Abstract
After exposure to DNA-damaging agents that block the progress of the replication fork, monoubiquitination of proliferating cell nuclear antigen (PCNA) mediates the switch from replicative to translesion synthesis DNA polymerases. We show that in human cells, PCNA is monoubiquitinated in response to methyl methanesulfonate and mitomycin C, as well as UV light, albeit with different kinetics, but not in response to bleomycin or camptothecin. Cyclobutane pyrimidine dimers are responsible for most of the PCNA ubiquitination events after UV-irradiation. Failure to ubiquitinate PCNA results in substantial sensitivity to UV and methyl methanesulfonate, but not to camptothecin or bleomycin. PCNA ubiquitination depends on Replication Protein A (RPA), but is independent of ATR-mediated checkpoint activation. After UV-irradiation, there is a temporal correlation between the disappearance of the deubiquitinating enzyme USP1 and the presence of PCNA ubiquitination, but this correlation was not found after chemical mutagen treatment. By using cells expressing photolyases, we are able to remove the UV lesions, and we show that PCNA ubiquitination persists for many hours after the damage has been removed. We present a model of translesion synthesis behind the replication fork to explain the persistence of ubiquitinated PCNA.
Keywords: DNA replication, translesion synthesis, UV damage
The replication of damaged DNA is a topic of much current interest after the discovery of the specialized Y-family of DNA polymerases, which are able to bypass lesions in DNA. There are four Y-family members in mammalian cells, DNA polymerase (pol) η, polι, polκ, and Rev1, each with a different substrate specificity (1–3).
Genetic studies using Saccharomyces cerevisiae have implicated ubiquitin-conjugating systems in the replication of damaged DNA, and the ubiquitination target is the DNA polymerase sliding clamp accessory protein, proliferating cell nuclear antigen (PCNA) (4). In response to DNA damage, Rad6 and Rad18 mediate the monoubiquitination of PCNA on lysine-164, and subsequent polyubiquitination is brought about by Ubc13-Mms2 and Rad5. Monoubiquitination appears to trigger translesion synthesis (TLS) to bypass DNA lesions, whereas polyubiquitination channels the damage into a poorly understood error-free damage-avoidance mechanism (4, 5).
In human fibroblasts, monoubiquitination on lysine-164 is by far the major modification of PCNA and is easily detectable on exposure of replicating cells to DNA damage induced by UV light or to replication arrest by hydroxyurea (HU) (6). Polyubiquitination has recently been detected at much lower levels (7, 8). Monoubiquitination of PCNA increases its affinity for polη, polι, and Rev1 (6, 9–12). The increased affinity of monoubiquitinated PCNA (Ub-PCNA) for Y-family polymerases is mediated by ubiquitin-binding domains that have been identified in all of the Y-family polymerases (10–13) and provides a mechanism for bringing about the polymerase switch, whereby the blocked replicative DNA polymerase is replaced by a TLS polymerase that can bypass the blocking lesion (1). Ub-PCNA activates the in vitro damage bypass activities of polη and Rev1 (14).
Whereas ubiquitination of PCNA is brought about by the Rad6-Rad18 system, it is kept in check in human cells by the deubiquitinating enzyme (DUB) USP1 (15). After high doses of UV, USP1 disappears from the cell (15, 16).
In this article we examine the response of PCNA ubiquitination to different DNA-damaging agents in human cells, we show that mutation of PCNA-K164 confers UV and methyl methanesulfonate (MMS) sensitivity to the cells, we demonstrate that PCNA ubiquitination and activation of cell cycle checkpoints are independent events, and we show that PCNA ubiquitination persists even after removal of the lesions.
Results
PCNA Ubiquitination After Exposure to Different Damaging Treatments.
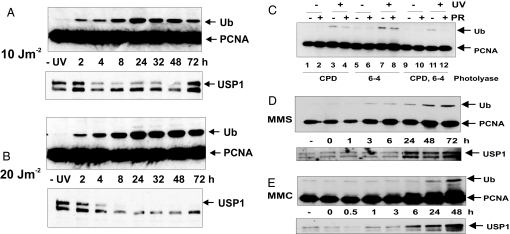
We showed that PCNA was monoubiquitinated in response to UV-irradiation or treatment with HU, but not with ionizing radiation (6). In our earlier work, PCNA ubiquitination remained elevated for at least 24 h after UV-irradiation, and our data shown in Fig. 1A and B Upper indicate that this elevated level persisted in MRC5V1 cells for >48 h after UV doses of 10 and 20 J m−2. In all experiments shown, we loaded the same proportion of the total cell population in each lane. Thus, the intensity of the band corresponding to Ub-PCNA represents the absolute level of Ub-PCNA in the culture rather than the amount relative to unmodified PCNA or per microgram of protein. We have analyzed the data in this way to avoid any apparent loss of PCNA ubiquitination by dilution when cells divide.
Fig. 1.
PCNA ubiquitination after DNA-damaging agents. (A and B) MRC5V1 cells were UV-irradiated with 10 (A) or 20 (B) J m−2, incubated for the indicated times, and analyzed by immunoblotting with anti-PCNA (Upper) or anti-USP1 antibody (Lower). −UV, mock-treated cells incubated for 6 h. (C) XP-A cells expressing the indicated photolyase were UV-irradiated (10 J m−2), exposed or not to photoreactivating light for 2 h (PR), incubated for a further 6 h, and analyzed as in A. (D and E) Cells were treated with 1 mM MMS for 1h (D) or 8 μg/ml mitomycin C for 30 min (E), followed by incubation for the indicated times before harvesting and analysis by immunoblotting.
UV-irradiation generates two major photoproducts in DNA, cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts (6-4PP). To determine which of these lesions is responsible for the ubiquitination of PCNA, we used XP-A cells that express photolyases specific for CPD, 6-4PP, or both (17). When UV-irradiated cells are exposed to visible light immediately after UV-irradiation, the photolyases reverse the cognate photoproducts in situ. More than 90% of the lesions disappear from the DNA upon exposure to visible light for 90–120 min [supporting information (SI) Fig. S1]. Removal of just the CPDs results in a significant reduction of PCNA ubiquitination (Fig. 1C, compare lanes 3 and 4). In contrast, removal of 6-4PP has a barely detectable effect (lanes 7 and 8), but this might be expected because 6-4PPs form only 20–30% of total photoproducts. Removal of both photolesions prevents the ubiquitination completely (lanes 11 and 12). We conclude that both photoproducts are able to elicit ubiquitination of PCNA.
In an attempt to understand the triggering structure(s) for PCNA monoubiquitination, we have exposed cells to different agents and measured PCNA ubiquitination for extended periods of time after damaging treatments. The monofunctional methylating agent, MMS, generates mainly 7-methylguanine and 3-methyladenine in DNA together with a small amount of O-6-methylguanine (18). Fig. 1D Upper shows that Ub-PCNA was detectable 3 h after a 1-h MMS treatment and increased in intensity at 24 h and later times. With the cross-linking agent mitomycin C (MMC), little Ub-PCNA was detectable in the first few hours after treatment, but as with MMS treatment, a strong band appeared at 24 h and increased in intensity up to 48 h (Fig. 1E Upper).
In contrast to these agents, which generate chemical alterations in DNA, bleomycin, like ionizing radiation, produces double-stranded breaks. With this agent, minimal Ub-PCNA was detected up to 48 h after treatment (Fig. S2A). Similar results were obtained with camptothecin, an inhibitor of topoisomerase I that generates double-stranded breaks in DNA during DNA replication (data not shown). No PCNA ubiquitination was detected after treatment with the microtubule spindle poison nocodazole (data not shown), confirming that agents that disrupt cell cycle progression without affecting DNA replication do not induce the ubiquitination of PCNA.
Failure to Ubiquitinate PCNA Confers UV and MMS Sensitivity.
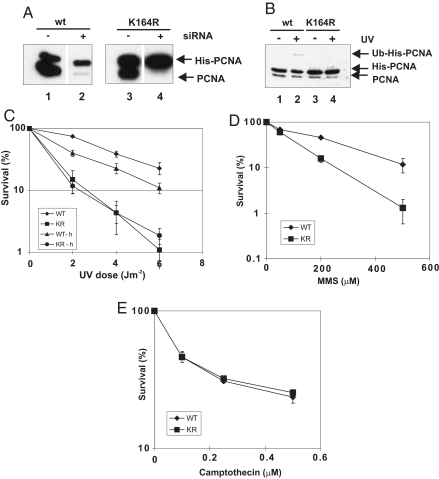
We have used SV40-transformed MRC5V1 human fibroblasts to generate cell lines expressing His-tagged PCNA, either wild-type or mutated at lysine-164, at levels similar to those of endogenous PCNA (Fig. 2A, lanes 1 and 3). The cDNA for the exogenous PCNA-contained silent mutations to make it refractory to targeting by siRNA directed against endogenous PCNA. By using siRNA, we were able to deplete the endogenous PCNA such that >80% of the PCNA is expressed from the transfected cDNA (Fig. 2A, lanes 2 and 4). The transfected wild-type His-PCNA is ubiquitinated after UV-irradiation (Fig. 2B, lane 2), whereas the K164R mutant His-PCNA is not (Fig. 2B, lane 4). Depletion of the endogenous PCNA results in a substantial sensitization of cells expressing mutant PCNA to UV-irradiation (Fig. 2C, KR) compared with those expressing wild-type PCNA (Fig. 2C, WT). Additional depletion of polη in cells expressing wild-type PCNA results in only a modest decrease in survival after UV-irradiation (Fig. 2C, WT-h), and in cells expressing mutant PCNA there is no further decrease in survival (Fig. 2C, KR-h). These data demonstrate the importance of PCNA ubiquitination for cell survival after UV-irradiation.
Fig. 2.
UV sensitivity of cells expressing PCNA-K164R. (A) MRC5V1 cell clones expressing His-tagged wild-type PCNA or PCNA-K164R were either mock-transfected or treated with PCNA-specific siRNA and PCNA levels measured after 72 h. (B) After transfection with PCNA siRNA, the cells were UV-irradiated (20 J m−2), incubated for 5 h, and analyzed for PCNA ubiquitination. (C–E) UV, MMS, and camptothecin survival curves of cells depleted for endogenous PCNA and expressing wild-type (WT) or mutant (KR) His-PCNA. Where indicated (-h), cells were also depleted for polη. Error bars, ±SEM of three or four experiments.
The cells expressing only PCNA-K164R are also sensitive to MMS (Fig. 2D) but not to camptothecin (Fig. 2E) or bleomycin (Fig. S2B), consistent with the patterns of ubiquitination of PCNA (Fig. 1).
PCNA Ubiquitination and Cell Cycle Checkpoints.
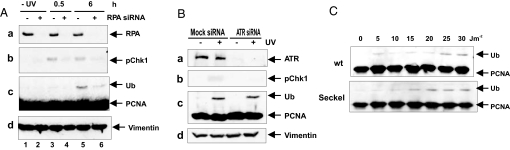
The spectrum of damaging agents giving rise to PCNA ubiquitination, its dependence on Rad18, and the single-stranded DNA-binding properties of Rad18 lead to the hypothesis that single-stranded DNA exposed at the site of stalled forks can trigger Rad18-mediated ubiquitination of PCNA. Single-stranded regions are also the trigger for cell cycle checkpoints mediated by the ATR protein kinase. To trigger the checkpoint, single-stranded DNA needs to be coated with the single-stranded DNA-binding protein, RPA (19). We depleted cells of RPA by using the same conditions as Zou and Elledge (19). In our initial experiments, we obtained substantial depletion of RPA without affecting the ubiquitination of PCNA after UV treatment (data not shown). However, when we altered our transfection conditions, we were able to reduce RPA to levels that were undetectable on Western blotting (Fig. 3Aa). Under these conditions, phosphorylation of Chk1 was reduced as described in ref. 19 (Fig. 3Ab), and we found that Ub-PCNA formation was also reduced substantially (Fig. 3Ac). We were concerned that depletion of RPA might deplete the S phase population of the cells and that the decreased ubiquitination of PCNA might be a secondary consequence of a lack of S phase cells. We therefore analyzed the cell cycle status of the RPA-depleted population. Flow cytometry showed that the cell cycle distribution of cells depeleted of RPA was very similar to that of undepleted cells (Table S1), suggesting that, although undetectable by immunoblotting, residual RPA is sufficient to permit DNA replication to continue. Our data suggest, therefore, that ubiquitination of PCNA depends on RPA.
Fig. 3.
PCNA ubiquitination in RPA or ATR knockdown cells. (A) MRC5V1 cells were transfected with RPA70 siRNA. After a 72-h incubation, cells were either irradiated or not with 10 J m−2 UV and incubated for 0.5 or 6 h. Cell extracts were analyzed by immunoblotting with (Top to Bottom) anti-RPA70, anti-Chk1-P-Ser-317, PC10, and anti-vimentin (loading control) antibody. Lanes 1, 3, and 5, nontargeting siRNA control. Lanes 2, 4, and 6, RPA siRNA-transfected samples. (B) MRC5V1 cells were transfected with nontargeting or ATR siRNA, UV-irradiated (20 J m−2) 72 h later, incubated for 6 h, and analyzed as in A. (C) Normal or Seckel syndrome lymphoblastoid cells were UV-irradiated with the indicated doses and incubated for 6 h before lysis and analysis.
To examine whether Ub-PCNA formation depends on checkpoint activation, we depleted MRC5V1 cells of ATR by using siRNA (Fig. 3Ba). This prevented the UV-induced phosphorylation of Chk1 (Fig. 3Bb), demonstrating that checkpoint activation had been abrogated. However, 6 h after exposure to 20 J m−2 UV-irradiation, depletion of ATR had no effect on the levels of Ub-PCNA (Fig. 3Bc). We also showed that the ubiquitination of PCNA in cells from a normal individual and from a child with Seckel syndrome caused by a mutation in the ATR gene and deficient in ATR signaling (20), was very similar (Fig. 3C). We conclude that ubiquitination of PCNA does not depend on a checkpoint response mediated by ATR.
Persistence of Ub-PCNA After Removal of the Damage.
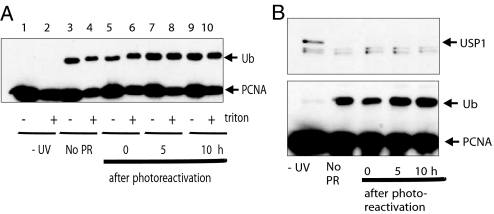
The results of Fig. 1 demonstrate that Ub-PCNA persists for a long time after formation of the DNA damage. However, many types of damage are known to persist for long periods, and the apparent persistence of Ub-PCNA may represent a dynamic equilibrium between cycles of ubiquitination and deubiquitination as the replication machinery encounters successive lesions. To test whether this is the case, we again used the XP-A cells that express both photolyases (17). These cells were UV-irradiated (20 J m−2) and then incubated for 6 h in the dark to permit replication forks to stall at damaged sites and Ub-PCNA to accumulate. The cells were then exposed to visible light for 2 h. Samples were taken at various times after photoreactivation and analyzed for Ub-PCNA. Fig. 4A, odd lanes, shows that despite the removal of nearly all of the damage, Ub-PCNA persisted for many hours. We obtained similar results after a lower dose of 5 J m−2 (data not shown). We considered the possibility that, after the damage has been removed, the Ub-PCNA is released from the chromatin into the PCNA pool. In the photoreactivated cells, however, although much of the unmodified PCNA was extracted by Triton X-100, most of the Ub-PCNA was refractory to Triton X-100 extraction (Fig. 4A, compare even with odd lanes), indicating that it remained associated with chromatin for many hours after removal of the damage.
Fig. 4.
Persistence of PCNA ubiquitination. PH-XPA cells were irradiated with 20 J m−2 UV, incubated for 6 h, and then photoreactivated for 2 h. After further incubation for the indicated times, PCNA in cell lysates was detected by immunoblotting. In A, duplicate samples were analyzed either with or without prior extraction with Triton X-100, as indicated. (B) Lysates were analyzed for both USP1 and PCNA ubiquitination.
Recently, USP1 was identified as a DUB that deubiquitinates Ub-PCNA. After high doses of UV-irradiation, USP1 was cleaved, and this permitted Ub-PCNA to accumulate (15). These data suggested that USP1 might regulate the level of PCNA ubiquitination. Given the existence of a DUB for Ub-PCNA, it seemed curious that Ub-PCNA persisted in our experiments. We therefore measured the level of USP1 and Ub-PCNA in the same cell pellet under different conditions. In agreement with the report of Huang et al. (15), we observed that USP1 disappeared from cell extracts after UV-irradiation of the cells. We found that this occurred even after relatively low UV doses (Fig. 1 A and B Lower). There was an ≈70% reduction 8 h after 10 J m−2 and recovery at 72 h, whereas, after 20 J m−2, as might be expected, the response was more severe. USP1 became barely detectable after 8 h and did not recover within the time of the experiment. After both doses, the ubiquitination of PCNA (Upper) correlated well with the disappearance of USP1. Furthermore in the photolyase experiments described above, we found that USP1 remained at undetectable levels for at least 24 h after reversal of the damage by photoreactivation (Fig. 4B Upper). The levels of USP1 in these experiments therefore show a good inverse correlation with those of Ub-PCNA, consistent with the idea that USP1 is an important regulator of Ub-PCNA (15).
We also measured USP1 levels after treatments with MMS and MMC. In striking contrast to the results with UV-irradiation, we were unable to detect any significant loss of USP1 after treatment with these chemicals (Figs. 1 D and E Lower). Indeed, there was an increase in USP1 at later times, more or less concomitant with the increase in PCNA ubiquitination.
Discussion
Ubiquitination of PCNA is a central control point for mediating the replication of damaged DNA, but many questions remain concerning the ubiquitination process. What is the trigger that turns it on, and what is the mechanism for turning it off? We and others have shown that PCNA is ubiquitinated efficiently after exposure to UV, MMS, MMC, and HU, but not by ionizing radiation, bleomycin, or camptothecin (this article and ref. 21) nor by daunomycin, actinomycin D, and neocarzinostatin (22). The former agents all cause stalling of the replication fork. A likely result of fork stalling is either the dissociation of the replicative helicase from the stalled replication machinery and exposure of single-stranded DNA ahead of the replication fork, or uncoupling of the synthesis on leading and lagging strands, exposing single-stranded regions on the leading strand (23). This single-stranded DNA likely binds Rad18, which together with either or both of the Rad6 orthologs carries out the ubiquitination process (24, 25). Ionizing radiation, bleomycin, neocarzinostatin, and camptothecin generate double-stranded breaks either directly or during replication and would not therefore be expected to generate regions of single-stranded DNA at the forks. MMC produces interstrand DNA cross-links. These will result in stalling of the fork, but the cross-links are likely to provide physical barriers to unwinding of the DNA ahead of the stalled forks. This may account for the lack of ubiquitination of PCNA at early times after treatment. The accumulation of Ub-PCNA at much later times is likely to be a result of secondary processes involved in the repair of the cross-links.
When we replaced PCNA with the K164R mutant form that cannot be ubiquitinated, the viability of the cells was unaffected. Consistent with this observation, Langerak et al. (26) recently generated a knockin PCNA-K164R mouse, which was viable. These mice were infertile and had an altered somatic hypermutation spectrum but were otherwise healthy. As in budding and fission yeasts (4, 27) and DT40 chicken cells (28), therefore, the inability to ubiquitinate PCNA is compatible with life in mammals. When treated with PCNA-specific siRNA, our “K164R cells” are, like DT40 cells expressing human PCNA-K164R as the sole source of PCNA (28), sensitive to UV-irradiation. At first sight, this may appear not unexpected. However, in response to UV, Ub-PCNA has been hypothesized to facilitate the switch from replicative to TLS polymerase to enable TLS past UV lesions (6). Interestingly, cells in which polη is depleted (Fig. 2C), like XP variant cells defective in polη (29), are barely sensitive to killing by UV light. The PCNA-K164R cells, in contrast, show much more pronounced UV sensitivity. This suggests that modification of PCNA has roles other than recruitment of polη in response to UV-irradiation. These roles could include recruitment of other polymerases involved in TLS past 6-4 photoproducts. For example, Rev1 also binds to Ub-PCNA (12) as well as to other Y-family polymerases and to polζ, suggesting that it might act as a platform for recruiting other polymerases (30, 31). In addition, polyubiquitination of PCNA might facilitate an error-free recombination-mediated process for bypassing lesions, as found in yeast (4, 5). A further possibility is that ubiquitination of PCNA has a role outside of S phase. We have shown in Schizosaccharomyces pombe that PCNA is ubiquitinated in G2 cells in response to DNA damage (27), and work by N. Zlatanou and PLK (unpublished data), has revealed that PCNA is ubiquitinated in response to DNA damage in quiescent human fibroblasts. The PCNA-K164R cells are also sensitive to MMS, implying an important role for Ub-PCNA in recovery from MMS-induced damage. Further studies will be required to determine whether this role involves TLS, recombination, or both.
Stalling of the replication fork also activates ATR-mediated cell cycle checkpoints and monoubiquitination of FANCD2 (32). This raises the question as to whether these processes are coordinated and interdependent. We found that depletion of RPA resulted in a reduction in PCNA ubiquitination, in agreement with findings of Bi et al. (33) and with recent observations in S. cerevisiae (34). However, we found that reduced levels of ATR had little effect on PCNA ubiquitination. These results are somewhat at variance with those of Bi et al., who reported some reduction of ubiquitination in ATR-deficient cells (33), but agree with a recent report showing no effect of reduction of ATR (35). They are also consistent with our earlier findings in S. pombe, in which deletion of the checkpoint kinase genes rad3 and tel1 (ATR and ATM orthologs) had no effect on PCNA ubiquitination (27) and with similar results in S. cerevisiae (34) and Xenopus laevis (24). We envisage therefore that PCNA ubiquitination and checkpoint activation are independently and automatically triggered by a “state of emergency” indicated by exposed single-stranded DNA at the replication fork.
Ubiquitination of PCNA is also regulated by the DUB USP1, which is able to remove ubiquitin from Ub-PCNA (15). This is at first sight difficult to reconcile with our finding that Ub-PCNA persisted for many hours even after replication blocks were removed. However, we have extended the original observations of Huang et al. (15) to demonstrate that, after UV-irradiation of MRC5V1 cells, USP1 disappears and is barely detectable during the periods when PCNA ubiquitination persists, even when the damage has been removed. In contrast, ubiquitination of PCNA after MMS or MMC treatment was not accompanied by a loss of USP1. This finding suggests that USP1 is an important regulator of PCNA ubiquitination in response to UV, whereas after chemical treatments, the Ub-PCNA is refractory to USP1. One possible explanation is that USP1 is sequestered away from the Ub-PCNA. Alternatively, USP1 is itself regulated and activated by association with a partner protein, UAF1 (16), and this activation might be differentially affected by different DNA-damaging treatments.
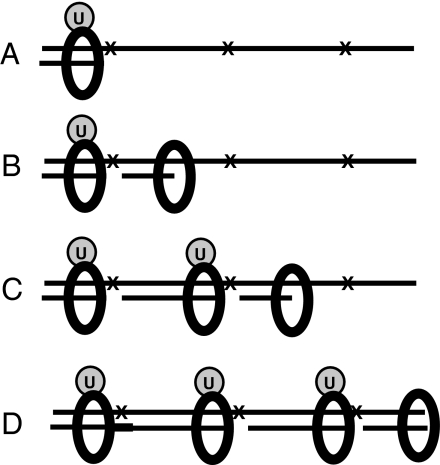
What is the explanation for the persistence of Ub-PCNA after UV-irradiation, even after UV damage has been removed? In Escherichia coli, DNA synthesized in UV-irradiated cells contains gaps opposite UV lesions, and these gaps are subsequently sealed (36). This finding led to a model in which the gaps were sealed behind the replication fork so that the bypass past the lesion was independent of replication fork progression. More recent models have, however, assumed that TLS occurs at the stalled forks and that fork progression and TLS are coordinated. This may not be the case, and recent work in yeast has provided direct support for the older model of gaps behind the replication fork (23). Furthermore, Heller and Marians (37) have shown, by using a bacterial in vitro system, that replication can restart downstream of a replication block, even on the leading strand. In addition, Waters and Walker (38) found high levels of the Y-family polymerase Rev1 in G2 cells in yeast and inferred that this was an indication of a postreplicative gap-filling step. We have developed this model to explain the persistence of Ub-PCNA in UV-irradiated cells (Fig. 5). We propose that when the replication fork stalls at a lesion, PCNA becomes ubiquitinated (Fig. 5A), and shortly afterward a new replication apparatus is assembled beyond the lesion, with a new molecule of PCNA (Fig. 5B). Synthesis continues up to the next lesion, where another Ub-PCNA molecule is deposited, and replication restarts again beyond the lesion (Fig. 5C). At some later time, the gaps are sealed by polη and/or maybe other Y-family polymerases, depending on the nature of the lesion (Fig. 5D), and the Ub-PCNA is left on the DNA, perhaps because there are no RFC molecules in the vicinity to unload it. The net result is that Ub-PCNA molecules remain on the DNA until they are disassembled at the next round of replication or, at least in the case of UV damage, deubiquitinated when USP1 levels are restored. Fig. 5 displays the proposed situation on the leading strand, but a similar process could occur on the lagging strand.
Fig. 5.
Model for persistence of Ub-PCNA. (A) On blocking of the replication fork at a lesion (X), PCNA becomes ubiquitinated (U). (Note that only one ubiquitin molecule is shown for simplicity, but it is likely that all three monomers of the homotrimeric ring become ubiquitinated.) (B) A new replication apparatus is assembled beyond the lesion, leaving a gap. (C) The process is repeated at the next lesion. (D) Some time later, the gap opposite the first lesion is filled, as indicated by the thick line.
Might the persistence of Ub-PCNA after the damage has been removed, together with its affinity for error-prone Y-family polymerases, result in inappropriate recruitment of these polymerases to the replication fork and an elevated mutation rate? This possibility is unlikely for two reasons. First, if the model proposed above is correct, the Ub-PCNA remaining on the chromatin will be behind the replication fork on DNA that has already been replicated and will therefore be harmless. Second, the replicative polymerases are much more efficient and processive than the Y-family members. Once the replicative polymerase is engaged and replicating an undamaged stretch of DNA, it is unlikely that a relatively inefficient Y-family polymerase will be able to compete effectively (39). It is only when passage of the replication fork is blocked and the replicative polymerases cannot proceed that engagement of the Y-family polymerases becomes an issue.
Is the above model of TLS behind the forks compatible with the findings in several reports that polη, polι, and Rev1 are localized in replication foci (40–42)? There is a widely held misconception that forks and foci are one and the same entity. In fact, foci are quite large structures that are thought to contain 5–20 replication forks. It is therefore perfectly plausible that the gapped structure, although behind the fork, remains associated with the focus.
Materials and Methods
SV40-transformed human fibroblast line MRC5V1, grown in Eagle's MEM with 10% or 15% FCS, was used in most experiments. For generation of cells expressing exogenous PCNA, His-PCNA constructs were used that contained mutations rendering them refractory to siRNA knockdown. Transfection and siRNA treatment used standard procedures. For photoreactivation, XP-A cells expressing photolyases were exposed to visible light at different times after UVC irradiation. Further details of procedures are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Tony Huang (New York University, New York) for the anti-USP1 antibody and Mark O'Driscoll and Penny Jeggo (University of Sussex, Brighton, UK) for the Seckel syndrome cells. This work was supported by Medical Research Council research grants, a European Union Integrated Project grant, and a European Science Foundation Eurodyna grant (to A.R.L.) and a Unilever studentship (to S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802727105/DCSupplemental.
References
- 1.Friedberg EC, Lehmann AR, Fuchs RP. Trading places: How do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann AR. Replication of damaged DNA by translesion synthesis in human cells. FEBS Lett. 2005;579:873–876. doi: 10.1016/j.febslet.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Hoege C, Pfander B, Moldovan G-L, Pyrolowakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 5.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 6.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase η with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 7.Chiu RK, et al. Lysine-63 polyubiquitination guards against translesion synthesis-induced mutations. PLOS Genet. 2006;2:e116. doi: 10.1371/journal.pgen.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motegi A, et al. Human SHPRH suppresses genomic instability through PCNA polyubiquitination. J Cell Biol. 2006;175:703–708. doi: 10.1083/jcb.200606145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe K, et al. Rad18 guides polη to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004;28:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bienko M, et al. Ubiquitin-binding domains in translesion synthesis polymerases. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- 11.Plosky BS, et al. Controlling the subcellular localization of DNA polymerases ι and η via interactions with ubiquitin. EMBO J. 2006;25:2847–2855. doi: 10.1038/sj.emboj.7601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C, et al. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol Cell Biol. 2006;26:8892–8900. doi: 10.1128/MCB.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Tang TS, Bienko M, Dikic I, Friedberg EC. Requirements for the interaction of mouse polκ with ubiquitin, and its biological significance. J Biol Chem. 2008;283:4658–4664. doi: 10.1074/jbc.M709275200. [DOI] [PubMed] [Google Scholar]
- 14.Garg P, Burgers PM. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proc Natl Acad Sci USA. 2005;102:18361–18366. doi: 10.1073/pnas.0505949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang TT, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8:341–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 16.Cohn MA, et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell. 2007;28:786–797. doi: 10.1016/j.molcel.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima S, et al. UV light-induced DNA damage and tolerance for the survival of nucleotide excision repair-deficient human cells. J Biol Chem. 2004;279:46674–46677. doi: 10.1074/jbc.M406070200. [DOI] [PubMed] [Google Scholar]
- 18.Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd Ed. Washington DC: ASM Press; 2005. [Google Scholar]
- 19.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 20.O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi N, et al. Human RAD18 is involved in S phase-specific single-strand break repair without PCNA monoubiquitination. Nucleic Acids Res. 2007;35:e9. doi: 10.1093/nar/gkl979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soria G, Podhajcer O, Prives C, Gottifredi V. P21(Cip1/WAF1) down-regulation is required for efficient PCNA ubiquitination after UV-irradiation. Oncogene. 2006;25:2829–2838. doi: 10.1038/sj.onc.1209315. [DOI] [PubMed] [Google Scholar]
- 23.Lopes M, Foiani M, Sogo JM. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Chang DJ, Lupardus PJ, Cimprich KA. Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and minichromosome maintenance helicase activities. J Biol Chem. 2006;281:32081–32088. doi: 10.1074/jbc.M606799200. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji Y, et al. Recognition of forked and single-stranded DNA structures by human RAD18 complexed with RAD6B protein triggers its recruitment to stalled replication forks. Genes Cells. 2008;13:343–354. doi: 10.1111/j.1365-2443.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 26.Langerak P, Nygren AO, Krijger PH, van den Berk PC, Jacobs H. A/T mutagenesis in hypermutated immunoglobulin genes strongly depends on PCNAK164 modification. J Exp Med. 2007;204:1989–1998. doi: 10.1084/jem.20070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frampton J, et al. Postreplication repair and PCNA modification in Schizosaccharomyces pombe. Mol Biol Cell. 2006;17:2976–2985. doi: 10.1091/mbc.E05-11-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson LJ, et al. RAD18-independent ubiquitination of proliferating-cell nuclear antigen in the avian cell line DT40. EMBO Rep. 2006;7:927–932. doi: 10.1038/sj.embor.7400777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arlett CF, Harcourt SA, Broughton BC. The influence of caffeine on cell survival in excision-proficient and excision-deficient xeroderma pigmentosum and normal human cell strains after ultraviolet light irradiation. Mutat Res. 1975;33:341–346. doi: 10.1016/0027-5107(75)90209-2. [DOI] [PubMed] [Google Scholar]
- 30.Guo C, et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi E, et al. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells. 2004;9:523–531. doi: 10.1111/j.1356-9597.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy RD, D'Andrea AD. The Fanconi anemia/BRCA pathway: New faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 33.Bi X, et al. Rad18 regulates DNA polκ and is required for recovery from S phase checkpoint-mediated arrest. Mol Cell Biol. 2006;26:3527–3540. doi: 10.1128/MCB.26.9.3527-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies AA, Huttner D, Daigaku Y, Chen S, Ulrich HD. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein A. Mol Cell. 2008;29:625–636. doi: 10.1016/j.molcel.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XH, Shiotani B, Classon M, Zou L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 2008;22:1147–1152. doi: 10.1101/gad.1632808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupp WD, Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968;31:291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- 37.Heller RC, Marians KJ. Replication fork reactivation downstream of a blocked nascent leading strand. Nature. 2006;439:557–562. doi: 10.1038/nature04329. [DOI] [PubMed] [Google Scholar]
- 38.Waters LS, Walker GC. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G2/M phase rather than S phase. Proc Natl Acad Sci USA. 2006;103:8971–8976. doi: 10.1073/pnas.0510167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang Z, et al. Regulation of polymerase exchange between polη and polδ by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc Natl Acad Sci USA. 2008;105:5361–5366. doi: 10.1073/pnas.0801310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannouche P, et al. Domain structure, localization and function of DNA polη, defective in xeroderma pigmentosum variant cells. Genes Dev. 2001;15:158–172. doi: 10.1101/gad.187501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannouche P, et al. Localization of DNA polymerases η and ι to the replication machinery is tightly coordinated in human cells. EMBO J. 2003;22:1223–1233. doi: 10.1093/emboj/cdf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tissier A, et al. Colocalization in replication foci and interaction of human Y-family members, DNA polymerase polη and Rev1 protein. DNA repair. 2004;3:1503–1514. doi: 10.1016/j.dnarep.2004.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.