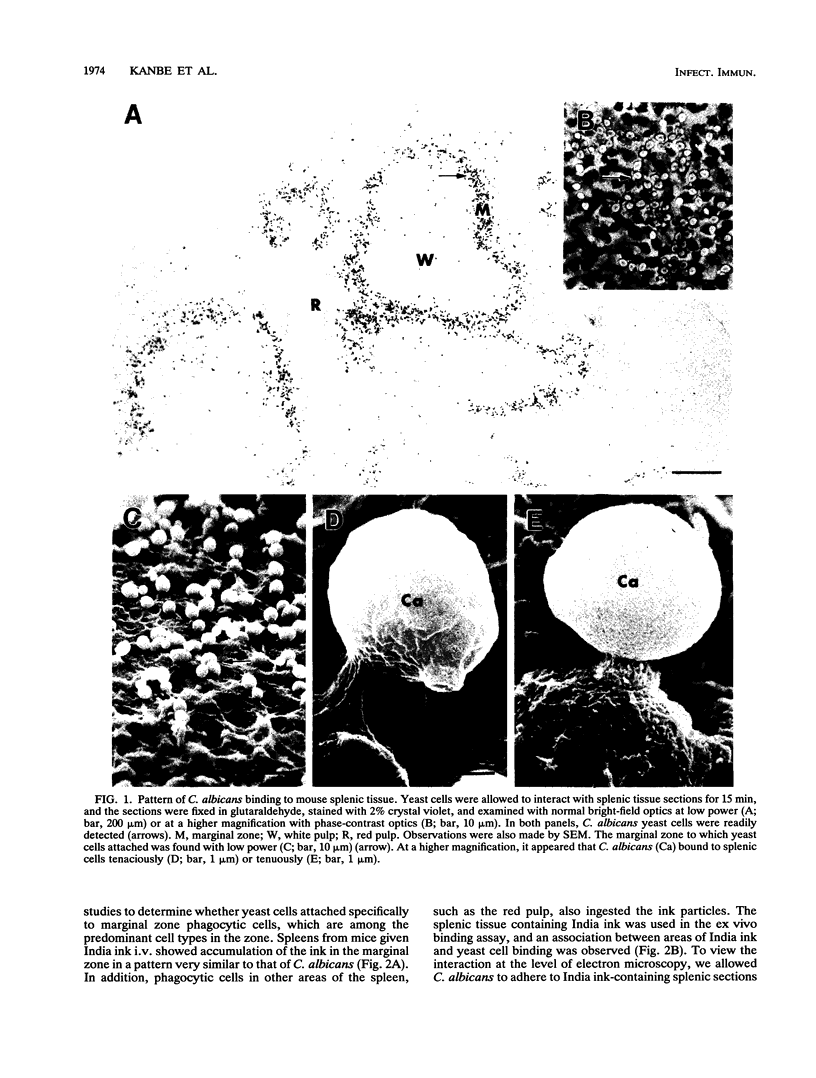
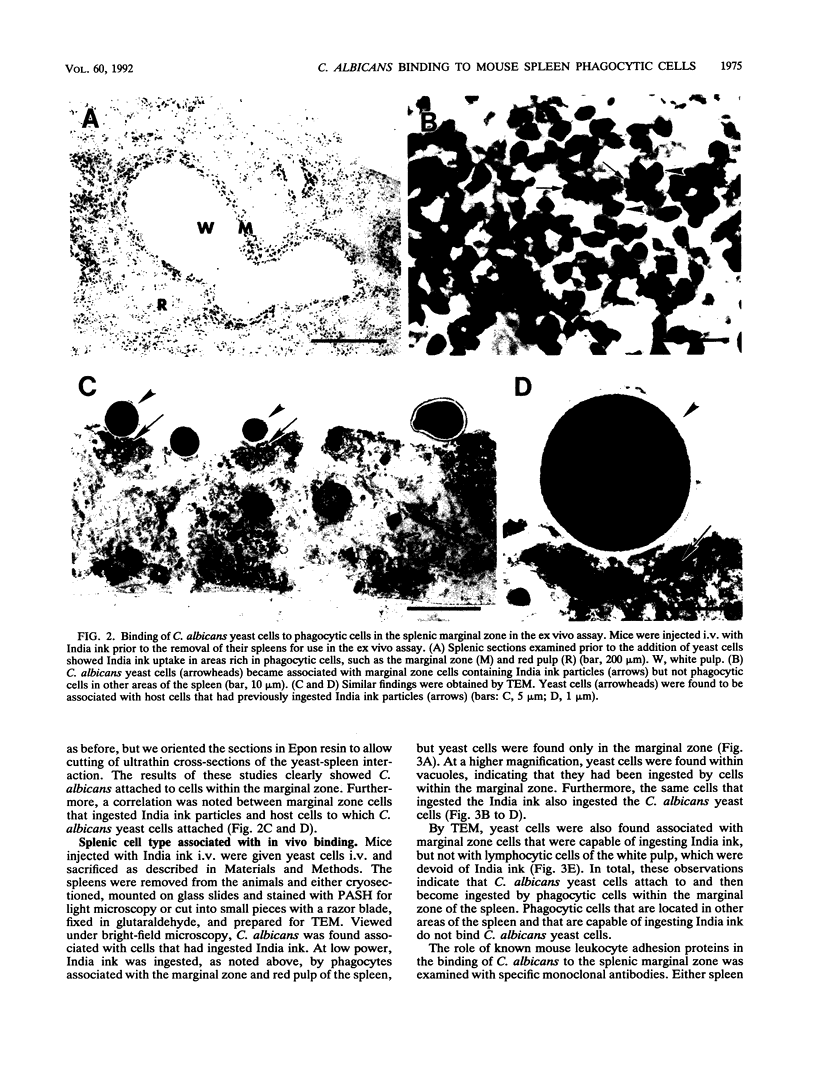
Abstract
We recently demonstrated by using an ex vivo adhesion assay that Candida albicans yeast cells exhibit a unique binding affinity for the marginal zone of the spleen. This binding event provides a working model for studying mechanisms of organ dissemination of the fungus from the blood. By using the ex vivo assays reported here, we showed by bright-field and electron microscopic techniques that mouse spleen marginal zone cells capable of ingesting India ink particles are also involved in yeast cell attachment. During splenic clearance of yeast cells from the circulation in vivo, C. albicans is also associated exclusively with marginal zone cells capable of ingesting India ink. The ability to ingest the ink particles is not necessarily related to yeast cell adherence, because the fungal cells did not bind to phagocytic cells in the splenic red pulp. In fact, the marginal zone phagocytic cells appear to have a unique binding system, because yeast cells also did not bind to phagocytes in other tissues, such as the thymus and peritoneum, or to seven different myeloid cell lines. In addition, antibodies to a number of well-characterized murine adhesion molecules, such as leukocyte integrins, LECAM-1, and CD44, had no effect on binding. On the basis of these results, we propose that splenic marginal zone phagocytes express a novel adhesion system that involves either a unique adhesion molecule or previously described adhesion molecules with unique binding activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen E., van Rooijen N. Evidence that macrophages in the marginal zone have no role in the migration of lymphocytes into the periarteriolar lymphocyte sheaths (PALS). Immunology. 1985 Dec;56(4):689–694. [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Edwards J. E., Jr Invasive candida infections--evolution of a fungal pathogen. N Engl J Med. 1991 Apr 11;324(15):1060–1062. doi: 10.1056/NEJM199104113241511. [DOI] [PubMed] [Google Scholar]
- Eigentler A., Schulz T. F., Larcher C., Breitwieser E. M., Myones B. L., Petzer A. L., Dierich M. P. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989 Feb;57(2):616–622. doi: 10.1128/iai.57.2.616-622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Migration of lymphocytes and thymocytes in the rat. I. The route of migration from blood to spleen and lymph nodes. J Exp Med. 1968 Jan 1;127(1):155–168. doi: 10.1084/jem.127.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991 Mar;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Riesselman M. H., Kanbe T., Cutler J. E. Improvements and important considerations of an ex vivo assay to study Candida albicans-splenic tissue interactions. J Immunol Methods. 1991 Dec 15;145(1-2):153–160. doi: 10.1016/0022-1759(91)90321-6. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Delga J. M., Wadsworth E., Walsh T. J., Kwon-Chung K. J., Calderone R., Lipke P. N. Isolation and characterization of cell surface mutants of Candida albicans. Infect Immun. 1990 Jun;58(6):1552–1557. doi: 10.1128/iai.58.6.1552-1557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. F., Ding J. F., Picker L. J., Bargatze R. F., Butcher E. C., Goeddel D. V. Molecular cloning and expression of Pgp-1. The mouse homolog of the human H-CAM (Hermes) lymphocyte homing receptor. J Immunol. 1989 Nov 15;143(10):3390–3395. [PubMed] [Google Scholar]
- von Eiff M., Essink M., Roos N., Hiddemann W., Büchner T., van de Loo J. Hepatosplenic candidiasis, a late manifestation of Candida septicaemia in neutropenic patients with haematologic malignancies. Blut. 1990 Apr;60(4):242–248. doi: 10.1007/BF01728792. [DOI] [PubMed] [Google Scholar]