Abstract
Historical datasets documenting changes to gene frequency clines are extremely rare but provide a powerful means of assessing the strength and relative roles of natural selection and gene flow. In 19th century Britain, blackening of the environment by the coal-fired manufacturing industry gave rise to a steep cline in the frequency of the black (carbonaria) morph of the peppered moth (Biston betularia) across northwest England and north Wales. The carbonaria morph has declined across the region following 1960s legislation to improve air quality, but the cline had not been comprehensively described since the early 1970s. We have quantified changes to the cline as of 2002, equivalent to an interval of 30 generations, and find that a cline still exists but that it is much shallower and shifted eastward. Joint estimation of the dominant fitness cost of carbonaria and dispersal parameters consistent with the observed cline change indicate that selection against carbonaria is very strong across the landscape (s ≈ 0.2), and that dispersal is much greater than previously assumed. The high dispersal estimate is further supported by the weak pattern of genetic isolation by distance at microsatellite loci, and it implies that in addition to adult dispersal, wind-dispersed first instar larvae also contribute to lifetime dispersal. The historical perspective afforded by this study of cline reversal provides new insight into the factors contributing to gene frequency change in this species, and it serves to illustrate that, even under conditions of high dispersal and strong reverse selection acting against it, complete erosion of an established cline requires many generations.
Keywords: Biston betularia, industrial melanism, cline change
Systematic changes in phenotype or genotype along an environmental gradient, or clines, are among the most visible signals of ongoing or recent selection in nature. If, through a change in environmental conditions, the selection producing the cline ceases to operate, gene flow, brought about by dispersal, will homogenize the differences, eventually eliminating the cline. The time required for this process can be predicted (1), but to do so it is necessary to evaluate the interacting effects of selection, gene flow, effective population size, and mutation. Industrial melanism in the peppered moth, Biston betularia, represents a textbook example of a pulse of gene frequency change that gave rise to a cline, followed by reduction in frequency after the selection reversed in direction. The purpose of this study is to quantify changes to the cline in northwest England and Wales during the latter phase spanning 30 years, and to evaluate the roles of selection and gene flow in producing this change.
The cline of melanic B. betularia arose in the second half of the 19th century, following the appearance of a completely black morph (known as carbonaria) first recorded from Manchester, in northwest England, in 1848, although a museum specimen of unknown provenance dates from before 1811 (2). A large body of evidence supports the view that the environmental gradient underlying the cline was the color of the background on which the moths rest, primarily branches and trunks of trees. The melanic morph was more cryptic to bird predators on dark backgrounds prevalent in the highly industrialized east, whereas the nonmelanic (typica) morph was more cryptic on lighter backgrounds in the less-polluted west (reviewed in ref. 3). This cline appeared stable up until the early 1970s when, in association with declining air pollution (primarily in the form of soot and SO2), there was a rapid fall in carbonaria frequency (3). A parallel trend in melanic frequency occurred in The Netherlands (4) and in the American subspecies, B. betularia cognataria (5, 6). Breeding experiments have shown that the polymorphism is controlled by a dominance series of alleles at a single locus, with carbonaria being fully dominant to typica (7). Up to five additional (insularia) morphs, characterized by intermediate levels of melanism, have been recognized. The insularia alleles are recessive with respect to carbonaria but dominant to typica (8).
When Bishop (9) examined the Welsh cline in 1972 (covering the western portion of the longer transect in our study), he found that melanic frequency was displaced to the west compared with estimates expected from selective predation experiments carried out along it. Subsequent modeling of the frequencies in this region and countrywide (10–14) showed that, given the existing results for dispersal and visual selection by bird predators, a fit could not be made unless an additional selective advantage arising from another (nonvisual) cause was included to counteract the strong selection against melanics in rural areas. The difficulty is that there is no convincing evidence for nonvisual components to the selection or for frequency-dependent predation. More recently, comparison of temporal change in carbonaria frequency at scattered locations in United Kingdom (3) suggested that since the mid-1970s selection pressure against the carbonaria morph has actually been stronger in postindustrial environments than in rural environments. A plausible explanation for the apparent association between disadvantage to carbonaria and its frequency before reduction of industrial fallout is that rural habitats provide greater heterogeneity in resting backgrounds than the postindustrial urban environment, and therefore enhanced crypsis for melanic moths. Crucially, however, these analyses did not explicitly consider the influence of gene flow.
Selection coefficients estimated from several independent predation experiments (3) in polluted and unpolluted habitats show clear fitness differences among typica and carbonaria morphs, in the expected direction, but are associated with large standard errors and have been criticized on the grounds that the resting positions used for experimental moths (tree trunks) were unrepresentative of natural resting positions (15). Although this might alter the exposure of adult moths to bird predators and the relative importance of different predator species, as there was a high degree of correlation in the color of all possible resting backgrounds in polluted versus unpolluted sites, the qualitative fitness differences from these experiments are not in doubt. On the other hand, these selection coefficients are unsuitable for quantitative predictions of cline change.
Quantitative information on gene flow in B. betularia populations has been limited to migration estimates for adult male moths derived from mark-release-recapture (MRR) experiments (9, 16, 17). The MRR studies indicate that Biston males are highly dispersive, able to fly 2 km per night, with about 20% traveling 3–5 km from their site of emergence in their lifetime. These estimates do not account for the greater propensity of males to disperse on the night of eclosion (17), and are therefore likely to be underestimates. Dispersal of adult females is thought to be minimal, but hatching larvae are behaviorally and morphologically adapted for wind dispersal (18), potentially making a greater contribution to lifetime dispersal than the adult phase. Estimates of adult male migration do not present the complete picture and are not a reliable measure of gene flow.
Given the deficiencies with the available information on selection coefficients and gene flow, we estimate selection and effective dispersal parameters directly from a comparison of the cline across north Wales and northwest England as it existed up to the early 1970s with the pattern in 2002. As B. betularia is univoltine in the United Kingdom, this is equivalent to about 30 generations. An independent measure of gene flow is provided by analysis of the spatial pattern of allelic variation at a set of neutral genetic loci, which we contrast with variation at the color morph locus among the same populations.
Results
Cline Change.
The “early” cline is derived from 150 sites sampled from 1959 to 1976, which provided records for 288 site/years [supporting information (SI) Table S1]. These data, together with the long-term continuous record at Caldy, 15 km from central Liverpool, and less complete ones from Manchester (19) and Leeds (20), suggest that change began to occur during the latter part of the 1960s, but the overall picture is one of stability throughout this early period, which we refer to as 1972 (SI Text). Morph frequencies for the cline around 2002 along a comparable transect of 200 km were recorded by assembly trapping at 20 sites in 2002, from eastern north Wales to Leeds, plus 2001–2006 records from an additional 15 sites in western north Wales (Fig. 1 and Table S2).
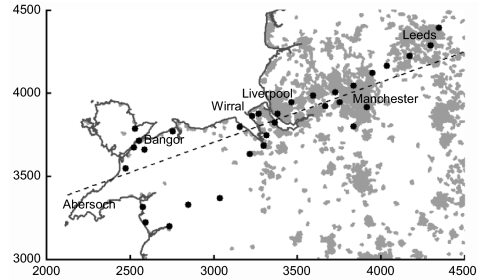
Fig. 1.
Map of northwest England and north Wales, showing 2000–2006 sampling locations (circles) and urban areas (shaded areas). The axis along which the cline is analyzed is shown as a dashed straight line (see Materials and Methods). Axes follow the United Kingdom ordnance survey grid reference system (in units of 0.1 km).
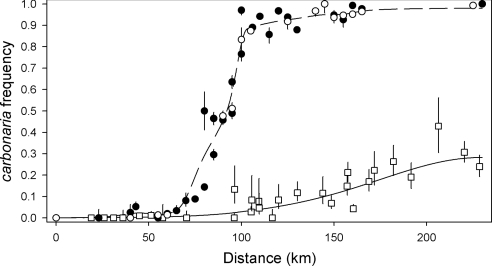
Major changes have occurred in the shape of the cline since the 1960s and early 1970s (Fig. 2). During the early period, a steep decline in melanic frequency occurred in eastern north Wales (just west of Wirral peninsula in Fig. 1), where carbonaria frequency fell from 85% to 15% over a distance of about 20 km (transect position 80–100 km in Fig. 2). The cline in 2002 is much shallower, broader, and shifted eastwards. From frequencies of greater than 95% east of Liverpool, approaching 100% east of Manchester, the carbonaria morph has declined to less than 10% in Liverpool, reaching a maximum of about 30–40% in the easternmost region. Nevertheless, a cline, albeit diminished, remains, raising the question of whether it is maintained by spatially varying selection or whether it is entirely a relic of the historical cline under uniform selection against the carbonaria morph.
Fig. 2.
Clines of the dominant melanic phenotype carbonaria of the moth B. betularia on a WSW to ENE transect across a region spanning Abersoch on the western edge of north Wales to Leeds in northern England (Liverpool and Manchester are located at positions 120 km and 160 km, respectively). Circles indicate the early cline up until 1975 (filled circles 1964–1969, open circles 1970–1975); squares show the cline in 2002; larger standard errors in the 2002 series are due to smaller sample sizes (standard errors were computed as 68.2% score confidence intervals, not the usual Gaussian approximation; ref. 49). Frequencies of intermediate morphs (insularia) have been included with typical frequencies. Throughout the period insularia has been uncommon, so that although sometimes proportional change in insularia has been quite great, the overall effect on melanic frequency is small. The picture seen is essentially unchanged if the melanic morphs are grouped together. The Bézier curves describing the early cline are indicated by the dashed line; the solid line shows the predicted cline after 30 generations, when the cost of carbonaria and dispersal are set to their maximum likelihood values (s = 0.19 and σ2 = 184 km2).
Estimating Selection and Dispersal from Cline Change.
Selection and dispersal parameters can be estimated from changes in cline shape over time (21). We estimated these parameters jointly by maximum likelihood (ML) (22) for a deterministic model of cline change, where the initial cline shape was given by interpolation from the compressed 1967 and 1972 data (Fig. 2 and Table S3), and we derived likelihood ratio confidence intervals (CIs) from profile confidence curves. To assess whether selection against melanics varies across the cline, we considered a linear predictor of the dominant cost s of melanism (i.e., the selection coefficient), where the predictor was the frequency of the carbonaria morph itself (carb) as a good descriptor of environmental condition. That is, we estimated the coefficients a and b of s(carb) = a + b carb. Initial carb is taken from the early cline and then changes iteratively. Dispersal was described by four parameters, including a fraction of spatially uniform dispersal (m) over the whole cline and, for the remainder of the population of males, a convenient three-parameter distribution of dispersal distances (23). The most important parameter of this distribution is the mean square parent–offspring distance (σ2). The two other parameters further describe the shape of the distribution but were found to have little effect on cline evolution (see Materials and Methods for further details).
As the typica allele is recessive, its increase in frequency when the selection for melanism is removed is highly sensitive to its initial frequency. Thus, the expected shape of the 2002 cline depends on the precise shape of the 1972 cline. However, the latter shape, and in particular the persistence of the typical phenotype at a few percent in polluted areas and of carbonaria at a few percent in unpolluted areas (Fig. 2), has been difficult to explain by dispersal at the level assumed at the time and selective predation (13). Indeed, preliminary computations showed that the cline should still clearly depart from equilibrium after 100 generations of evolution. Hence, we did not attempt to predict or to fit the 1972 cline to any theoretical prediction, but described it using a polynomial interpolation method (Bézier curves). Three Bézier curves corresponding to west-southwest (WSW), center, and east-northeast (ENE) sections of the cline were used to fit the compressed 1967 and 1972 data (Table S3). To test the sensitivity of the conclusions to variation in the frequency of the typical form in the extreme ENE of the cline, the carbonaria morph frequency P was either taken to be 0.995 (first three samples in Table S1), or more conservatively assumed to be the mean of the first six samples in Table S1 (0.98).
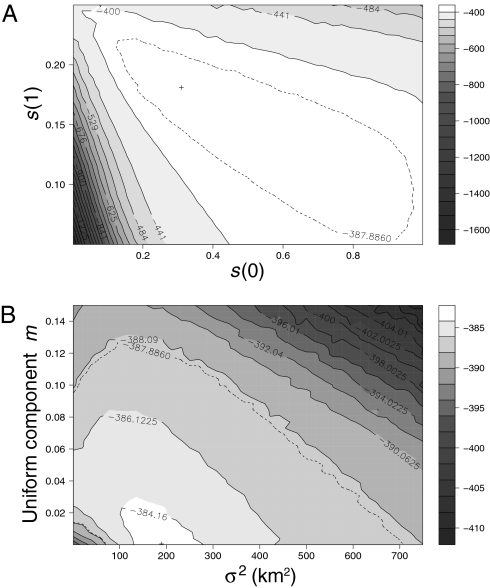
With initial extreme ENE carbonaria frequency P = 0.98, a profile likelihood surface for s(0) and s(1) (cost in locations where initial cabonaria frequencies were 0 and 1, respectively; Fig. 3A) shows that s is always high. The ML estimate of s(1) is 0.186 and can be decreased only if the decrease is compensated by a very large increase in s(0). Low values of s(0) are clearly rejected, with a lower confidence limit of 0.163 and an ML estimate of 0.28. The data are compatible with much higher values of s(0), for the simple reason that these also result in null carbonaria frequencies in the WSW part of the cline.
Fig. 3.
Profile likelihoods for (A) the coefficient of selection s acting against the carbonaria morph of B. betularia at carbonaria frequencies of 0 and 1, and (B) dispersal parameters: variance of dispersal distance σ2 and uniform component m. They were derived from 4 million simulated points. In each plot the cross is the ML estimate and the dashed line is the contour of the 95% confidence region, computed from the likelihood ratio corrected for over-dispersion. The overdispersion of the fitted model was moderate, 1.534 when estimated by the Pearson residuals method as described by Venables and Ripley (50).
Overall, there is only very weak evidence for variation in s along the cline; however, the confidence intervals for dispersal parameters derived below do not rest on an assumption of no such variation. By their nature, profile confidence intervals for each parameter take into account the uncertainty in estimates of all other parameters. The results imply large dispersal, and the best fit suggests that this dispersal is in the form of nonuniform dispersal with a large σ2 (ML estimate of σ2 = 184.5 km2; Fig. 3B), rather than with some uniform component of dispersal over the landscape. Confidence regions are large but always imply more dispersal than has previously been inferred, either in the form of some uniform component of dispersal or as a confidence interval of 85–450 km2 for σ2 in the absence of uniform dispersal. The main conclusions are only strengthened when an initial ENE frequency of P = 0.995 is assumed, as the main effects are to shift upward the confidence region for selection at the ENE end [s(1)] by approximately 0.015, and to shift upward the lower boundary of the confidence region for dispersal parameters (σ2 > 125 km2 in the absence of a uniform component).
The importance of uniform versus nonuniform dispersal could, in principle, be further distinguished from the shape of equilibrium clines, as the steepness of such clines depends mainly on the σ2 value for the nonuniformly dispersing fraction of individuals (24). The observed steepness of the initial cline is much closer to that of the initial clines predicted with the ML estimates (without a uniform component) than it is to the predictions with a uniform component of dispersal and a small σ2. This suggests that a large uniform component of dispersal, although consistent with the recent cline evolution, could be ruled out by additional analyses of the initial clines, but such analyses would be complicated by the fact that these clines were probably not at equilibrium.
Independent Estimates of Dispersal.
To further test our conclusions, we have derived an independent estimate of gene flow from the pattern of genetic isolation by distance, in which the product of effective population density De and variance of dispersal distance σ2 (neighborhood size) can be inferred from the slope of the regression of a standardized measure of genetic similarity between all pairs of individuals or populations on the Euclidean distance between those pairs. For purposes of illustration we use pairwise FST/(1 − FST) between population samples (25), but we prefer the individual-based estimator ê for estimating σ2, which in general should perform better for highly mobile species (26). Genetic similarity estimators were computed from nine unlinked microsatellite loci (27) in a subsample of 367 male moths from 20 sites in the 2002 cline survey.
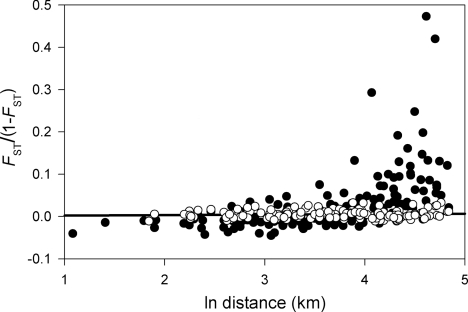
Pairwise FST for microsatellite loci is uniformly low (pairwise mean = 0.005, global FST = 0.004), and the pattern of isolation by distance reveals a very shallow slope (Fig. 4), indicating low levels of neutral genetic differentiation along the cline and suggesting concomitantly high gene flow. The estimated slope for microsatellite loci based on ê is 0.0008, but with a very wide confidence interval (0 to 0.0018), thus only allowing us to reject low estimates of σ2. In this respect it is consistent with the cline evolution analysis, but it does not bring further information. A nonsignificant Mantel test confirms that the signal of isolation by distance is very weak (P = 0.37). Taking De = 44 individuals/km2 (see Materials and Methods for further details), the 95% confidence interval for σ2 is consistent with any σ2 greater than 1 km2.
Fig. 4.
Contrasting pattern of pairwise isolation by distance for putatively neutral microsatellite loci (white circles) and color morph locus (black circles) in B. betularia along a 130-km (2002) transect in northern Britain. The black line shows the linear regression for microsatellite loci, reflecting the level of gene flow. Because melanic (carbonaria and insularia) alleles are dominant to the typica allele, the genotype at the color locus is fully specified by phenotype only in the case of typica, which are always homozygous for the t allele. As insularia is rare throughout the transect (and recessive to carbonaria); all insularia were assumed to be heterozygous for insularia and typica alleles (it), allowing the local frequency of i and c, the carbonaria allele, to be estimated. Expected numbers of heterozygous (ct) and homozygous (cc) carbonaria then were calculated for each site, assuming Hardy-Weinberg equilibrium, which is supported by microsatellite loci (27).
Discussion
Thirty generations of strong selection against the carbonaria morph have left a much diminished cline to that described by Bishop and coworkers (Fig. 2; refs. 9 and 28). The greatest absolute change in carbonaria frequency has taken place in Liverpool (0.96 to 0.08) and the Wirral peninsula (0.86 to 0.03). The cline is much flatter and shifted eastward, but a progressive increase in frequency from west to east has persisted. Near York, about 40 km northeast of Leeds, Cook et al. (20) obtained 65.3% carbonaria in 1990–1994 (n = 118) and 27% in 2000–2004 (n = 131). These figures suggest that frequencies near York fell over the decade of collecting but have remained higher than those found near Leeds. The recent figures, therefore, probably rise toward a quite extensive region, including the west Yorkshire conurbation and the more rural country to the east, where carbonaria frequency may still be as high as about 30%. Our model predicts that even in this region, carbonaria frequency will fall to approximately 1% by 2018. This means that from the first signs of carbonaria decline in the late 1960s, the near-complete erosion of the cline will have taken roughly 50 generations.
The dominant cost of melanism was allowed to vary over the position along the cline, but very little evidence was found for such variation. The fitness cost estimate was approximately 0.2 (CI: 0.19–0.215) if assumed constant, and could be reduced in the ENE only by accepting an implausibly large increase at the opposite end of the cline. The magnitude of our estimate is consistent with estimates obtained using a different method (29) from records of continuous change at seven United Kingdom sites (including three on our transect). Previous models constructed to account for the spatial variation in carbonaria frequencies have assumed a value for adult male migration of 2.5 km/day (10, 13), liberally derived from one MRR experiment (9), and that effects of gene flow in different directions tend to cancel out (29, 30). In contrast to earlier studies we have jointly estimated selection and dispersal parameters from temporal changes along a cline. Our analysis suggests that the true scale of dispersal is much greater and has played a crucial role in the evolution of this cline. The ML estimate for σ2 of 184 km2 implies a median dispersal distance of 9 km (CI: 6.2–14.3 km), approximately five times greater than mean dispersal distance of adult males implied by the MRR experiment by Bishop (9). Part of this discrepancy is probably due to a failure to account for the greater propensity to disperse of newly eclosed males, as revealed by a subsequent MRR experiment (17).
We also propose that wind-assisted dispersal of newly hatched larvae is additionally necessary to account for the higher level of lifetime dispersal. Although this has not been measured directly, a series of morphological and behavioral characters strongly suggest that newly hatched larvae disperse by “ballooning.” Silk threads allow the very small (<2 mm) and light (<0.5 mg) larvae to be carried by the gentlest breeze (18). Furthermore, the majority of a brood do not feed during the first 2 days after hatching, but actively crawl toward light at a rate of ≈7 m/hr (N. Edmonds, unpublished results), a behavior that is expected to take them to the outer tree canopy, from where they can be most easily transported into the turbulent boundary layer, where summer wind speeds average 5 m/s. Given these facts, an average dispersal distance of 10 km is entirely plausible and is indirectly supported by a model of aerial dispersal for ballooning linyphiid spiders (31).
An elevated level of dispersal resolves two key questions about the evolution of the cline between 1972 and 2002. In particular, it greatly facilitates the rapid response in ENE, where typicals were very rare. Second, the WSW data per se are incompatible with both high dispersal and low cost in rural areas [s(0)], as the homogenizing effect of dispersal would tend to increase the frequency of melanics in the middle to WSW part of the cline, so that selection must be strong there. Thus, although our results are consistent with some large variation in cost (Fig. 3A), it is neither necessary to invoke spatially varying cost (3) nor to invoke some hypothetical pleiotropic nonvisual cost (13) to account for changes in carbonaria frequency. The estimates of selection and dispersal would be affected, however, if there were a partially dominant nonvisual cost of carbonaria (resulting in a much faster response to selection from low initial typica frequencies), or if dispersal was directional and predominantly toward ENE [so that high dispersal would be consistent with persistent low carbonaria frequencies in the middle/WSW part of the cline, without the need for high s(0)].
Comparison of pairwise FST among numerous loci is an effective and popular means of identifying genomic regions under selection (32). At face value, the contrast between the pattern of differentiation between microsatellite and melanism loci (Fig. 4) would be taken as a clear signature of strong spatially varying selection currently operating on the carbonaria switch locus to counteract the effect of high gene flow. In the present case, however, by virtue of the exceptional historical record available for this polymorphism, we have shown that the contemporary pattern of genetic differentiation at this locus is not maintained by ongoing selection, but rather is a gradually dissipating signature of past selection in a rapidly transformed industrial environment. A measure of the change to resting backgrounds is provided by a survey of samples of trees along the cline in 1973 showing low light reflectance and little epiphyte cover to the east, where carbonaria frequency was 80% or more, with much greater extent and variety of epiphytes to the west; in a 1986 resurvey, epiphyte cover at the eastern end had increased, and differences along the cline had diminished (33).
Predation experiments by Kettlewell (34) and others (3) imply that carbonaria is strongly deleterious in unpolluted rural settings. Using this assumption, the precise shape of the north Wales cline as existed around 1970 could not be accounted for without including a nonvisual advantage to melanics, applied either to melanic heterozygotes only (9) or to heterozygous and homozygous melanic genotypes (12). The only other possibility was that migration occurred at a rate then considered to be “unreasonably high” (12). As data on declines in melanic frequency in various parts of the country accumulated, it appeared that selection against carbonaria was lower in rural areas than in historically polluted areas (summarized in refs. 3 and 29). As before, this assumes relatively low migration, and it provides selection against carbonaria in rural areas of 3–4%, obviating the need for a nonvisual component. In the present study, however, we have a second cline to compare with the first. The analysis, which takes a new approach to estimating jointly the contributions of dispersal and selection, suggests that the migration rate is indeed much larger than hitherto supposed. As a result, strong selection against melanics is necessary in the rural part of the cline to counteract the influence of gene flow from the section where melanic frequency is higher. We therefore reinstate the assumption of strong selection in rural conditions, but couple it with a revised, greatly enlarged estimate of migration rate.
Materials and Methods
Morph Frequencies.
1960s and 1970s.
Records given by Kettlewell (34) for 1952–1956 showed carbonaria at a high frequency in northwest England in the region around Liverpool, with frequencies from 90–98%. To the east in Manchester and Bradford, near Leeds, he gives 98% and 96%. Working from Liverpool, Clarke and Sheppard (35, 36) extended the survey into north Wales to study the decline in frequency as one moves southwestward from this highly industrialized region into a rural setting. Samples were collected from 1959 to 1965. Subsequently these authors, Bishop, and others (9, 28, 37, 38) added further study locations and more data from the same sites. A list of 158 locations was compiled, including a few of Kettlewell's 1962–1966 records, with the majority dating between 1962 and 1975 (28). The most westerly site is at Abersoch on the west Welsh coast, and the most easterly is near Leeds. We have condensed the Bishop et al. (28) data, combining records within sites for the periods 1962–1969 and 1970–1975, to produce Table S1.
2000–2006.
Twenty samples were collected between June 20 and July 24, 2002, to establish the recent pattern along the transect. At each site one assembling trap was suspended from the lower branches of a tree at ≈3 m from the ground. Traps were baited with 1- to 2-day-old virgin females and usually were visited every other day, with fresh females generally being added twice a week. There was some variation in trapping effort among sites, with 25 nights being the average. Three samples from Liverpool, which were small and close to each other, have been combined. Three additional datasets have been included: two from the Manchester area at Wilmslow (1998–2003) and Flixton/Urmston (1998–2003) recorded in Cook et al. (39), and one for 11 sites in western north Wales (2001–2006). This provides the list shown in Table S2.
Axis transformation.
The combined transect in Bishop et al. (28) runs from WSW to ENE, and samples with equal frequency occur approximately at right angles to this axis. When frequency is plotted on distance west–east, however, the high frequencies close to Liverpool are the same distance east as the much lower frequencies to the south in Wales, so as to obscure the pattern of the cline. To avoid this overlap and to form a base for comparison with the 2002 data, we have therefore transformed the main axis by calculating C1 = E cosα + N sinα, where E and N are the grid references east and north and α is 20°. This provides a set of locations along an axis running WSW to ENE with a compass bearing of 70°. Plotted on this axis (Fig. 1), the data show the transition more clearly (Fig. 2). The line chosen passes through GR 3350 3830, near Bebington on the Wirral peninsula. The distance of sites from C1, at right angles to it, is given by C2 = −E sinα + N cosα. The C1 transect has been divided into 5-km intervals (or bands). Band 0–1 contains the most westerly site at Abersoch (GR 2315 3285), and band 45–50 contains the most easterly in Leeds (GR 4377 4366). Samples within each band have been divided into those made before 1970 (with a mean collection date of about 1967) and those from 1970 onward (mean collection date about 1972). Within each period, homogeneous samples have been combined, and the resulting realigned and compressed dataset is listed in Table S3.
Modeling Cline Change.
We numerically analyzed models of clines out of equilibrium by using grid space approximations with a step size of 1 km. These computations are based on iterations of standard selection equations for diploid populations (e.g., ref. 40) and dispersal according to a specified dispersal distribution. Selection either before or after dispersal was considered, and the order of these events did not significantly affect the results. The probability of a sample in a given location then was computed according to binomial sampling, with the expected frequency given by the deterministic cline model. The likelihood computation procedure was independently written in Mathematica (41) and in C++, and checked against standard results for equilibrium clines (e.g., ref. 42) and spatial diffusion processes.
Likelihoods computed in this way are a function of six parameters. Confidence intervals for subsets of these parameters were derived by the profile likelihood method, where the profile likelihood for given values of some parameters is the maximum value of the likelihood over all possible values of the other parameters (which are therefore not fixed to their maximum likelihood estimates). This method properly takes into account uncertainty in other parameters. In particular, confidence regions can be determined by the usual likelihood ratio χ2 method, with the number of degrees of freedom given as the number of parameters retained in the profile (22).
Dispersal was modeled as the sum of a uniform dispersal component over the whole area and of a mixture of discretized Gaussian distributions with different variances. The shifted binomial B(n), with terms
is a discrete version of the Gaussian distribution, with zero mean and variance n/4, and can also be viewed as a n-convolution of stepping stone steps. A convenient mixing distribution is the Sichel distribution (23). The Sichel mixture of binomial distributions is a three-parameter distribution family, which allows dispersal to be modeled with three given constraints: a σ2 value, a kurtosis value, and a given power exponent for the tail of the distribution. Wide ranges of the three parameters were considered, but the likelihood was found to be sensitive only to the σ2 of the distribution.
Estimating Gene Flow from Genetic Markers.
Genotypes at 12 microsatellite loci were determined in 375 male moths following the method described in Daly et al. (27). From the 20 locations sampled in 2002, the sample from Greasby had only eight genotypes and was removed from the analysis, and the three Liverpool sites were combined, leaving 367 genotyped individuals from 17 locations, with an average of 22 individuals per location. Genotype frequencies at two loci (Biston 8 and 13) deviate significantly from Hardy-Weinberg expectations (permutation tests of FIS using FSTAT 2.9.3; ref. 43). Furthermore, two groups of loci (group 1 = Biston 4, 8, and 14; group 2 = Biston 6 and 13) were in strong linkage disequilibrium. Exclusion of Biston 4, 8, and 13 left nine independent loci for further analysis.
Microsatellite genotypes were used to calculate ê, an estimator of genetic similarity between pairs of individuals (26), using GENEPOP′007 (44). The slope of the regression of all pairwise ê against ln geographic distance was interpreted as 1/(4πDeσ2), where De is effective density and σ2 is the variance of the axial distance between parent and offspring positions (25, 26).
Effective Density.
To interpret the isolation-by-distance slope for microsatellite loci in terms of a distribution of dispersal distances, it is necessary to estimate the long-term effective density of moths across the region (25). In Britain, B. betularia is known to be widely distributed, with a low to intermediate abundance of adults relative to other common macromoths (45). The 43-year record of B. betularia light trap and assembling catches from 1959–2002 at one site (Caldy) on the transect (46) suggests that in this region, yearly density fluctuations are low (207–1057 males per season, harmonic mean 360). The 2002 transect sample shows definite differences in density across the landscape, presumably due to variation in habitat quality, but these are not extreme. MRR studies (9) provide a correlation between density and light trap catch that we used to calibrate the long-term average seasonal catch across the transect. The estimate so derived is 113 moths/km2 emerging per season over the entire area of the cline. Additionally, the long-term effective density is expected to be strongly influenced by yearly fluctuations in number and by variance in reproductive success. The former effect was estimated by the ratio of the harmonic to the arithmetic mean of mercury vapor light catches at Caldy (0.78); the latter effect is unknown for Biston, but a conservative ratio based on other Lepidoptera (47) and theoretical considerations (48) is 0.5. The combined effect is to reduce the estimate of effective density by a factor of 0.39 to 44 moths/km2.
Supplementary Material
Acknowledgments.
We thank Andrew Graham and John Harold, compilers of the north Wales moth database. Kevin Waltham, Rob Aspden, and John Mulley assisted with sample collection. Some computations were done on the Institut des Sciences de l'Evolution, Montpellier cluster. Comments made by two anonymous referees helped improve a previous version of this paper. Financial support was provided by The Nuffield Foundation and Natural Environment Research Council (NE/C003101/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803785105/DCSupplemental.
References
- 1.May RM, Endler JA, McMurtrie RE. Gene frequency clines in the presence of selection opposed by gene flow. Am Nat. 1975;109:659–676. doi: 10.1086/283036. [DOI] [PubMed] [Google Scholar]
- 2.Berry RJ. Industrial melanism and peppered moths (Biston betularia (L.)) Biol J Linn Soc London. 1990;39:301–322. [Google Scholar]
- 3.Cook LM. The rise and fall of the carbonaria form of the peppered moth. Q Rev Biol. 2003;78:399–417. doi: 10.1086/378925. [DOI] [PubMed] [Google Scholar]
- 4.Brakefield PM, Liebert TG. Evolutionary dynamics of declining melanism in the peppered moth in The Netherlands. Proc R Soc B. 2000;267:1953–1957. doi: 10.1098/rspb.2000.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant BS, Owen DF, Clarke CA. Parallel rise and fall of melanic peppered moths in America and Britain. J Hered. 1996;87:351–357. [Google Scholar]
- 6.Grant B. Recent history of melanism in American peppered moths. J Hered. 2002;93:86–90. doi: 10.1093/jhered/93.2.86. [DOI] [PubMed] [Google Scholar]
- 7.Grant B. Allelic melanism in American and British peppered moths. J Hered. 2004;95:97–102. doi: 10.1093/jhered/esh022. [DOI] [PubMed] [Google Scholar]
- 8.Cook LM, Muggleton J. The peppered moth, Biston betularia (Linnaeus, 1758) (Lepidoptera: Geometridae): A matter of names. Entomol Gaz. 2003;54:211–221. [Google Scholar]
- 9.Bishop JA. An experimental study of the cline of industrial melanism in Biston betularia (L.) (Lepidoptera) between urban Liverpool and rural North Wales. J Anim Ecol. 1972;41:209–243. [Google Scholar]
- 10.Cook LM, Mani GS. A migration-selection model for the morph frequency variation in the peppered moth over England and Wales. Biol J Linn Soc London. 1980;13:179–198. [Google Scholar]
- 11.Mani GS. A theoretical study of morph ratio clines with special reference to melanism in moths. Proc R Soc B. 1980;210:299–316. [Google Scholar]
- 12.Mani GS. A theoretical analysis of the morph frequency variation in the peppered moth over England and Wales. Biol J Linn Soc London. 1982;17:259–267. [Google Scholar]
- 13.Mani GS. Theoretical models of melanism in Biston betularia - a review. Biol J Linn Soc London. 1990;39:355–371. [Google Scholar]
- 14.Mani GS, Majerus MEN. Peppered moth revisited: Analysis of recent decreases in melanic frequency and predictions for the future. Biol J Linn Soc London. 1993;48:157–165. [Google Scholar]
- 15.Majerus MEN. Melanism: Evolution in Action. Oxford, UK: Oxford Univ Press; 1998. [Google Scholar]
- 16.Bishop JA, Cook LM, Muggleton J. The response of two species of moths to industrialization in northwest England. II. Relative fitness of morphs and population size. Philos Trans R Soc London B. 1978;281:517–542. [Google Scholar]
- 17.Brakefield PM, Liebert TG. The reliability of estimates of migration in the peppered moth Biston betularia and some implications for selection-migration models. Biol J Linn Soc London. 1990;39:335–341. [Google Scholar]
- 18.Liebert TG, Brakefield PM. Behavioral studies on the Peppered Moth Biston betularia and a discussion of the role of pollution and lichens in industrial melanism. Biol J Linn Soc. 1987;31:129–150. [Google Scholar]
- 19.Cook LM, Dennis RLH, Mani GS. Melanic morph frequency in the peppered moth in the Manchester area. Proc R Soc B. 1999;266:293–297. [Google Scholar]
- 20.Cook LM, Sutton SL, Crawford TJ. Melanic moth frequencies in Yorkshire, an old English industrial hot spot. J Hered. 2005;96:522–528. doi: 10.1093/jhered/esi082. [DOI] [PubMed] [Google Scholar]
- 21.Lenormand T, Raymond M. Analysis of clines with variable selection and variable migration. Am Nat. 2000;155:70–82. doi: 10.1086/303295. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Principles of Statistical Inference. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 23.Chesson P, Lee CT. Families of discrete kernels for modeling dispersal. Theor Popul Biol. 2005;67:241–256. doi: 10.1016/j.tpb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Rousset F. In: Dispersal. Clobert J, Danchin E, Dhont AA, Nichols JD, editors. New York: Oxford Univ Press; 2001. pp. 18–28. [Google Scholar]
- 25.Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts PC, et al. Compatible genetic and ecological estimates of dispersal rates in insect (Coenagrion mercuriale: Odonata: Zygoptera) populations: Analysis of “neighbourhood size” using a more precise estimator. Mol Ecol. 2007;16:737–751. doi: 10.1111/j.1365-294X.2006.03184.x. [DOI] [PubMed] [Google Scholar]
- 27.Daly D, et al. Trinucleotide microsatellite loci for the Peppered Moth (Biston betularia) Mol Ecol Notes. 2004;4:179–181. [Google Scholar]
- 28.Bishop JA, Cook LM, Muggleton J. The response of two species of moths to industrialization in northwest England. I. Polymorphisms for melanism. Philos Trans R Soc London B. 1978;281:491–515. [Google Scholar]
- 29.Cook LM, Turner JRG. Decline of melanism in two British moths: Spatial, temporal and inter-specific variation. Heredity. 2008 doi: 10.1038/hdy.2008.105. in press. [DOI] [PubMed] [Google Scholar]
- 30.Cook LM, Mani GS, Varley ME. Postindustrial melanism in the peppered moth. Science. 1986;231:611–613. doi: 10.1126/science.231.4738.611. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CFG, Brain P, Jepson PC. Aerial activity of linyphiid spiders: Modelling dispersal distances from meteorology and behaviour. J Appl Ecol. 2003;40:912–927. [Google Scholar]
- 32.Grahame JW, Wilding CS, Butlin RK. Adaptation to a steep environmental gradient and an associated barrier to gene exchange in Littorina saxatilis. Evolution. 2006;60:268–278. [PubMed] [Google Scholar]
- 33.Cook LM. Changing views on melanic moths. Biol J Linn Soc. 2000;69:431–441. [Google Scholar]
- 34.Kettlewell HBD. The Evolution of Melanism. Oxford, UK: Clarendon; 1973. [Google Scholar]
- 35.Clarke CA, Sheppard PM. Frequencies of the melanic forms of the moth Biston betularia (L.) on Deeside and in adjacent areas. Nature. 1963;298:1279–1282. [Google Scholar]
- 36.Clarke CA, Sheppard PM. A local survey of the distribution of industrial melanic forms in the moth Biston betularia and estimates of the selective values of these in an industrial environment. Proc R Soc B. 1966;165:424–439. [Google Scholar]
- 37.Whittle PDJ, Clarke CA, Sheppard PM. Further studies on the industrial melanic moth Biston betularia (L.) in the north west of the British Isles. Proc R Soc B. 1976;194:467–480. doi: 10.1098/rspb.1976.0089. [DOI] [PubMed] [Google Scholar]
- 38.Askew RR, Cook LM, Bishop JA. Atmospheric pollution and melanic moths in Manchester and environs. J Appl Ecol. 1971;8:247–256. [Google Scholar]
- 39.Cook LM, Dennis RLH, Dockery M. Fitness of insularia morphs of the peppered moth Biston betularia. Biol J Linn Soc London. 2004;82:359–366. [Google Scholar]
- 40.Hartl DL, Clark AG. Principles of Population Genetics. Sunderland, MA: Sinauer Associates; 1997. [Google Scholar]
- 41.Wolfram S. The Mathematica Book. Chapmpaign, IL: Wolfram Media; 2003. [Google Scholar]
- 42.Barton N, Gale KS. In: Hybrid Zones and the Evolutionary Process. Harrison RG, editor. New York: Oxford Univ Press; 1993. pp. 13–45. [Google Scholar]
- 43.Goudet J. FSTAT (1.2): A computer program to estimate F-statistics. J Hered. 2001;86:485–486. [Google Scholar]
- 44.Rousset F. GENEPOP′007: A complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resour. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 45.Cook LM, Riley AM, Woiwod IP. Melanic frequencies in three species of moths in post industrial Britain. Biol J Linn Soc Lond. 2002;75:475–482. [Google Scholar]
- 46.Clarke CA, Grant B, Clarke FMM, Asami T. A long term assessment of Biston betularia (L.) in one UK locality (Caldy Common near West Kirby, Wirral), 1959–1993, and glimpses elsewhere. Linnean. 1994;10:18–26. [Google Scholar]
- 47.Brakefield PM, et al. Effective population size, reproductive success and sperm precedence in the butterfly, Bicyclus anynana, in captivity. J Evol Biol. 2001;14:148–156. doi: 10.1046/j.1420-9101.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- 48.Nunney L. Measuring the ratio of effective population size to adult numbers using genetic and ecological data. Evolution. 1995;49:389–392. doi: 10.1111/j.1558-5646.1995.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 49.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 50.Venables WN, Ripley BD. Modern Applied Statistics with S. New York: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.