Abstract
Lateral organ polarity in Arabidopsis is regulated by antagonistic interactions between genes that promote either adaxial or abaxial identity, but the molecular basis of this interaction is largely unknown. We show that the adaxial regulator ASYMMETRIC LEAVES2 (AS2) is a direct target of the abaxial regulator KANADI1 (KAN1), and that KAN1 represses the transcription of AS2 in abaxial cells. Mutation of a single nucleotide in a KAN1 binding site in the AS2 promoter causes AS2 to be ectopically expressed in abaxial cells, resulting in a dominant, adaxialized phenotype. We also show that the abaxial expression of KAN1 is mediated directly or indirectly by AS2. These results demonstrate that KAN1 acts as a transcriptional repressor and that mutually repressive interactions between KAN1 and AS2 contribute to the establishment of adaxial–abaxial polarity in plants.
Adaxial–abaxial polarity in plants is specified by interactions between genes that individually specify either adaxial or abaxial identity (1, 2). In Arabidopsis, adaxial identity is specified by class III homeodomain leucine zipper (HD-ZIPIII) transcription factors (3, 4), the transcription factor ASYMMETRIC LEAVES2 (AS2) (5, 6), and the transacting siRNA tasiARF (7), whereas abaxial identity is specified by KANADI (KAN) (8, 9), YABBY (YAB) (10, 11), AUXIN RESPONSE FACTOR (ARF) (12), and LITTLE ZIPPER (ZPR) (13) transcription factors and by the miRNAs miR165/miR166 (14–16). Genetic analysis indicates that many of these genes interact antagonistically: loss-of-function mutations in adaxial genes typically produce an abaxialized phenotype that is accompanied by the expanded expression of abaxial genes, whereas loss-of-function mutations in abaxial genes produce an adaxialized phenotype that is associated with the expanded expression of adaxial genes; mutations or transgenes that produce ubiquitous expression of these genes have a phenotype opposite to that of the loss-of-function mutations. miR165/166 and tasiARF repress the expression of their targets, respectively, HD-ZIPIII genes and ARF3/ETTIN, by directing the cleavage of the transcripts of these genes (14, 17). The molecular basis for the antagonistic interactions between other adaxial and abaxial regulators is unknown.
KAN1, KAN2, and KAN3 are members of the GARP family of transcription factors and act redundantly to promote abaxial identity in both lateral organs and the shoot axis. These genes are expressed in abaxial cells of lateral organs and peripheral cells of the hypocotyl and stem (9, 16). Loss-of-function mutations in individual genes have relatively weak effects on organ polarity (9, 18), but kan1 kan2 (8) and kan1 kan2 kan3 (16) mutants are strongly adaxialized and resemble plants ectopically expressing the HD-ZIPIII genes PHB, PHV, and REV (3, 15) or the LOB gene AS2 (5, 6). Consistent with this observation, PHB is abaxially expressed in kan1 kan2 kan3 triple mutants (16). The effect of kan on the expression of AS2 has not been examined.
Here, we show that KAN1 promotes abaxial identity by directly repressing the transcription of AS2 in abaxial tissue. Specifically, we demonstrate that KAN1 binds to a site in the promoter of AS2, and that a single nucleotide mutation in this site interferes with KAN1 binding and produces an adaxialized phenotype that is associated with the inappropriate expression of AS2 in abaxial tissue. These results indicate that KAN1 acts as a transcriptional repressor and provide evidence for a direct interaction between transcription factors involved in the specification of adaxial–abaxial polarity. In addition, we show that AS2 represses the expression of KAN1 in adaxial tissue, suggesting that these transcription factors may interact in a mutually repressive fashion.
Results and Discussion
To identify genes involved in the specification of adaxial–abaxial polarity, we took advantage of the observation that adaxialized mutants often have flat or upwardly curled leaves. Screens for ethyl methane sulfonate (EMS)-induced mutations with this leaf phenotype identified a dominant mutation that was mapped to a region on chromosome 1 containing AS2. Because this new mutation had a phenotype that strongly resembled transgenic plants that ectopically express AS2 (5, 6, 19) we immediately focused on this gene. Sequencing of the region surrounding AS2 revealed a G-to-A substitution 1,484 bp upstream of the AS2 ATG (Fig. 1A). Existing ESTs and cDNAs (www.arabidopis.org) and the results of 5′ RACE (data not shown) indicate that AS2 has several variable 5′ exons and multiple transcription start sites, three of which are illustrated in Fig. 1A. The mutation, which we refer to as as2–5D, is located in the first intron of one of these transcripts, but is in the promoter of most of the transcripts produced from this locus (Fig. 1A). To confirm that this mutation is responsible for the as2–5D phenotype, wild-type (gAS2) and as2–5D (gas2–5D) genomic sequences extending from the 5′ end of the upstream gene At1g65610 to the 3′ end of the AS2 transcript (Fig. 1A) were transformed into wild-type plants and as2–1 loss-of-function mutants (Fig. 1B). Ninety-seven percent (n = 422) of as2–1 plants transformed with the wild-type genomic sequence had a wild-type phenotype, indicating that this sequence contains all of the regulatory information necessary for AS2 function. Interestingly, introduction of the AS2 sequence into a wild-type background did not produce an AS2 gain-of-function phenotype, demonstrating that additional copies of this locus are insufficient to direct ectopic adaxial development. In contrast, 99% (n = 572) of as2–1 plants and 97% (n = 570) of wild-type plants transformed with the as2–5D genomic construct exhibited a dominant cupped leaf phenotype (Fig. 1B). These results demonstrate that the as2–5D phenotype is attributable to the G-to-A mutation in the AS2 promoter sequence and that this mutation is a gain-of-function mutation.
Fig. 1.
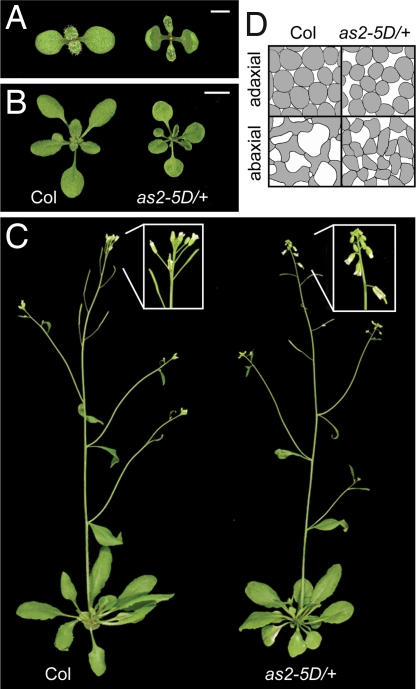
as2–5D affects a predicted KAN1 binding site. (A) AS2 transcripts share exons 2 and 3, but have variable first exons. The location of the as2–5D mutation is indicated in red. Exons are indicated as boxes or arrows. (B) The phenotype of wild-type and as2–1 plants transformed with wild-type (gAS2) and mutant (gas2–5D) genomic constructs. (Scale bar: 1 cm.)
Plants homozygous or heterozygous for as2–5D produced erect, cupped-up cotyledons, which remained cupped throughout their development (Fig. 2 A and B). The true leaves of mutant plants were strongly up-rolled early in development, but became flatter as they expanded and at maturity were quite similar to wild-type leaves (Fig. 2 A–C). as2–5D also affected the polarity of the mesophyll in the leaf blade (Fig. 2D). In wild-type leaves the adaxial layer of the mesophyll is occupied by round, densely packed cells, whereas the middle and lower (abaxial) mesophyll layers consist of irregularly shaped, loosely packed cells. In as2–5D leaves, cells in the adaxial mesophyll had more intercellular air space than normal, whereas cells in the abaxial layers of the mesophyll were more regular in shape and more densely arrayed than in wild-type leaves. Thus, as2–5D reduces the polarization of the mesophyll by affecting the differentiation of both adaxial and abaxial tissue. as2–5D plants also exhibited a reduction in the size of leaves and floral organs and had flowers and siliques that pointed horizontally or downward (Fig. 2C). In contrast to other adaxializing mutations, as2–5D did not affect the production of trichomes on the abaxial surface of the lamina; wild-type plants first produced abaxial trichomes on leaf 6.2 ± 0.2 (n = 10), and as2–5D/+ plants were not significantly different (6.4 ± 0.2; n = 10).
Fig. 2.
as2–5D has an adaxialized phenotype. (A–C) Eight-day-old (A), 21-day-old (B), and mature (C) wild-type Columbia and as2–5D/+ plants. as2–5D causes immature leaves and cotyledons to curl upward, and flowers and siliques to bend downward. (D) Camera-lucida drawings of the adaxial and abaxial mesophyll of leaf 3, demonstrating the loss of tissue polarity in as2–5D/+. (Scale bars: A, 1 mm; B, 1 cm.)
To examine the effect of the as2–5D mutation on the expression of AS2, we generated promoter–reporter constructs with wild-type or mutated AS2 regulatory sequences fused to ß-glucuronidase (GUS). For this purpose we used a 4.1-kb genomic fragment that is capable of rescuing the as2–1 phenotype when fused to an AS2 cDNA [supporting information (SI) Fig. S1]. Constructs with the wild-type fragment (pAS2:GUS) or mutated versions of this fragment containing either the as2–5D mutation (pas2–5D:GUS) or a nearby mutation in the second G in the AAGAATAAGAATAA sequence (pAS2m:GUS) were introduced into plants by Agrobacterium-mediated transformation. Paralleling the distribution of AS2 mRNA observed by in situ hybridization (19, 20), pAS2:GUS embryos and seedlings displayed GUS activity exclusively in the adaxial domain of cotyledons and leaf primordia (Fig. 3 A and D). This pattern was also observed in plants transformed with pAS2m:GUS (Fig. 3 C and F). In contrast, plants transformed with pas2–5D:GUS expressed GUS throughout the cotyledons, leaf primordia, and the hypocotyl (Fig. 3 B and E). These results suggest that as2–5D interferes with the binding of a factor that represses the transcription of AS2 in abaxial tissue and the hypocotyl.
Fig. 3.
as2–5D causes ectopic expression of AS2. The expression pattern of pAS2:GUS (A and D), pAS2–5D:GUS (B and E), and pAS2m:GUS (C and F) in Columbia. (A–C) Embryos. (D–F) Eight-day-old seedlings with cotyledons removed to show leaves 1 and 2. The sequence of the region surrounding the as2–5D mutation in each construct is shown, with the mutated nucleotide shown in red. The as2–5D mutation (B and E) causes AS2 to be expressed in the abaxial domains of cotyledons and leaves and in the hypocotyl. (Scale bars: A–C, 20 μm; D–F, 100 μm.)
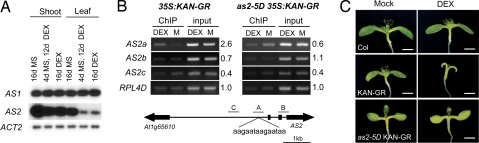
The phenotypic similarity between as2–5D and loss-of-function mutations of KAN1 (9) suggested that KAN1 might correspond to this negative regulator. To test this hypothesis, we examined the expression of AS2 and several other polarity genes in plants transformed with 35S:KAN1-GR, a construct that constitutively expresses a dexamethasone (DEX)-inducible form of KAN1. Semiquantitative RT-PCR analysis of 35S:KAN-GR plants grown continuously on DEX or on basal medium followed by DEX revealed that DEX reduced the amount of AS2 mRNA, but had no effect on the expression of AS1, FIL1, or YAB3 (Fig. 4A, data not shown). To determine whether KAN1 binds directly to the AS2 promoter we examined the chromatin fragments that immunoprecipitate with KAN1-GR in DEX- and mock-treated 35S:KAN1-GR seedlings. A 300-bp region surrounding the site of as2–5D mutation and two adjacent 300-bp regions upstream of the translation start site were amplified by PCR from immunoprecipitated material. KAN1 binding (as judged by enrichment after chromatin immunoprecipitation) was detected only for the fragment containing the as2–5D mutation (Fig. 4B). No enrichment was observed in 35S:KAN1-GR plants homozygous for as2–5D, demonstrating that the as2–5D mutation prevents or greatly reduces the binding of KAN1 to this site. Consistent with this result, we found that as2–5D suppresses the morphological defects produced by continuous DEX treatment of 35S:KAN1-GR plants. Nine-day-old KAN-GR seedlings treated with DEX have unexpanded leaves and cotyledons and poorly gravitropic roots, whereas DEX-treated as2–5D KAN-GR seedlings closely resemble untreated as2-5d KAN-GR plants and display no defect in root gravitropism (Fig. 4C; and Fig. S2). These results indicate that AS2 is directly repressed by KAN1 via the site that is mutated in as2–5D.
Fig. 4.
KAN1-GR directly represses AS2. (A) RT-PCR analysis of AS1 and AS2 transcripts in control and DEX-treated 35S:KAN-GR plants; AS2 is repressed in DEX-treated plants, primarily in leaves. (B) ChIP assays performed on DEX- and mock (M)-treated 35S:KAN1-GR and 35S:KAN1-GR as2–5D seedlings using antibodies specific for GR. Chromatin from three different regions 5′ of AS2 (A, B, and C indicated by gray bars) were analyzed with semiquantitative PCR before (input) and after ChIP. Fragment A contains the as2–5D point mutation. Average fold enrichment is indicated on the right of each image and was calculated for three replicates in DEX versus mock samples after normalizing the band intensity to that of the control gene RPL4D and to the input sample. (C) Phenotype of the 9-day-old seedlings used for ChIP. as2–5D blocks the binding of KAN1-GR to the AS2 promoter and the effect of KAN-GR on seedling morphology. (Scale bars: 1 mm.)
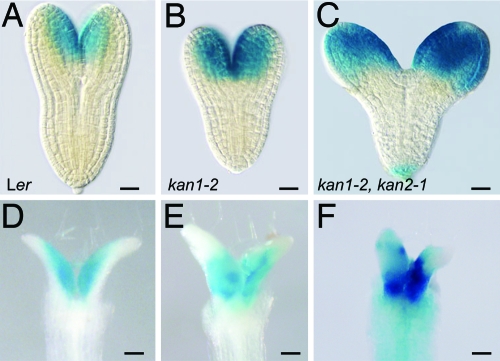
If the effect of as2–5D on the pattern of AS2 expression is attributable to a defect in KAN1 binding, loss of KAN1 activity should have the same effect on AS2 expression as as2–5D. To test this hypothesis, we examined the effect of loss-of-function mutants of KAN1 and KAN2 on the expression of pAS2:GUS in embryos and 8-day-old seedlings. Similar results were obtained with mutations isolated in the Landsberg erecta (kan1–2, kan2–1) and Columbia (kan1–11, kan2–5) genetic backgrounds. Consistent with their subtle morphological phenotype, mutations in KAN2 had no apparent effect on pAS2:GUS expression (data not shown). In contrast, mutations in KAN1 had little effect on the expression of pAS2:GUS in cotyledons (Fig. 5B), but caused this reporter to be expressed on both the adaxial and abaxial sides of leaf primordia (Fig. 5E). Plants mutant for both KAN1 and KAN2 expressed pAS2:GUS ubiquitously in cotyledons, leaf primordia, and the hypocotyl (Fig. 5 C and F). These results indicate that KAN1 and KAN2 redundantly repress AS2 in the abaxial domain of cotyledons and the hypocotyl, and that KAN1 has a nonredundant function in the repression of AS2 in leaf primordia. The observation that loss of KAN1 and KAN2 has essentially the same effect on the expression of pAS2:GUS (Fig. 5 C and F) as pas2–5D:GUS (Fig. 3 B and E) suggests that both of these proteins act via the site that is mutated in as2–5D.
Fig. 5.
AS2 is ectopically expressed in kan1 kan2 mutants. The expression of pAS2:GUS in wild-type Landsberg erecta (Ler) (A and D), kan1–2 (B and E), and kan1–2 kan2–1 (C and F). (A–C) embryos, (D–F) Eight-day-old seedlings with cotyledons removed to show leaves 1 and 2. The expression pattern of pAS2:GUS in kan1 kan2 is similar to expression pattern of pas2–5D:GUS (Fig. 3 B and E). (Scale bars: A–C, 20 μm; D–F, 100 μm.)
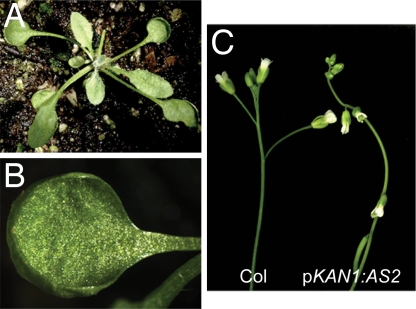
To determine whether misexpression of AS2 in the KAN1 expression domain accounts for the phenotype of as2–5D, we generated plants expressing AS2 under the control of the KAN1 promoter. Plants transformed with pKAN1:AS2 were nearly indistinguishable from as2–5D. In addition to possessing cupped cotyledons and leaves, they displayed the downward-pointing flowers characteristic of this mutation (Fig. 6). This result demonstrates that the phenotype of as2–5D is indeed a consequence of the misexpression of AS2 in the KAN1 expression domain and, along with the results described above, supports the conclusion that the expression domain of AS2 is defined by KAN1 and other abaxially expressed KAN genes. The floral phenotype of as2–5D and pKAN1:AS2 is strikingly similar to the phenotype of loss-of-function mutations in the KNOX gene BREVIPEDICELUS (BP) (21, 22). Because AS2 represses BP (5), this floral phenotype is likely caused by the repression of BP in the abaxial region of the pedicel by the inappropriate expression of AS2 in this domain.
Fig. 6.
Transgenic plants expressing AS2 under the regulation of the KAN1 promoter resemble as2–5D. (A) Rosette of pKAN1:AS2 plant. (B) Cotyledon of pKAN1:AS2 showing cupped margin. (C) The flowers and siliques of pKAN1:AS2 plants point downward.
kan1 kan2 double mutants have a very severe adaxialized phenotype with many features that are not observed in as2–5D plants. To determine whether the ectopic expression of AS2 contributes to this phenotype, we generated a kan1–12 kan2–4 as2–1 triple mutant. We found that triple mutants had nearly the same phenotype as kan1 kan2, although their leaf blades were shorter and more irregular than kan1 kan2 (Fig. S3); a similar result has been obtained by other investigators (23, 24). The observation that as2–1 is unable to ameliorate the adaxialized phenotype of kan1 kan2 indicates that the aberrant expression of AS2 in kan1 kan2 mutants is not responsible for this phenotype and implies that KAN1 and KAN2 have other targets with important roles in leaf polarity. Possible candidates include LBD genes that are closely related to AS2, such as LBD36/ASL1. Overexpression of ASL1 produces a phenotype that is similar to that of as2–5D, suggesting that AS2 and ASL1 have overlapping functions (25, 26). If AS2 and ASL1 contribute redundantly to the kan1 kan2 phenotype, loss-of-function mutations in only one of these mutations may have little or no effect on this phenotype. In addition to AS2, KAN1 appears to directly regulate the transcription of at least a dozen genes in Arabidopsis, several of which are involved in auxin and gibberellin metabolism and response (T.H, Y. Harrar, W.C. Lin, and R.A.K., unpublished results). Some of these genes may be primarily responsible for the phenotype of kan1 kan2 double mutants.
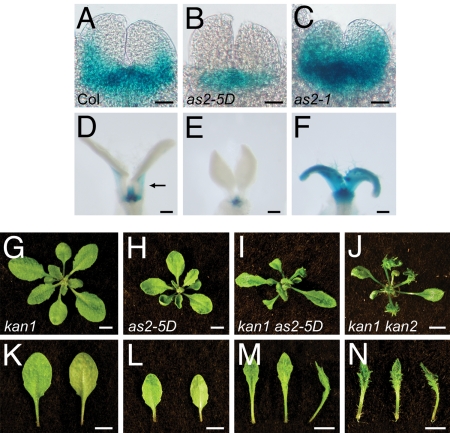
Plants transformed with 35S:AS2 have reduced levels of KAN1 and KAN2 mRNA and have a leaf phenotype reminiscent of the kan1 kan2 double mutant, whereas as2–1 mutants have elevated levels of KAN2 (5, 20). These observations suggest that AS2 and KAN genes may mutually repress each other's transcription. To test this hypothesis, we examined the effect of as2–1 and as2–5D on the expression of the KAN1 reporter pKAN1:GUS. In wild-type plants, pKAN1:GUS expression was restricted to the abaxial side of young leaf primordia and disappeared quickly as the leaf expanded (Fig. 7 A and D). In as2–1, pKAN1:GUS expression extended to the adaxial side of young leaf primordia and persisted late in leaf development (Fig. 6 C and F). as2–5D had the opposite effect: in as2–5D plants pKAN1:GUS expression was excluded from leaf primordia (Fig. 6 B and E). Thus, AS2 directly or indirectly represses KAN1 expression. Repression of KAN genes by AS2 is further supported by the phenotype of plants doubly mutant for kan1–11 and as2–5D (Fig. 7 I and M). Leaves of these double mutants display abaxial outgrowths that are a characteristic feature of kan1 kan2 double mutants (Fig. 7 J and N) but are not present in either kan1 (Fig. 7 G and K) or as2–5D single mutants (Fig. 7 H and L); that is, kan-11 as2–5D plants resemble kan1 kan2 double mutants. A reasonable interpretation of this result is that as2–5D represses KAN2 as well as KAN1.
Fig. 7.
AS2 represses KAN1 and KAN2. (A–F) The expression of pKAN1:GUS in the leaf primordia of 3-day-old (A–C) and 9-day-old (D–F) seedlings. (A and D) Wild type. (B and E) as2–5D. (C and F) as2–1. Cotyledons were removed to reveal the leaf primordia. The arrow in D indicates abaxial GUS expression in the petiole. The expression domain of pKAN1:GUS is reduced by the gain-of-function as2–5D mutation (B and E) and expanded by the loss-of-function as2–1 mutation (C and F). (G–N) The rosette (G–J) and fifth leaf (K–N) of 18-day-old plants. (G and K) kan1–11. (H and L) as2–5D. (I and M) kan1–11 as2–5D. (J and N) kan1–11 kan2–5. Adaxial, abaxial, and side views of the leaf are shown from left to right. The phenotype of kan1–11 as2–5D resembles that of kan1–11 kan2–5. (Scale bars: A–C, 20 μm; D–F, 100 μm; G–N, 0.5 cm.)
These results provide strong evidence that the spatial expression patterns of AS2 and KAN1 are specified by mutual repression and that AS2 is a direct target of KAN1. We do not have evidence that KAN2 also binds to the AS2 promoter. However, this seems likely because of the similarity of these proteins, and the observation that the expression of pAS2:GUS in a kan1 kan2 background is essentially identical to the expression pattern of pas2–5D:GUS; single kan1 mutations have a much less significant effect on pAS2:GUS expression. The result is surprising because it suggests that KAN1 and KAN2 regulate AS2 entirely via the site that is mutated in pas2–5D. Multiple binding sites are usually required to confer regulation by a specific transcription factor; indeed, we are unaware of another case in which a single nucleotide mutation in a plant promoter has such a profound effect on gene expression. Although it is clear that KAN1 and KAN2 play a major role in the regulation of AS2, these factors probably do not act alone. In particular, the observation that mutations of ARF3/ETT are capable of suppressing the floral phenotype of AP3:KAN1 suggests that ARF3/ETT cooperates with KAN1 and KAN2 to promote abaxial identity (12). This result is interesting in light of the observation that overexpression of ARF3 produces a phenotype nearly indistinguishable from that of as2–1 (27). This latter result is consistent with experimental evidence indicating that ARF3 acts as transcriptional repressor (28) and suggests that ARF3/ETT may work with KAN1 and KAN2 to repress AS2 transcription in some tissues. Defining the functional relationships between KAN family members and these and other major regulators of adaxial–abaxial polarity is an important goal for future research.
Materials and Methods
Genetic Stocks and Growth Conditions.
Seeds of the Col ecotype were mutagenized by suspending in 0.4% EMS at room temperature for 10 h, and then rinsing them five times with water. Bulked M2 progeny of mutagenized seeds were screened for defects in leaf expansion. Phenotypic characterization was carried out on the progeny of the second backcross of as2–5D to wild-type Col.
Unless otherwise noted, all mutants stocks were in a Col background. kan2–4 (SALK_095643) and as2–1 seed were obtained from the Arabidopsis Biological Resource Center; the as2–1 stock was crossed to Col two times before analysis. kan1–2 and kan2–1 were obtained from John Bowman (Monash University, Melbourne), and are in Ler. kan1–11 and -12 have been described (9). The lesion in kan2–5 is identical to that in kan2–1 (8) but was independently isolated in Col. Seeds were sown on Metromix 360 (Sun-Gro) and kept at 4°C for 2 days before transfer to growth chambers. Plants were grown in 96-well flats under continuous fluorescent light (100 μE/min per m2; Sylvania VHO) at 22°C. Abaxial trichomes were scored with a stereomicroscope 2–3 weeks after planting.
as2–5D Was Mapped in the F2 Progeny of a Cross to Ler.
A total of 179 wild-type plants in the F2 were used to map the mutation to a site between the BACs F5I14 and F12P19 on chromosome 1.
Transgenic Plants.
The AS2 ORF and the entire 5′ intergenic region were PCR-amplified from wild-type and as2–5D genomic DNA by using the 5′ primer 5′-CGCGGATCCAATTTTGGTTAATGATCGGTGAGAG-3′, which incorporates a BamHI site, and the 3′ primer 5′-CGCATGCCATGGATCAATTAAGAGAGCAAGTCCATAAA-3′, which incorporates an NcoI site, and the products were cloned into pCAMBIA3301. These clones were transformed into wild-type and as2–1 plants to confirm the identity of the as2–5D mutation. To examine the effect of this mutation on gene expression, the wild-type AS2 promoter sequence was amplified from wild-type genomic DNA by using primers pAS2-F (5′-GGATCCTGGTAGCTAGCGTTGTTGACA-3′) and pAS2-R (5′-GGATCCGTGAACGTTTGCGAATTTTTG-3′), which contained introduced BamHI sites, and the resulting PCR products were cloned into the binary vector pCB308 (29), generating a translational fusion to the GUS gene in the pAS2:GUS construct. The pas2–5D:GUS and pAS2-m:GUS constructs were generated by using site-directed mutagenesis, by amplification of the pAS2:GUS template with primers containing introduced mutations: pas2–5D-F (5′-CCCTAGACAAA-AAAAATAAGAATAAAAAGAGC-3′) and pas2–5D-R (5′-GCTCTTTTTAT-TCTTATTTTTTTTGTCTAGGG-3′) or pAS2-m-F (5′-CCCTAGACAAAAAG-AATAAAAATAAAAAGAGC-3′) and pAS2-m-R (5′-GCTCTTTTTATTTTTA-TTCTTTTTGTCTAGGG-3′) with PfuTurbo DNA polymerase (Stratagene). The PCR products were digested with DpnI to restrict the parental DNA template, and dsDNA containing the introduced mutation was transformed into TOP10 Escherichia coli cells (Invitrogen). The p35S:KAN1-GR construct was produced as follows: the p35S:KAN1-mGFP5 plasmid (9) was digested with BglII and partially digested with NheI to remove the fragment encoding mGFP5. In its place, a DNA fragment was ligated that encoded amino acids 508–795 of the rat glucocorticoid receptor isolated from pBS-GR (a gift from Doris Wagner, University of Pennsylvania, Philadelphia) by digestion with BamHI and XbaI. To generate pKAN1:GUS, a 7.7-kb BglII fragment from P1 clone MQK4 was ligated into the BamHI site of pCAMBIA2300 (www.cambia.org). A 3.5-kb XbaI fragment carrying the KAN1 promoter was subcloned into pBI101.2 to generate a translational fusion between the first 8 aa of the KAN1 coding region and GUS. The resulting constructs were sequenced to confirm their integrity and introduced into Arabidopsis plants by using the floral dip method. To generate the pKAN1:AS2 construct, the KAN1 promoter was amplified from genomic DNA by using the primers pKAN1-F (5′-ACTAGTAAGACCAACACAAACAAATTACC-3′) and pKAN1-R (5′-CCATGGAATTAAAGAAACCTTTCTCTTGCT-3′), which contained introduced SpeI and NcoI restriction sites, respectively. The amplified promoter fragment was fused to the AS2 coding sequence and an OCS3′ terminator in pCB302 (29).
Histology.
GUS staining was conducted according to the method of Donnelly et al. (30), with or without acetone pretreatment. Siliques were opened along the valve margin with a needle and stained with GUS staining buffer. After staining, developing seeds were placed on a glass slide, and the embryos were removed from the seed by applying gentle pressure with a needle.
RT-PCR and ChIP Analysis.
Semiquantitative RT-PCR analysis of transcript levels in 35S:KAN1-GR plants was performed as described (5), using the AS2 primers LBD6-F (5′-ATTTCCCCTCTGAGCAACAG-3′) and LBD6-R (5′-AAGACGGATCAACAGTACGG-3′), the AS1 primers AS1-F (5′- GGAAGTTGCTCTTGAGTTTGG-3′) and AS1-R (5′- ACTTCCCTAACCGCTTTGCCG-3′), and the ACT2 primers ACT2-C (5′-ACTCACCACCACGAACCAG-3′) and ACT-N (5′-AAAATGGCCGATGGTGAGG-3′). For ChIP analysis, ≈200 surface-sterilized seeds of as2–5D 35S:KAN1-GR, as2–5D, AS2 35S:KAN1-GR, or wild-type were sown on 1/2X MS media without sucrose. Nine-day-old seedlings were treated with 10 μM DEX or mock solution for 4 h. Seedlings were harvested, washed with deionized water, and cross-linked with 1% formaldehyde. Cross-linking was quenched by adding 0.125 M glycine. Nuclear extract preparation and immunoprecipitation procedures were adapted (31) with the following modifications: Sonication conditions for nuclear extracts were empirically determined to obtain an average DNA size of 600 bp. Sonication was performed on ice at power setting 6 (40% duty cycle and 20% input) for 12 s repeated four times with 1-min pauses between for 35S:KAN1-GR and wild-type or 9 s repeated four times with 1-min pauses between for as2–5D 35S:KAN1-GR and as2–5D samples. After chromatin shearing, 10 μl of anti-GR P-20 (Santa Cruz Biotechnology) was added to immunopreciptate KAN1-GR proteins. After reversing cross-links, DNA was purified by phenol/chloroform extraction, ethanol-precipitated, and resuspended in 50 ml of Tris-EDTA. One microliter of immunoprecipitated DNA was used in ChIP PCR. Input DNA was diluted 120 times to achieve comparable PCR product band intensities. Primers recognizing different regions in the promoter of AS2 indicated in Fig. 3 were as follows: AS2A-F (5′-TTTGACCGAAGAAACTTTGAGGAC-3′), AS2A-R (5′-AACCCTTGGACCCTAGCATAGACT-3′), AS2B-F (5′-CGAAAAGCACCTTCATGTTACTCA-3′), AS2B-R (5′-AAGTTGCTCAATCTTTATTGTTGTCA-3′), AS2C-F (5′-TTGACAATCGCTTAACCCAAAGTT-3′), and AS2C-R (5′-GGCACCGTTTCTTAGATGCTTTAG-3′). Control primers were RPL4-F (TGTGTTTGTTCATTACTGTGCTATGC), RPL-R (5′-ATAAAGCTGGCGGTTCGAGT-3′), IAA2-L (5′-CGGGTCGGCCGATAGAAT-3′), and IAA2-R (5′-TCGGAAGCATGAAAGGCAAG-3′). PCR conditions were 33 cycles of 94°C for 30 s, 56°C (except AS2C, which was 53°C) for 30 s, and 72°C for 30s. ChIP analysis performed on wild-type and as2–5D seedlings lacking KAN1-GR displayed no detectable differences (data not shown). DNA band intensity was measured by using the Gaussian Curve method with background subtraction with Molecular Imaging Software 4.0 (Eastman Kodak). To compare the abundance of promoter fragments in DEX- versus mock-treated plants, band intensities were first normalized to the negative-control gene RPL4D. The band intensities of DEX- and mock-treated samples were then divided by the band intensities of the input samples to obtain input-normalized values. The relative abundance of a fragment in DEX- versus mock-treated samples was calculated by determining the ratio of these input-normalized values.
Supplementary Material
Acknowledgments.
We thank Stewart Gillmor and Li Yang for helpful discussions. This work was supported by Department of Energy Grant DE-FG02-99ER20328) (to R.S.P.) and National Science Foundation Grants IBN-0318822 (to P.S.S.) and IBN-0343179 (to R.A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803997105/DCSupplemental.
References
- 1.Bowman JL, Eshed Y, Baum SF. Establishment of polarity in angiosperm lateral organs. Trends Genet. 2002;18:134–141. doi: 10.1016/s0168-9525(01)02601-4. [DOI] [PubMed] [Google Scholar]
- 2.Kidner CA, Timmermans MC. Mixing and matching pathways in leaf polarity. Curr Opin Plant Biol. 2007;10:13–20. doi: 10.1016/j.pbi.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 3.McConnell JR, et al. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- 4.Prigge MJ, et al. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin WC, Shuai B, Springer PS. The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial–abaxial patterning. Plant Cell. 2003;15:2241–2252. doi: 10.1105/tpc.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, et al. Novel as1 and as2 defects in leaf adaxial–abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development. 2003;130:4097–4107. doi: 10.1242/dev.00622. [DOI] [PubMed] [Google Scholar]
- 7.Garcia D, Collier SA, Byrne ME, Martienssen RA. Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol. 2006;16:933–938. doi: 10.1016/j.cub.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 9.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- 10.Sawa S, et al. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 1999;13:1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegfried KR, et al. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 12.Pekker I, Alvarez JP, Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenkel S, Emery J, Hou BH, Evans MM, Barton MK. A feedback regu-latory module formed by LITTLE ZIPPER and HD-ZIPIII Genes. Plant Cell. 2007;19:3379–3390. doi: 10.1105/tpc.107.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallory AC, et al. MicroRNA control of PHABULOSA in leaf development: Importance of pairing to the microRNA 5′ region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emery JF, et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131:2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- 17.Allen E, Xie Z, Gustafson AM, Carrington JC. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Eshed Y, Baum SF, Bowman JL. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell. 1999;99:199–209. doi: 10.1016/s0092-8674(00)81651-7. [DOI] [PubMed] [Google Scholar]
- 19.Iwakawa H, et al. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol. 2002;43:467–478. doi: 10.1093/pcp/pcf077. [DOI] [PubMed] [Google Scholar]
- 20.Iwakawa H, et al. Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J. 2007;51:173–184. doi: 10.1111/j.1365-313X.2007.03132.x. [DOI] [PubMed] [Google Scholar]
- 21.Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002;14:547–558. doi: 10.1105/tpc.010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venglat SP, et al. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:4730–4735. doi: 10.1073/pnas.072626099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha CM, Jun JH, Nam HG, Fletcher JC. BLADE-ON-PETIOLE1 and 2 control Arabidopsis lateral organ fate through regulation of LOB domain and adaxial–abaxial polarity genes. Plant Cell. 2007;19:1809–1825. doi: 10.1105/tpc.107.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y, et al. Genetic interactions between leaf polarity-controlling genes and ASYMMETRIC LEAVES1 and 2 in Arabidopsis leaf patterning. Plant Cell Physiol. 2007;48:724–735. doi: 10.1093/pcp/pcm040. [DOI] [PubMed] [Google Scholar]
- 25.Chalfun-Junior A, et al. ASYMMETRIC LEAVES2-LIKE1 gene, a member of the AS2/LOB family, controls proximal–distal patterning in Arabidopsis petals. Plant Mol Biol. 2005;57:559–575. doi: 10.1007/s11103-005-0698-4. [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa M, et al. Activation tagging, a novel tool to dissect the functions of a gene family. Plant J. 2003;34:741–750. doi: 10.1046/j.1365-313x.2003.01758.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunter C, et al. Trans-acting siRNA-mediated repression of ETTIN and ARF4 regulates heteroblasty in Arabidopsis. Development. 2006;133:2973–2981. doi: 10.1242/dev.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiwari SB, Hagen G, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16:533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. A mini binary vector series for plant transformation. Plant Mol Biol. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 31.Gendrel AV, Lippman Z, Yordan C, Colot V, Martienssen RA. Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science. 2002;297:1871–1873. doi: 10.1126/science.1074950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.