cyclase gene [acy-1(js127)] is specifically expressed in the GABAergic neurons, it significantly suppresses the Exp defects of aex-2, aex-4, and aex-5 mutants. (B) Activation of GABAergic neurons by photoactivatable ChR2 suppresses the Exp defects in aex mutants. The photoactivatable cation channel ChR2 was expressed specifically in GABAergic neurons under the Punc-47 promoter in aex-2, aex-4, and aex-5 mutants. In the presence of all-trans retinal and blue-light activation, the ChR2 fully suppresses the Exp defects in all the aex mutants. In contrast, in the absence of all-trans retinal or blue light, the ChR2 transgene does not rescue the defecation mutant phenotypes to WT levels. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 significantly different from the respective mutant. +, P > 0.05 not significantly different from WT. Error bars represent SEM. See SI Text for transgenes and clones.
GABAergic Neurons Act Downstream of AEX-4 and AEX-5 to Control Defecation.
Although aex-2 acts in AVL and DVB GABAergic neurons, aex-4 and aex-5 function in the intestine to regulate Exp. We wished to determine if aex-2 signaling acts downstream of intestinal aex-4 and aex-5. Because we have not identified the ligand for AEX-2, we asked whether gain-of-function mutations in the aex-2 pathway could suppress the aex-4 and aex-5 Exp defects. GABAergic expression of the gain-of-function acy-1 gene is sufficient to rescue the Exp defects seen in aex-4 and aex-5 mutants (Fig. 4A). As seen in aex-2 mutants, overexpression of gain-of-function acy-1 in aex-4 and aex-5 mutants also causes ectopic Exps. These data suggest that GABAergic signaling of aex-2 acts downstream of intestinal signaling of aex-4 and aex-5.
Activation of GABAergic Neurons by a Light-Activatable Channel Can Bypass the Requirement of AEX-2, AEX-4, and AEX-5 for Defecation.
We wished to demonstrate that the role of aex-2, aex-4, and aex-5 is specifically to activate GABAergic neurons controlling the Exp step. We used YFP-tagged ChR2 under a GABAergic promoter to bypass the loss of these aex genes (SI Text, section VI). ChR2, from the green alga Chlamydomonas reinhardtii, is a light-activatable nonselective cation channel that, in the presence of all-trans-retinal, will depolarize excitable cells (25). We hypothesized that the activation of GABAergic neurons using the ChR2 transgene would suppress the behavioral defect of each of the aex mutants. Indeed, activation of ChR2 in GABAergic neurons by brief (≈1 sec) pulses of blue light ≈2 sec after pBoc is sufficient to rescue the Exp defects of aex-2, aex-4, and aex-5 mutants completely (Fig. 4B and Movie S2). These results strongly suggest that aex-2, aex-4, and aex-5 regulate the activity of GABAergic neurons AVL and DVB to induce Exp during the DMP.
Discussion
Several lines of evidence suggest that a group of exocytic genes function in the C. elegans intestine to control secretion of a signal to regulate the aBoc and Exp steps of the DMP. aex-1, aex-3, aex-4, and aex-6 are each homologous to the genes that regulate exocytosis in secretory cells (11, 12). Here, we present data that these genes regulate the secretion of a signal from the intestine to induce aBoc and Exp. This model is further supported by the observation that aex-4 mutants prevent the secretion of an intestinal AEX-5::VENUS into the pseudocoelom and its subsequent endocytosis by coelomocytes. Therefore, aex-4 (and likely aex-1, aex-3, and aex-6) regulates the secretion of AEX-5 and, arguably, its substrate from the intestine. Although our data indicate aex-3 and aex-6 function in intestine, they do not exclude the possibility that these genes also function in AVL and DVB to regulate the DMP (see Fig. S8 for model).
The model of the intestine secreting a signal to regulate Exp prompted us to search for the receptor for the signal. aex-2 encodes a putative GPCR, and our data suggest that this receptor likely functions in AVL and DVB GABAergic neurons to regulate Exp. Interestingly, when using a gain-of-function Gsα or an activated adenylyl cyclase that is expressed in GABAergic neurons, we suppressed the Exp defect not only in aex-2 but in the intestinal aex-4 and aex-5 mutants. These results are consistent with aex-2 encoding the receptor of the intestinal signal and acting downstream of intestinal aex genes.
We speculate that Gsα and adenylyl cyclase act downstream of aex-2 to excite AVL and DVB during the Exp. In support of this model, some gsa-1 loss-of-function animals, with a mosaic rescuing transgene, were reported to exhibit a defecation defect (26). We also observed a robust defect in the Exp step in these animals (11% ± 3%, P < 0.0005). In contrast, acy-1 loss-of-function mutants, with a rescuing transgene expressing in muscle (27), do not have a significant defect in the Exp step (81% ± 7%, P > 0.05). This may be attributable to redundancy of the three other adenylyl cyclase genes in C. elegans (28) and/or the action of other gsa-1 effectors acting in parallel to acy-1.
Activation of ChR2 in GABAergic neurons is sufficient to suppress the Exp defects of aex-2, aex-4, and aex-5 mutants. The aBoc defect of these aex mutants, however, was not rescued. Activation of GABAergic ChR2 causes the worm to become paralyzed as a result of muscle relaxation; therefore, it may be difficult to observe the aBoc contractions under these conditions. These findings indicate that the aex genes likely act to activate AVL and DVB GABAergic neurons through aex-2.
Although aex-2 is likely involved in AVL and DVB activation, it remains unclear how aex-2 is activated. Because aex-5 encodes a pro-protein convertase and aex-5 mutants are defective in neuropeptide production (13), we suspect that aex-2 encodes a neuropeptide-like receptor. There are ≈100 genes in the C. elegans genome encoding more than 250 peptides (29, 30), many of which are expressed in the intestine. The identification of aex-2 ligand(s) will shed new light on our understanding of this GPCR's signaling and the regulation of neuronal functions by nonneuronal tissues.
Several of our observations suggest that the Exp step may be regulated by more than one signal. First, we noticed that activation of ChR2 in WT or aex mutant animals does not induce ectopic Exps. Only temporally correct light activation of AVL and DVB rescues the Exp defect of aex-2, aex-4, and aex-5. This observation indicates that activation of AVL and DVB (at least via ChR2) is permissive, but not completely instructive, to drive the Exp step of the DMP. Second, although constitutive activation of the Gsα pathway causes ectopic Exps in aex mutants, there is a strong tendency for Exps to occur at the proper time (≈3 sec after pBoc). If aex genes were the sole connection between the intestinal pacemaker and activation of Exp, then one would expect to see a weaker tendency for Exps to occur at the proper time. These observations suggest that a second signal may function to regulate the Exp step. This signal could, for example, consist of a permissive signal that allows enteric muscles to be excited at the right time point in the DMP.
On first glance, one cannot help but notice the similarities between the intestinal aex genes and the neuronal secretory apparatus. SNARE proteins (AEX-4) are thought to create a membrane fusion structure at the nerve terminal, with SNARE regulators (AEX-1) playing a critical role in exocytosis (31). Although the precise function of Rabs (AEX-6) in exocytosis, per se, is unclear, they play an important role in synaptic transmission (11). Interestingly, although the regulator for AEX-6, AEX-3, has a similar defecation phenotype when mutated, the effector of AEX-6, RBF-1, does not (11). Perhaps AEX-6, also known as Rab27, functions through a novel effector to regulate the DMP. Given the similarities between the genes involved in the DMP and those involved in synaptic transmission, the C. elegans intestine could be seen as an alternative means for investigating the mechanisms governing regulated exocytosis and, by analogy, synaptic transmission. When one adds on the observation that RNAi is highly ineffective in C. elegans neurons (32) but very effective in the intestine (17), the intestine becomes an attractive model for discovering genes and genetic pathways regulating exocytosis.
Our work shows that the intestine may secrete a signal to activate the AVL and DVB neurons to induce the DMP (Fig. S8). This might explain why the Ca2+ oscillations are necessary for the timing of the DMP, because excitable cells use increased Ca2+ to induce secretion. Our work provides the first explanation of how the intestine may regulate this behavior by activating the AVL and DVB neurons.
Materials and Methods
Refer to SI Text for detailed materials and methods and for a description of transgenes and clones.
Behavioral Assay.
For detailed methods, refer to SI Text, section IX. Briefly, 8–20 1-day-old adults were scored for 10 defecation cycles (i.e., 10 pBocs). Statistical significance was determined by using unpaired two-tailed Student t tests, with unequal variance.
ChR2 Experiments.
For detailed methods, refer to SI Text, section X. Briefly, L4 larval-staged animals were grown in the presence or absence of 500 μM all-trans retinal (Sigma) overnight at 22°C. The next day, defecation was scored on a Leica MZ16F fluorescent stereomicroscope with an x-Cite 120 excitation light source (EXFO) and a standard GFP filter. During the DMP, animals were stimulated with a brief ≈1-sec pulse of blue light, ≈2 sec after pBoc.
Supplementary Material
Acknowledgments.
Some strains used in this work were obtained from the Caenorhabditis elegans Genetics Center, which is supported by the National Institutes of Health National Center for Research Resources. This work was supported by grants from the U.S. Public Health Service (M.L.N). T.M. was supported by a training grant from the U.S. Public Health Service. This work was partly supported by National Institutes of Health Neuroscience Blueprint Core Grant NS057105 to Washington University and the Bakewell Family Foundation. We thank Kevin Strange, Ken Miller, and Jim Rand for providing strains.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data Deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. FJ165552 and FJ165553).
This article contains supporting information online at www.pnas.org/cgi/content/full/0803617105/DCSupplemental.
References
- 1.Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–872. doi: 10.1093/genetics/124.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGhee JD The C. elegans Research Community, editor. The C. elegans intestine. WormBook. 2007 Available at www.wormbook.org.
- 3.Peters MA, Teramoto T, White JQ, Iwasaki K, Jorgensen EM. A calcium wave mediated by gap junctions coordinates a rhythmic behavior in C. elegans. Curr Biol. 2007;17:1601–1608. doi: 10.1016/j.cub.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol triphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- 5.Teramoto T, Iwasaki K. Intestinal calcium waves coordinate a behavioral motor program in C. elegans. Cell Calcium. 2006;40:319–327. doi: 10.1016/j.ceca.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C. elegans. Cell. 2008;132:149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- 8.Liu DW, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beg AA, Jorgensen EM. EXP-1 is an excitatory GABA-gated cation channel. Nat Neurosci. 2003;6:1145–1152. doi: 10.1038/nn1136. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney TR, et al. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–2625. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi M, Iwasaki K. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron. 2002;33:249–259. doi: 10.1016/s0896-6273(01)00587-6. [DOI] [PubMed] [Google Scholar]
- 13.Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): Mutant analysis by mass spectrometry. J Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- 14.Thacker C, Rose AM. A look at the Caenorhabditis elegans Kex2/Subtilisin-like proprotein convertase family. Bioessays. 2000;22:545–553. doi: 10.1002/(SICI)1521-1878(200006)22:6<545::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 17.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: Role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai T, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 19.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 20.Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169:631–649. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen G, et al. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- 22.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 23.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saifee O. St. Louis, MO: Washington Univ; 2003. A genetic analysis of molecular machinery regulating synaptic transmission. PhD thesis. [Google Scholar]
- 25.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Korswagen HC, Park JH, Ohshima Y, Plasterk RH. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev. 1997;11:1493–1503. doi: 10.1101/gad.11.12.1493. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds NK, Schade MA, Miller KG. Convergent, RIC-8-dependent Galpha signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics. 2005;169:651–670. doi: 10.1534/genetics.104.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastiani C, Mendel J The C. elegans Research Community, editor. Heterotrimeric G proteins in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.75.1. Available at www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 29.Nathoo AN, Moeller RA, Westlund BA, Hart AC. Identification of neuropeptide-like protein gene families in Caenorhabditiselegans and other species. Proc Natl Acad Sci USA. 2001;98:14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husson SJ, Clynen E, Baggerman G, De Loof A, Schoofs L. Discovering neuropeptides in Caenorhabditis elegans by two dimensional liquid chromatography and mass spectrometry. Biochem Biophys Res Commun. 2005;335:76–86. doi: 10.1016/j.bbrc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 32.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 33.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc London B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.