Abstract
Tobacco smoking is frequently abused by schizophrenia patients (SZP). The major synaptically active component inhaled from cigarettes is nicotine, hence the smoking habit of SZP may represent an attempt to use nicotine self-medication to correct (i) a central nervous system nicotinic acetylcholine receptor (nAChR) dysfunction, (ii) DNA-methyltransferase 1 (DMT1) overexpression in GABAergic neurons, and (iii) the down-regulation of reelin and GAD67 expression caused by the increase of DNMT1-mediated hypermethylation of promoters in GABAergic interneurons of the telencephalon. Nicotine (4.5–22 μmol/kg s.c., 4 injections during the 12-h light cycle for 4 days) decreases DNMT1 mRNA and protein and increases GAD67 expression in the mouse frontal cortex (FC). This nicotine-induced decrease of DNMT1 mRNA expression is greater (80%) in laser microdissected FC layer I GABAergic neurons than in the whole FC (40%), suggesting selectivity differences for the specific nicotinic receptor populations expressed in GABAergic neurons of different cortical layers. The down-regulation of DNMT1 expression induced by nicotine in the FC is also observed in the hippocampus but not in striatal GABAergic neurons. Furthermore, these data show that in the FC, the same doses of nicotine that decrease DNMT1 expression also (i) diminished the level of cytosine-5-methylation in the GAD67 promoter and (ii) prevented the methionine-induced hypermethylation of the same promoter. Pretreatment with mecamylamine (6 μmol/kg s.c.), an nAChR blocker that penetrates the blood–brain barrier, prevents the nicotine-induced decrease of FC DNMT1 expression. Taken together, these results suggest that nicotine, by activating nAChRs located on cortical or hippocampal GABAergic interneurons, can up-regulate GAD67 expression via an epigenetic mechanism. Nicotine is not effective in striatal medium spiny GABAergic neurons that primarily express muscarinic receptors.
Keywords: antagonists, epigenetic mechanisms, nicotinic acetylcholine receptor agonists, schizophrenia
Tobacco smoking is frequently abused by schizophrenia patients (SZP) (for reviews see refs. 1 and 2). Because nicotine is a potent cholinergic receptor agonist that is inhaled with tobacco smoking and both the expression and function of nicotinic acetylcholine receptors (nAChRs) are down-regulated in the brain of SZP, one may conclude that the high level of tobacco smoking in these patients represents an attempt to self-medicate; i.e., correction of some disease-associated abnormalities of cholinergic (nicotinic) neurotransmission (3, 4), possibly related to the decrease of GABAergic function occurring in the brain of SZP (5–9).
Typically, plasma nicotine levels in heavy smokers (≈20–30 cigarettes a day) oscillate between 0.3 and 0.6 μM. Because in humans nicotine half-life is ≈2 h, the nicotine plasma levels in heavy smokers progressively increase during the day but fluctuate in a “peak and trough” fashion after each cigarette (10, 11). These submicromolar concentrations of nicotine, which act at heterooligomeric α4β2 high-affinity and homomeric α7 low-affinity nAChR abundantly expressed in GABAergic interneurons of the frontal cortex (FC) and hippocampus (12–14), may improve cognitive function in laboratory animals and also in normal human subjects (1, 2).
Although the effect of submicromolar concentrations of nicotine on cognition and perception is reduced with chronic treatment with nicotine because of nAChR desensitization (1, 10, 12), repeated exposure to nicotine also results in a long-term homeostatic increases of several synaptic proteins (15) including: (i) α4β2 and α7 nAChR (3, 4) and (ii) AMPA, NMDA, and GluR1 receptors (15). Chronic nicotine treatment also results in an increase of (i) dendritic length and spine density in the pyramidal neurons of the cingulate cortex (16) and (ii) long-term potentiation of hippocampal neuronal responses (17). Hence, by stimulating nAChR, chronic cigarette smoking could elicit long-term synaptic plasticity caused by changes of specific receptor expression, a response that may correct the impaired cognitive function in SZP.
When postmortem brains of SZP are compared with those of nonpsychiatric subjects, in addition to the above-mentioned decrease of high- and low-affinity nAChR subtypes in the hippocampus, cortex, and caudate, a GABAergic neuropathology is detected in these brain areas. This neuropathology includes the decreased expression of glutamic acid decarboxylase (GAD) 67, one of the two enzymes (GAD65 and GAD67) that synthesize GABA in the brain (5–9).
Recent studies suggest that the decrease of GAD67 and other GABAergic genes expression in cortical, hippocampal, and caudate GABAergic neurons of SZP may be caused by promoter hypermethylation (18, 19). Such hypermethylation is very likely caused by the overexpression of DNA methyltransferase 1 (DNMT1) in cortical BA9, BA10, and BA17 layers I and II but not in layer V and VI GABAergic interneurons of SZP (18, 19).
Here, we investigated in mice whether repeated nAChR stimulation by s.c. nicotine injections that increase their plasma concentrations by an extent comparable to that reported by heavy cigarette smokers (10, 11, 20) results in a down-regulation of DNMT1 and an increased expression of GAD67 in GABAergic neurons of cortical, hippocampal, and striatal brain areas. Furthermore, to test the concept that protracted nAChR stimulation up-regulates GAD67 by reducing its promoter methylation via a reduction of DNMT1 expression, we investigated whether the same nicotine treatment that induces FC GAD67 expression also decreases the level of GAD67 promoter methylation or prevents the methionine-induced hypermethylation of this specific promoter. The data support a role for nicotine as a treatment to correct (through a normalization of an altered epigenetic function) the down-regulation of GAD67 expression present in cortical and hippocampal GABAergic neurons of SZP.
Results
DNMT1 Expression in the Brain of Mice Treated s.c. with Nicotine.
Selection of nicotine dose and time schedule.
A recent study (20) showed that in Swiss albino male mice treated with an s.c. nicotine injection of 16 μmol/kg (2.5 mg/kg), the plasma concentration of this alkaloid reaches ≈0.8 μM during the first hour and thereafter declines with a half-life of ≈15 min. These pharmacokinetic parameters suggest that to establish the nicotine plasma concentration ranges achieved by heavy cigarette smokers (0.5–0.6 μM for 2–3 h in a mouse model), mice should receive nicotine (4.5–22 μmol/kg s.c, corresponding to 0.75–3.5 mg/kg) once every 3 h during a 12-h light cycle.
These nicotine doses injected s.c. into mice elicit a transient (10–15 min) increase of sympathetic tone characterized by an increase of respiratory rate and locomotor activity. At the highest dose (22 μmol/kg s.c.) of nicotine tested there is an intermittent tremor beginning a few minutes after the injection and terminating within 15–20 min. One should also note that in heavy cigarette smokers when plasma nicotine concentrations reach values of 0.6 μM or higher, there are signs of an increased sympathetic tone (i.e., increased rate of respiration, tachycardia, and hypertension) and frequently, increased hand tremor as well (21). This finding suggests that repeated s.c. injections of nicotine in mice elicit a pharmacological response comparable to that of heavy cigarette smokers. Our preliminary experiments showed that a single injection of nicotine (22 μmol/kg s.c.) or four injections in a day (22 μmol/kg, one injection every 3 h during the 12-h light cycle) failed to induce a significant decrease of DNMT1 mRNA or protein content in mouse FC. In contrast, doses of 22 μmol/kg (four injections per 12 h) of nicotine given for 2 or 4 days result in a significant decrease of FC levels of DNMT1 mRNA or protein. The DNMT1 mRNA values ± SE expressed as fmol/0.1 pmol neuronal-specific enolase (NSE) mRNA were: 3.2 ± 0.2 in the vehicle-treated group (n = 5), 2.6 ± 0.1 (P < 0.05) in the group treated for 2 days, and 2.0 ± 0.2 (P = 0.001) in the group treated for 4 days. Nicotine treatment for 5 or 8 days also produced a decrease of DNMT1 mRNA expression but the decrease was not significantly greater than that observed after 4 days of nicotine treatment.
DNMT1 protein levels estimated by Western blot and referred to β-actin blots used to estimate sampling variation were decreased by 19 ± 1.4% in the group treated with nicotine for 1 day and 37 ± 1.6% (P < 0.05) in the group treated with nicotine for 4 days. It is important to notice that β-actin used as a reference protein failed to change after nicotine treatment.
Nicotine decreases DNMT1 mRNA expression in cortical and hippocampal but not in striatal GABAergic neurons.
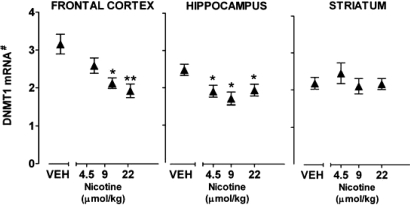
Fig. 1 shows that nicotine in doses between 4.5 and 22 μmol/kg (0.75–3.5 mg/kg for four injections per 12 h for 4 days) elicits a significant decrease (30–40%) of DNMT1 mRNA expression in the FC and hippocampus but not in the striatum. The decrease of cortical and hippocampal DNMT1 mRNA expression reached almost maximal level at doses of 9 μmol/kg (1.5 mg/kg) nicotine and became significant in the hippocampus after a dose of 4.5 μmol/kg (0.75 mg/kg). In these experiments, DNMT1 mRNA was decreased when measured 2 h after the last injection of nicotine and the DNMT1 mRNA decrease persisted unabated up to 12 h after the last nicotine treatment (41% decrease after 2 h and 35% decrease 12 h after the last s.c. injection of 22 μmol/kg nicotine).
Fig. 1.
Nicotine reduces DNMT1 mRNA expression in mouse FC and hippocampus but not in striatum. Mice were injected with nicotine s.c. four times a day during 12-h light cycle for 4 days. DNMT1 mRNA was measured 2 h after the last nicotine injection. Each value is the mean ± SE of five mice. Overall one-way ANOVA for DNMT1 mRNA levels in vehicle and nicotine treatment yielded a P < 0.003 for the FC and hippocampus. *, P < 0.01; **, P < 0.003 for Student-Newman-Keuls comparison between vehicle and nicotine. #, Data are expressed as fmol DNMT1mRNA/0.1 pmol NSE mRNA.
DNMT1 mRNA content in the liver failed to change after nicotine treatment. DNMT1 mRNA attomol/μg RNA in liver tissue of vehicle-treated mice is 4.9 ± 0.84, and in nicotine-treated mice (22 μmol/kg four times a day for 4 days) is 4.2 ± 0.57.
Mecamylamine but not hexamethonium blocks nicotine-induced down-regulation of DNMT1 expression.
To establish whether nicotine-induced reduction of DNMT1 expression in cortical neurons is mediated by an activation of CNS nAChRs, we used mecamylamine, a noncompetitive nAChR open-channel blocker that crosses the blood–brain barrier (22). In our strain of mice, mecamylamine (6 μmol/kg s.c.) attenuates or virtually abolishes the effect of 22 μmol/kg of nicotine on behavior and on the FC decrease of DNMT1 protein (Table 1). Importantly, doses of mecamylamine in a range of 6 (Table 1) and 24 μmol/kg (data not shown) failed to modify per se the expression level of DNMT1 in FC.
Table 1.
Mecamylamine but not hexamethonium prevents the nicotine-induced DNMT1 down-regulation in mouse FC
| Treatment | DNMT1 OD /β-actin OD |
|
|---|---|---|
| Vehicle | Nicotine | |
| Vehicle | 100 | 69 ± 5* |
| Mecamylamine | 94 ± 20 | 93 ± 10 |
| Hexamethonium | 112 ± 15 | 59 ± 10* |
Mice were treated four times per 12 h for 4 days with nicotine (22 μmol/kg s.c.), mecamylamine (6 μmol/kg s.c.), hexamethonium (19 μmol/kg s.c.), or their combination. DNMT1 protein levels (OD ratio to β -actin) are expressed as percentage of controls. Each value is the mean ± SE of five mice. Overall one-way ANOVA yielded a P = 0.004. *, P < 0.05 for Student-Newman-Keuls multiple comparison.
To distinguish between the susceptibility of the CNS and ganglionic nAChRs in the action of nicotine, we attempted to block the effects of nicotine on cortical DNMT1 expression by administering hexamethonium. This ganglionic blocking agent acts only on peripheral nAChRs (23) and, injected in a dose (19 μmol/kg s.c.) known to block several cardiovascular, gastrointestinal, and respiratory responses induced by nicotine (23), fails to block the decrease of DNMT1 expression elicited by nicotine (Table 1).
In the FC, the nicotine-induced reduction of DNMT1 expression occurs in a subset of GABAergic neurons.
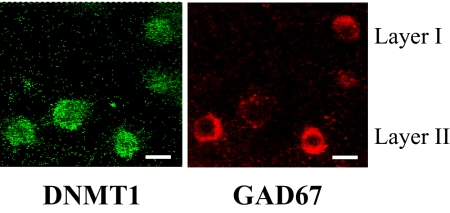
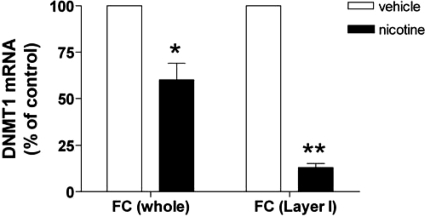
Using confocal fluorescence microscopy, we found that DNMT1 is primarily expressed in GAD67-positive neurons in layers I and II of the mouse FC (Fig. 2). Hence, to study whether nicotine down-regulates the expression of DNMT1 in GABAergic neurons, we laser microdissected layer I neurons of the FC (19). This cortical layer only expresses a distinct population of GABAergic neurons (19). Fig. 3 shows that nicotine induces a significantly greater reduction of DNMT1 mRNA in laser-microdissected layer I samples (≈80%) than in samples including all FC layers (≈40%).
Fig. 2.
DNMT1 and GAD67 colocalize in layers I and II of mouse frontal cortex. Double fluorescence microscopy images show DNMT1 immunoreactivity (green) and GAD67 immunoreactivity (red). (Scale bars: 10 μm.)
Fig. 3.
Nicotine-induced reduction of DNMT1 mRNA is expressed at a higher level in laser-microdissected layer I FC than in the whole FC samples. Mice were treated with nicotine (22 μmol/kg s.c. four times a day for 4 days) and were killed 2 h after the last injection. DNMT1 mRNA values in vehicle treated mice are as follows: whole FC, 3.2 fmol DNMT1 mRNA/0.1 pmol NSE mRNA; layer I, 18 fmol DNMT1 mRNA/0.1 pmol NSE mRNA. Each value is the mean ± SE of five mice. *, P < 0.01; **, P < 0.001, Student's t test between vehicle and nicotine treatment.
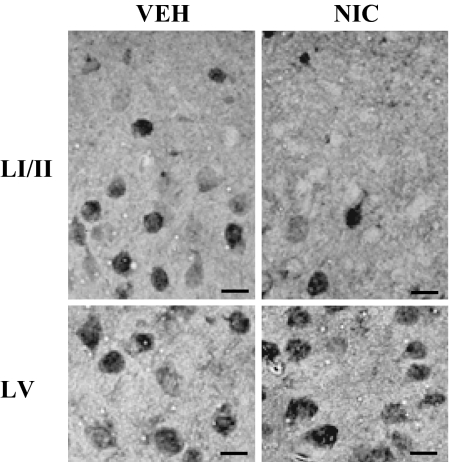
In situ hybridization studies (a typical example is given in Fig. 4) show that in mice treated with nicotine (22μmol/kg four times per 12 h for 4 days), the number of DNMT1 mRNA-positive neurons is specifically decreased in layer I (DNMT1-positive neurons ± SE = 340 ± 50/mm2 in vehicle and 220 ± 20/mm2 in nicotine-treated animals; n = 10, P < 0.01). In contrast, in layer V the number of DNMT1-positive neurons fails to decrease (820 ± 70/ mm2 in vehicle and 850 ± 120/ mm2 in mice treated with nicotine). As shown in Fig. 4, in layers I and II, not only the neuronal number but also the signal intensity of the neuronal DNMT1 mRNA staining is considerably decreased. Note that the number of NeuN-positive neurons in FC layer I was not modified after nicotine treatment (350 ± 50/mm2 in vehicle and 330 ± 60/mm2 in mice treated with nicotine).
Fig. 4.
DNMT1 in situ hybridization in the FC layer I/II (Upper) and layer V (Lower) in vehicle (Left) and nicotine-treated (Right) mice. Nicotine was given 22 mmol/kg s.c. four times per 12 h for 4 days. Brains were fixed 2 h after the last injection. (Scale bars: 10 μm).
Nicotine Up-Regulates Cortical but Not Striatal GAD67 Expression.
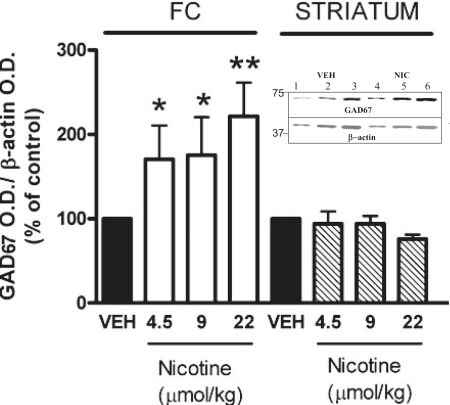
Mice exposed to nicotine doses that reduce the expression of DNMT1 in the FC exhibit a significant increase of GAD67 protein expression in the same brain area (Fig. 5). In this brain area, the increase is inversely related to the decrease of DNMT1 mRNA expression (r = 0.6, P = 0.03). In the same samples, we failed to observe an increase of GAD65 protein expression. However, the increase of GAD67 induced by nicotine in the FC fails to occur in the striatum of the same animals.
Fig. 5.
Nicotine increases GAD67 expression in FC but not in striatum. Mice were treated with nicotine four times a day during the 12 h of light cycle for 4 days. GAD67 was measured 2 h after the last nicotine injection. Each value is the mean ± SE of 5–8 mice. **, P < 0.02; *, P < 0.05 Student's t test between vehicle (VEH) and nicotine treatment. (Inset) Typical Western immunoblot of GAD67 and β-actin after 4–12% SDS/PAGE. Comparison between FC vehicle (lanes 1–3, serial dilutions of the same sample) and nicotine-treated (lanes 4–6, serial dilutions of the same sample) mice.
Nicotine Decreases the Level of GAD67 Promoter Methylation in the FC.
Previous studies suggest that GAD67 expression can be regulated by changes in the levels of promoter cytosine-5 methylation. For example, administration of histone deacetylase inhibitors, such as valproic acid (VPA) and MS-275, in doses that reduce GAD67 or reelin promoter methylation in mouse FC (24–27), increase their transcription. On the contrary, repeated administration of the essential amino acid methionine, a precursor of S-adenosyl-methionine (SAM) that serves as a methyl group donor, resulted in an increased methylation of GAD67 promoter and a decrease of GAD67 expression (24–27).
To determine whether the GAD67 up-regulation induced in mouse FC by protracted (4 days) nicotine treatment is associated with a decrease in the level of GAD67 promoter methylation presumably mediated by an action related to DNMT1 changes, we quantified the ratio of 5′methylated-cytosines (5mC) to the unmethylated cytosines (C) of the mouse GAD67 (−760, − 311 bp) CpG-enriched promoter region by measuring the fraction of GAD67 promoter immunoprecipitated by specific 5mC antibodies (MeDIP). In previous studies, we reported that the ratio 5mC/C for GAD67 or reelin promoters measured with MeDIP technology is virtually identical to the ratio obtained with the MeCP2 ChIP procedure (27). Moreover, MeDIP values are about proportional to the number of methylated CpG dinucleotides measured with sodium bisulfite (compare refs. 26 and 27).
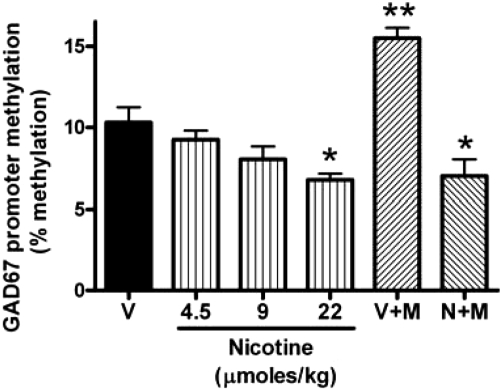
As inferred from previous studies (24–27), we found that the methylated cytosines expressed by the GAD67 promoter detected in the FC of control mice are ≈10% of the total promoter cytosines (Fig. 6). Nicotine treatment causes a small but significant dose-dependent decrease in the methylation level of the CpG-rich dinucleotides present in the GAD67 promoter (Fig. 6). This down-regulation is in line with the observed increase of GAD67 expression and the associated decrease of DNMT1 mRNA (r = 0.97, P = 0.03).
Fig. 6.
Nicotine reduces GAD67 promoter methylation in the FC. V: received saline for four times a day for 5 days. N: received nicotine (4.5, 9, or 22 μmol/kg s.c. four times a day for 4 days) and saline on the fifth day. V+M: received saline for 2 days followed by methionine (5 mmol/kg, twice a day) for 3 days. N+M: received nicotine (22 μmol/kg) for 4 days. On the third and fourth days of nicotine treatment, mice also received methionine. On the fifth day, nicotine was suspended and mice received only methionine. Each value is the mean ± SE of three mice. Overall one-way ANOVA for GAD67 promoter methylation yielded a P < 0.001. **, P < 0.01 for Student-Newman-Keuls multiple comparison between V versus V+M and V+M versus N+M; *, P < 0.05 for V versus N and V versus N+M.
To obtain further support for the hypothesis that nicotine up-regulates GAD67 expression because it reduces promoter (cytosine-5) methylation, we investigated the effect of nicotine in mice in which the GAD67 promoter methylation was increased by injecting methionine (5mmol/kg s.c., twice a day for 3 days) (Fig. 6). In these mice, nicotine administration (22μmol/kg four times a day) for 4 days before l-methionine treatment completely prevents l-methionine-induced GAD67 promoter hypermethylation (Fig. 6). Importantly, the decreased GAD67 promoter methylation induced by nicotine cannot be caused by the decreased level of the methyl donor SAM. In fact, when measured 2 h after the last methionine injection, the levels of SAM in the FC are similar in mice receiving methionine + nicotine (52 ± 3.2 pmol/mg tissue) and mice receiving only methionine (50 ± 2.7 pmol/mg tissue).
Discussion
Nicotine Down-Regulates DNMT1 and Up-Regulates GAD67 Expression in Cortical and Hippocampal but Not Striatal GABAergic Neurons.
If repeated for 2–4 days, nicotine, injected in mice in doses that yield nicotine plasma concentrations comparable to those found in the plasma of compulsive cigarette smokers (0.5–0.6 μM) (10, 11, 20), induces a significant decrease of DNMT1 mRNA and protein content in cortical layers I and II (Figs. 3 and 4) and hippocampal GABAergic interneurons but not in striatal medium spiny GABAergic neurons.
In the FC, the down-regulation of DNMT1 expression in GABAergic interneurons is abolished by mecamylamine but not by hexamethonium, suggesting that the effects of nicotine are mediated by a CNS nAChR-dependent mechanism. In fact, in the medium spiny neurons of the striatum that express mostly muscarinic AChRs and are virtually devoid of nAChRs (28), the administration of nicotine failed to decrease DNMT1 mRNA expression (Fig. 1).
In the same mice in which multiple nicotine injections induce a decrease of cortical DNMT1, the expression of GAD67 is significantly increased (Fig. 5). In contrast, in the striatum where nicotine failed to down-regulate DNMT1 expression, GAD67 also failed to change. Note that to the best of our knowledge, an effect of repeated nicotine administration on the FC expression density of GAD67 has never before been reported. Hence, our experiments suggest an important role for CNS nAChRs in the regulation of gene expression in cortical and hippocampal GABAergic interneurons.
These experiments do not allow the identification of the affinity constants of the nAChRs that are responsible for the nicotine-induced down-regulation of cortical DNMT1 or the increase of cortical GAD67 expression. In preliminary experiments, we found that the decrease of DNMT1 and the increase of GAD67 expression elicited by nicotine in FC can be mimicked by s.c. injections of A-85380 [3-(2S)-azetidinylmethoxy pyridine dihydrochloride], an α4β2 nAChR selective agonist (29). In contrast, PNU-282987 (N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride), an α7 nAChR selective agonist (30), injected in doses that enhance the hippocampal-dependent form of learning, is inactive on FC DNMT1 and GAD67 expression. If confirmed, the data suggest that α4β2 high-affinity nAChRs located in cortical layer I and II GABAergic interneurons may play an important role in the nicotine-induced regulation of gene expression.
nAChR Stimulation Selectively Down-Regulates DNMT1 mRNA Expression in Cortical Layer I GABAergic Neurons.
Consistent with previous studies conducted in the human brain (18, 19), we found that in layers I and II of the mouse FC and in the striatum, DNMT1 appears to be selectively expressed in GABAergic neurons. However, in cortical and hippocampal homogenates the maximal reduction of DNMT1 mRNA and protein expression induced by administration of nicotine never exceeded 30–40% and DNMT1 mRNA never decreased in the striatum. Hence, it may be inferred that AChRs on GABAergic neurons in cortical layers I and II and the hippocampus differed from that in the striatum (28) and deeper cortical layers.
To strengthen this hypothesis, in the present experiment we show that the decrease of DNMT1 mRNA expression induced by nicotine administration was significantly higher in FC layer I (≈80%) vs. the whole FC (30–40%). Accordingly, in situ hybridization studies showed that after 4 days of nicotine treatment (see Materials and Methods for details), the number of DNMT1 mRNA-positive neurons in the cortex was significantly decreased in layers I and II but not in layers V and VI (Fig. 3). In striatal medium spiny GABAergic neurons that express high levels of muscarinic AChRs but low levels of nAChRs (28), the injections of nicotine failed to change the levels of DNMT1 mRNA and protein. Thus, it appears that DNMT1 levels decrease only in GABAergic neurons that express nAChRs.
In addition to DNMT1, DNMT3A and DNMT3B, have been identified in mammalian CNS (31). To ascertain whether the nicotine-induced down-regulation of DNMT1 is associated with changes of DNMT3A or DNMT3B, in preliminary experiments we measured the mRNA expression of both enzymes in the FC. DNMT3A mRNA, unlike DNMT1 mRNA, is not down-regulated by nicotine treatment and therefore may be expressed in neurons devoid of nAChRs. DNMT3B does not seem to be expressed in significant amounts in the CNS of adult mice.
Nicotine Induces GAD67 Promoter Hypomethylation.
To test the hypothesis that the nicotine-induced increase of GAD67 expression could be a consequence of the reduction of DNMT1, we studied the effect of nicotine on the methylation status of a specific GAD67 promoter fragment enriched with CpG dinucleotides. As reported (24), promoter methylation, likely through the recruitment of methyl-binding proteins, is a mechanism that regulates GAD67 promoter activity and therefore GAD67 expression. In previous studies with VPA, we reported that a decrease of basal GAD67 promoter methylation was associated with an up-regulation of GAD67 transcriptional activity and therefore of GAD67 expression (24–27). In mice FC extracts, using a DNA methylation assay with an antibody that specifically immunoprecipitates DNA nucleosomal fragments containing 5-methyl-cytosines, we showed that protracted nicotine treatment (4.2–22 μmol/kg four times a day for 4 days) reduces the levels of methylation of GAD67 promoters in a dose-dependent manner.
Furthermore, the same doses of nicotine that induce GAD67 promoter demethylation and GAD67 up-regulation in normal mice were shown to completely prevent the l-methionine-induced hypermethylation of GAD67 promoters (Fig. 6). Further studies with specific nAChR agonists or antagonists and chromatin remodeling agents such as VPA are required to define the molecular mechanisms whereby stimulation of high-affinity nAChRs decreases DNMT1 expression and reduces GAD67 or other promoter methylation selectively in GABAergic neurons of cortex and hippocampus.
Conclusions
Current research on SZP suggests that the overexpression of DNMT1 in the upper cortical layers of GABAergic neurons is responsible for the epigenetic hypermethylation of specific GABAergic gene promoters, including GAD67 and reelin (18, 19). The expression down-regulation of these genes in SZP brains likely leads to a GABAergic transmission defect, which presumably plays an important role in the pathogenetic mechanisms that underlie the cognitive, behavioral, and auditory gating system impairments expressed in psychotic patients (8, 9). This evidence suggests that a reversal of the epigenetically induced transcriptional down-regulation of GAD67 and other genes in cortical GABAergic neurons of SZP should be attempted by using drugs that directly or indirectly target DNMT1.
Nicotinic agonists are one of several treatment options for SZP currently under investigation (1, 2, 32). Hence, the use of synthetic nAChR ligands to selectively down-regulate DNMT1 in GABAergic interneurons of cortical layers I and II and the hippocampus may represent an innovative attempt to control the hypermethylation of GAD67 and other gene promoters operative in selected populations of telencephalic GABAergic neurons of SZP while leaving the function of DNMT1 in cells that do not express nAChRs intact. To be investigated is whether α4β2 or α7 nAChR agonists are better suited to correct the epigenetic alterations of GABAergic neurons operative in SZP.
Materials and Methods
Animals and Drug Administration.
Male Swiss albino mice (Harlan Breeders) weighing 20–25 g were used in this study. The following drugs were used: nicotine bitartrate, mecamylamine hydrochloride, hexamethonium hydrochloride, l-methionine, all from Sigma–Aldrich. All drugs were dissolved in a volume of saline solution corresponding to 0.05 ml/10 g body weight and were injected s.c.
Immunohistochemistry.
DNMT1 in situ hybridization, NeuN immunolabeling, and neuronal counts.
In sections (15–20 μm thick), DNMT1 in situ hybridization was carried out as described (18, 19). NeuN immunolabeling was carried out with a mouse anti-NeuN mAb (Chemicon) diluted 1:500 for 72 h and subsequently with biotinylated goat anti-mouse antiserum and 3–3′ diaminobenzidine (Sigma) as described (18, 19). Five sections were taken from each brain area and a bidimensional cell counting method was used to count DNMT1- or NeuN-positive cells at ×40 magnification in a square area of 100 × 100 μm (33).
Confocal fluorescence microscopy.
Sections were incubated with a rabbit polyclonal anti-GAD65/67 antibody (diluted 1:500; Chemicon) and a mouse anti- DNMT1 mAb (diluted 1:500; Imgenex) for 72 h. Sections were then incubated with Cy-5-conjugated goat anti-rabbit IgG (Amersham Biosciences; 1:1,000) to label the antibody reacting with GAD65/67 and with Cy2-conjugated streptavidin (1:1,000) to label the antibody directed against DNMT1 (for further details see refs. 18 and 19).
Laser-Assisted Microdissection.
From each brain, 20-μm coronal sections were mounted on polyethylene terephthalate membrane frame slides (Leica). Sections were rapidly fixed in cold 70% ethanol and stained with 0.1% toluidine blue to visualize the cortical layers. Layer 1 sections from eight FC coronal sections (between +1.1 and +0.9 mm anterior to the bregma) were microdissected from each brain sample by using the Application Solution Laser Microdissection system (Leica) to collect an ≈3 × 105-μm2 area from layer I. Samples were collected directly in 50 μl of guanidine-isothiocynate solution containing Triton X-100 (1%) and β-mercaptoethanol (0.8%) as described (19).
RNA Isolation and Quantitative RT-PCR Analysis.
Total RNA was extracted by cesium chloride (CsCl) density gradient centrifugation as described (19). DNMT1, DNMT3A, and NSE mRNA content were measured in each sample by competitive RT-PCR. The following amplification primers were used: DNMT1, forward, base pairs 4231–4254; reverse, base pairs 4667–4644; GenBank accession no. X14805.1; DNMT3A, forward, base pairs 1827–1850; reverse, base pairs 2245–2268; GenBank accession no. NM_007872.2); and NSE, forward, base pairs 382–405; reverse, base pairs 769–792; GenBank accession no. M22349.1. This technique is based on the simultaneous amplification of the target mRNA and a specific internal standard having the same sequence as the target except for a deletion of 58 bp for DNMT1 (between 4,461 and 4,518 bp), 84 bp for DNMT3A (between 2,000 and 2083 bp), and 85 bp for NSE (between 552 and 636 bp) (34).
Western Blot.
Extraction of nuclear DNMT1.
Nuclear protein extraction was conducted according to a procedure described by Cupers et al. (35). Briefly, tissue samples (10–15 mg) were homogenized in 200 μl of 0.32 M sucrose, 50 mM Tris·HCl, 0.1 mM EDTA, and 1 mM PMSF at pH 7.4 and centrifuged at 1,000 × g for 10 min. The pellet containing the nuclei was lysed on ice for 30 min in 200 μl of 150 mM NaCl, 50 mM Tris·HCl, 0.1 mM EDTA, and 0.1% Triton X-100 at pH 7.4. After centrifugation at 20,000 × g for 20 min, the supernatant containing the soluble nuclear material was separated, and proteins were precipitated in 1.5 ml of methanol at −20°C overnight. After centrifugation at 20,000 × g for 20 min at 4°C, the pellets were resuspended in loading buffer (120 mM Tris·HCl/20% glycerol/8% SDS/0.01% blue bromophenol/0.1% DTT) and denatured at 100°C for 5 min.
Extraction of GAD65/67.
These proteins were extracted directly from the FC samples in Laemmli buffer (100 μl/10 mg tissue) (7).
SDS/PAGE.
Proteins were separated by 4–12% SDS/PAGE (Invitrogen), blotted onto nitrocellulose membranes (Invitrogen), and developed overnight at 4°C with anti-DNMT1 mAb (Imgenex) diluted 1:1,000 or anti-GAD65/67 rabbit polyclonal antibody diluted 1:2,000 (Chemicon) (26). Membranes were then washed and reblotted with a β-actin mAb (1:5,000; Sigma–Aldrich) for 2 h (26). Two major bands were detected for DNMT1, one at 180 kDa corresponding to the full-length DNMT1 protein molecular mass, and the second at 70 kDa (a possible metabolite or processing product). The specificity of the DNMT1 antibody was tested by preabsorption with a corresponding antigen in 0.005 M NaPHO4 buffer (pH 7.2), 0.2 M NaCl, and 5% BSA. All of the immunoreactive bands detected in the membrane after incubation with the nonpreabsorbed DNMT1 antibody disappeared when the antibody was previously preabsorbed with the recombinant antigen.
Changes in DNMT1 expression induced by various treatments were determined by assigning the value of 100 to the control samples and expressing the values of each treatment sample as percentages of the controls. Five serial dilutions were run for every sample to identify the linear range for protein quantification.
DNA Methylation Assay.
Genomic DNA was extracted from the mouse FC and sonicated to produce fragment sizes of 200–1,000 bp. After ethanol precipitation, 3 μg of sonicated DNA were heat-denatured at 95°C for 10 min. An aliquot of the solution (50 μl) was removed and stored at −20°C to be used as input. The remaining solution was incubated overnight at 4°C, with a mouse anti-5mC mAb (1:250) (Calbiochem). The DNA–antibody complex was then added to 50 μl of protein A agarose beads (Invitrogen) and incubated on a rotating platform for 2 h at 4°C. The resulting DNA–antibody–beads complex was isolated, and the DNA was released by proteinase K digestion. After phenol-chloroform extraction and ethanol precipitation, the DNA pellet was resuspended in 20 μl of diethyl pyrocarbonate water.
A CpG-rich GAD67 promoter fragment (base pairs 760–311) (27) was quantified by quantitative PCR analysis. The following amplification primers were used: forward, base pairs 760–737; reverse, base pairs 311–334 (27). The internal standard used for the quantification was an oligonucleotide with the same sequence as the target except for a deletion of 100 bases (between 390 and 490 bp) (27). The level of methylation of GAD67 promoter is expressed as percentage of the input DNA that is immunoprecipitated by the anti-5mC antibody.
Acknowledgments.
We thank Francine M. Benes (McLean Hospital, Harvard University, Belmont, MA) and Dr. Bryan Roth (St. Louis University School of Medicine and University of North Carolina, Chapel Hill) for constructive criticism and suggestions in the preparation of the manuscript. This work was supported in part by National Institute of Mental Health Grants MH071667 (to E.C.) and MH070855 (to A.G.).
Footnotes
Conflict of interest statement: M.H. is associated with Pfizer Global Research and Development.
References
- 1.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: Potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–56. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 2.Leonard S, et al. Smoking and schizophrenia: Abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 3.Breese CR, et al. Abnormal regulation of high-affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 4.Besson M, et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc Natl Acad Sci USA. 2007;104:8155–8160. doi: 10.1073/pnas.0702698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes FM, et al. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbarian S, et al. GABAA receptor subunit gene expression in human prefrontal cortex: Comparison of schizophrenics and controls. Cereb Cortex. 1995;6:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 7.Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti A, et al. GABAergic dysfunction in schizophrenia: A new treatment target on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 10.Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 11.Pidoplichko VI, DeBiasis M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 12.Picciotto MR, et al. Neuronal nicotinic acetylcholine receptor subunit knockout mice: Physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 13.Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- 14.Kofalvi A, Sperlagh B, Zelles T, Vizi ES. Long-lasting facilitation of 4-amino-n-[2,3-(3)H]butyric acid ([(3)H]GABA) release from rat hippocampal slices by nicotinic receptor activation. J Pharmacol Exp Ther. 2000;295:453–462. [PubMed] [Google Scholar]
- 15.Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteosomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- 17.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 18.Veldic M, et al. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruzicka WB, et al. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 20.Damaj MI, Siu ECK, Sellers EM, Tyndale RF, Martin BR. Inhibition of nicotine metabolism by methoxysalen: Pharmacokinetic and pharmacological studies in mice. J Pharmacol Exp. 2007;320:250–257. doi: 10.1124/jpet.106.111237. [DOI] [PubMed] [Google Scholar]
- 21.Goodman A, Gilman . The pharmacological basis of therapeutics. In: Goodman A, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon; 1990. pp. 180–181. [Google Scholar]
- 22.Collins AC, Evans CB, Miner LL, Marks MJ. Mecamylamine blockade of nicotine responses: Evidence for two brain nicotine receptors. Pharmacol Biochem Behav. 1986;24:1767–1773. doi: 10.1016/0091-3057(86)90518-6. [DOI] [PubMed] [Google Scholar]
- 23.Caulfield MP, Higgins GA. Mediation of nicotine induced convulsions by central nicotinic receptors of the C6 type. Neuropharmacology. 1983;22:347–351. doi: 10.1016/0028-3908(83)90251-4. [DOI] [PubMed] [Google Scholar]
- 24.Dong E, et al. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci USA. 2005;102:12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremolizzo L, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate nuclear DNA demethylation in the brain. Proc Natl Acad Sci USA. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 29.Smith JW, et al. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalize to the nicotine discriminative stimulus in the rat. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- 30.Walker DP, et al. Design, synthesis, structure–activity relationship, and in vivo activity of azabicyclic aryl amides as α7 nicotinic acetylcholine receptor agonists. Bioorg Med Chem. 2006;14:8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Bird A. DNA methylation de novo. Science. 1999;286:2287–2288. doi: 10.1126/science.286.5448.2287. [DOI] [PubMed] [Google Scholar]
- 32.Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- 33.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: A practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 34.Auta J, Chen Y, Ruzicka WB, Grayson DR. Nucleic acid quantitation using the competitive polymerase chain reaction: Practical neurochemistry methods. In: Baker G, Dunn S, Holt A, Lajtha A, editors. The Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer; 2007. pp. 341–361. [Google Scholar]
- 35.Cupers P, Orlans I, Craessaerts K, Annaert W, De Strooper B. The amyloid precursor protein (APP)-cytoplasmic fragment generated by γ-secretase is rapidly degraded but distributes partially in a nuclear fraction of neurons in culture. J Neurochem. 2001;78:1168–1178. doi: 10.1046/j.1471-4159.2001.00516.x. [DOI] [PubMed] [Google Scholar]