Abstract
The mechanisms underlying in vivo activation of indoleamine 2,3-dioxygenase (IDO), a tryptophan-catabolizing enzyme that mediates in the brain, the induction of depressive-like behavior by peripheral innate immune system stimulation are still poorly understood. By monitoring how cytokines parallel IDO mRNA expression in the brain in response to intraperitoneal lipopolysaccharide injection in mice, we report a time-dependent induction of IDO expression in both the hippocampus and hypothalamus that was associated with a specific structure-dependent expression of proinflammatory cytokines, particularly interferon-γ. This study suggests that different mechanisms regulate the activation of IDO by lipopolysaccharide in various brain structures.
Keywords: Interferon-γ, tumor necrosis factor-α, interleukin-6, interleukin-1, hypothalamus, hippocampus
1. Introduction
During an infection, proinflammatory cytokines produced in the periphery by activated innate immune cells induce the production of the same molecular signals by microglial cells and macrophage-like cells in the brain (Dantzer, 2006). These brain cytokines organize the subjective, behavioral and metabolic components of the sickness response that allows the organism to cope with infectious micro-organisms (Konsman et al., 2002). The host response to infection can be induced experimentally by the administration of lipopolysaccharide (LPS), the active component of endotoxin from Gram-negative bacteria. LPS induces a strong production of both peripheral and brain proinflammatory cytokines such as interleukin-1β (IL-1β, interleukin-6 (IL-6) and tumor-necrosis factor-α (TNFα) (Castanon et al., 2004; Gatti and Bartfai, 1993; Laye et al., 1994; van Dam et al., 1998). Brain IL-1β is a key cytokine for orchestrating the development of sickness behavior (Kent et al., 1992; Laye et al., 2000). When immune activation continues unabated, exacerbation of the sickness response can, in some conditions, culminate in the development of depressive symptoms (Capuron and Dantzer, 2003). Since the intensity of these symptoms is correlated to a drastic fall in plasma levels of the essential amino acid tryptophan, it has been proposed that this drop could be due to enhanced activation of indoleamine 2,3-dioxygenase (IDO) (Dantzer et al., 2008). In mice, the acute behavioral symptoms of sickness that are induced by LPS are replaced by depressive-like behaviors appearing 24 h post-treatment (Frenois et al., 2007). These changes coincide with maximal stimulation of brain IDO activity by LPS (Lestage et al., 2002). The role of IDO in the development of depressive-like behavior is apparent from experiments involving direct blockade of LPS-induced IDO activation with 1-methyltryptophan. This IDO antagonist abrogates depressive-like behavior, but not sickness behavior (O'Connor et al., 2008).
In mammals, the extrahepatic enzyme, IDO, is the first and rate-limiting enzyme of tryptophan catabolism along the kynurenine pathway (Taylor and Feng, 1991). It is expressed in human and mouse macrophages and dendritic cells, as well as in brain endothelial cells, astrocytes, microglia and neurons (Carlin et al., 1989; Guillemin et al., 2005; Kwidzinski et al., 2005). Mounting evidence indicates that interferon-gamma (IFNγ) is an essential factor for the induction of IDO (Brown et al., 1989; Byrne et al., 1986). Conversely, IFNγ-induced IDO activation is postulated to be one of the key mechanisms underlying the anti-microbial, anti-viral and anti-proliferative properties of this cytokine (King and Thomas, 2007; Mellor and Munn, 2004). The activation of IDO following a peripheral parasite infection is abolished in IFNγ-deficient mice (Fujigaki et al., 2002; Silva et al., 2002), whereas intraperitoneal injections of IFNγ stimulate IDO activity (Saito et al., 1991; Saito et al., 1992). In addition, the IDO gene promoter possesses the different elements required for its induction by IFNγ (Sotero-Esteva et al., 2000). However, both in vitro and in vivo studies show that in some conditions other proinflammatory cytokines can also contribute to the induction of IDO by acting either in synergy with IFNγ, as shown for TNFα (Babcock and Carlin, 2000; Robinson et al., 2006; Robinson et al., 2003), or by IFNγ-independent mechanisms (Fujigaki et al., 2001; Saito et al., 1996). In particular, a transcriptional synergistic activation of IDO by IL-1β, TNFα and IL-6 has been reported in human monocytic THP-1 cell cultures exposed to LPS (Fujigaki et al., 2006).
Although these data provide important insights into the mechanisms underlying increased IDO activity in peripheral immune cell lines, much less is known about the mechanisms involved in the in vivo induction of brain IDO by stimulation of peripheral innate immune cells. We have recently shown that in LPS-treated mice increased brain IDO activity was associated with an increased transcription of IDO mRNA concomitant with increased production of plasma IFNγ and IL-6 (Godbout et al., 2007; Lestage et al., 2002). Moreover, targeting peripheral and brain proinflammatory cytokine expression via the administration of the anti-inflammatory tetracycline derivative minocycline blocked LPS-induced brain IDO activation (O'Connor et al., 2008). Based on these findings, we hypothesized that brain IDO activation by peripheral LPS administration could be mediated by local expression of IFNγ and/or one or more of the other proinflammatory cytokines induced by LPS, particularly TNFα, IL-1β or IL-6. In order to test this hypothesis, the present set of experiments was carried out to determine the effect of LPS on the temporal pattern of expression of cytokines and IDO in two key structures for brain cytokine expression and action: the hypothalamus and hippocampus (Castanon et al., 2004; Frenois et al., 2007; Laye et al., 1994; Schiepers et al., 2005) up to 24 h post-LPS. We report here that LPS induced a time-dependent increase in IDO expression in both the hippocampus and hypothalamus that was associated with a specific structure-dependent induction of proinflammatory cytokines by LPS.
2. Material and methods
2.1. Animals and treatment
Male CD1 mice 7-week old were purchased from Charles River Laboratories. They were housed individually under a normal 12 h light/dark cycle (7:00 on). Food and water were available ad libitum and room temperature was controlled (23 ± 1°C). Mice were handled daily for at least one week before the onset of the experiment to minimize stress reactions to manipulation. All animal care and use were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC) and approved by the Institutional Animal Care and Use Committee.
Lipopolysaccharide (LPS) from E.coli (serotype 0127:B8) was purchased from Sigma and dissolved in sterile endotoxin-free isotonic saline on the injection day to obtain 830 μg/kg. This dose of LPS was used because it induces the full spectrum of sickness and depressive-like behavior (Frenois et al., 2007; O'Connor et al., 2008), together with a marked increase of brain IDO activity (Lestage et al., 2002).
2.2. Experimental procedures
Mice were injected intraperitoneally (i.p.) with sterile physiological saline or LPS and were immediately returned to their home cage. Body weight loss was used as a marker of sickness (Dantzer, 2006). Therefore, mice were weighed just before the LPS treatment and at the different time points of sacrifice (2, 6, 12 or 24 h after LPS) to control the efficiency of the response to LPS. Mice were killed by CO2 inhalation and blood samples were immediately collected via cardiac puncture into EDTA (10%)-coated chilled tubes. After centrifugation (10 min, 3000 g, 4°C), aliquots of plasma were stored at −80°C until assayed for determination of cytokine levels. Mice were perfused with PBS at 4°C via the ascending aorta to remove all traces of blood from tissues, then lungs were rapidly collected and kept at −80°C until analysis of IDO activity. Although IDO is present in most extrahepatic tissues (Wirleitner et al., 2003), lung IDO activity is often assayed to serve as positive control for the efficacy of treatment with LPS, parasites or mycobacteria (Fujigaki et al., 2001; Fujigaki et al., 2002; Moreau, 2008; Moreau et al., 2005; Silva et al., 2002). Additionally, lung IDO activation observed in these conditions is positively correlated with brain IDO activation. As a positive control in the present study, we checked that there was a significant increase in lung IDO activity in response to LPS, as previously reported (Moreau et al., 2008). Brains were also rapidly extracted from the skulls and carefully dissected on glass plate over ice by a trained person to immediately collect the hypothalamus and hippocampus, as previously described (Dunn, 1988; Glowinski and Iversen, 1966) and routinely performed in our laboratory (Castanon et al., 2004; Layé et al., 1994). This method readily allows dissection of several brain structures, including the entire hypothalamus and hippocampus, but not a fine neuroanatomical discrimination of the different sub-regions or nuclei within the dissected brain structures. After collection, the hypothalamus and hippocampus were immediately dry frozen and stored at −80°C for subsequent determination of cytokines and IDO mRNA levels.
2.3. Reverse transcription and real time PCR
Total RNA was isolated from the hypothalamus and hippocampus using a RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions and eluted in 30 μl RNase free water as previously described (Mingam et al., 2008). Yields were determined by spectrophotometer at λ 260 nm. 1μg of RNA samples was reverse transcribed to cDNA using a RetroScript kit (Ambion, Austin, Tx). Resultant first-strand cDNA was amplified by the Taqman Universal PCR Master Mix with sequence-specific primers and the FAM-labeled Taqman MBG probe assay mix (Applied Biosystems, Foster city, CA) according to the manufacturer's instructions. Real time PCR was performed on a ABI Prism 7900 using Taqman® gene expression assays for IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), IFNγ (Mm00801778_m1), TNFα (Mm00443258_m1), IDO, (Mm00492586_m1) and for the house keeping gene GAPDH (Mm999999_g1) (O'Connor et al., 2008). Samples were run in duplicate. Amplifications without reverse transcription or template were included as negative controls. Data were analyzed using the comparative threshold cycle method, as described elsewhere (Applied Biosystems user bulletin n°2).
2.4. KYN and TRP measurements
Kynurenine (KYN) and tryptophan (TRP) levels were determined in lungs as previously described (Moreau et al., 2005). The KYN/TRP ratio served to assess lung IDO activity. Briefly, lungs were homogenized using ice cold potassium 0.14 M KCl, 20 mM phosphate buffer pH 7.0 with an UltraTurrax T25 homogenizer at 1000 rpm. Homogenates were then centrifuged at 14,000 g for 30 min at 4°C. 200 μl of supernatants were precipitated in trichloroacetic acid (2 mM) and then centrifuged twice (15 and 5 min) at 1300 g at 4°C. Supernatants were injected onto a 5-μm C18 HPLC column (Lichrospher, Alltech, Deerfield, IL, USA) at a flow rate of 1.0 ml/min with mobile phase containing 0.1 M ammonium acetate/acetic acid buffer and 5 % acetonitrile (pH 4.65). Levels of KYN were evaluated by UV absorbency at 360 nm. Levels of TRP were detected by fluorescent detector at 285 nm excitation and 365 nm emission wavelengths.
2.5. Plasma cytokine measurements
Plasma IL-1β, IL-6, TNF-α, IFN-γ were measured using a mouse multiplex-4 bead array assay kit for Luminex™ (Linco Research Inc., St. Charles, MO, USA) as previously described (Moreau, 2008). The assay was conducted according to manufacturer instructions. Briefly, the assay is based on Luminex™ technology using carboxylated polystyrene beads with a distinct emitting fluorescence pattern and coupled covalently with capture antibodies specific for individual cytokines. A Luminex 100 IS instrument (Biosource) was used to process the data. The service was provided by the “Functional Exploration Platform” from the Toulouse Genopole, France (http://ifr31.toulouse.inserm.fr/PFT/ExplPhysioPatho/). All samples were run in duplicate.
2.6. Statistical analysis
Data are represented as means ± SEM. All measures were analyzed using one-way (treatment) or two-way (treatment × time) analysis of variance (ANOVA) with repeated measurement on the time factor where appropriated, followed by a Fischer's LSD post hoc test if the interaction treatment × time was significant.
3. Results
3.1. Effect of peripheral LPS challenge on body weight and lung IDO enzymatic activity
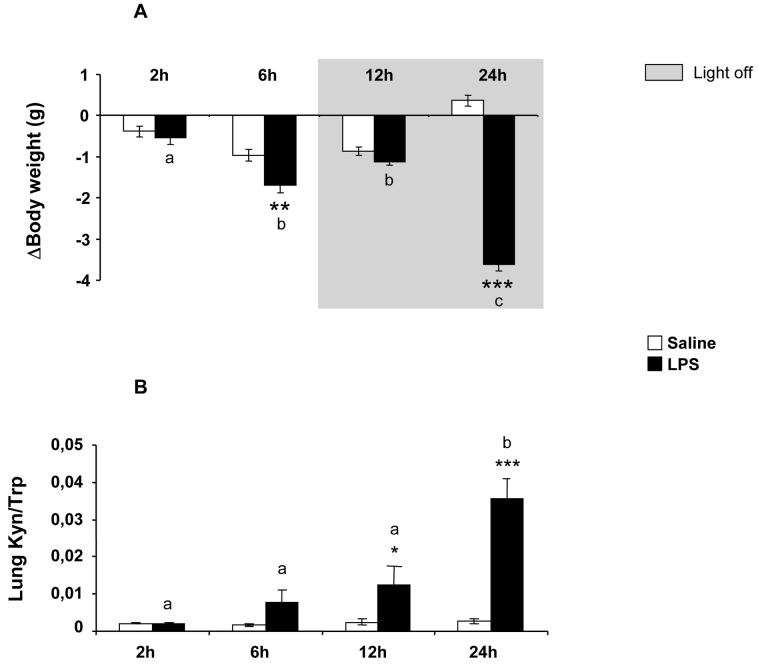
In order to verify the effectiveness of LPS treatment, we measured in saline- and LPS-treated mice both the time course of body weight change, over time, compared to pre-treatment body weight (Fig. 1A) and lung IDO activity as assessed by the KYN/TRP ratio 2, 6, 12 and 24 h after LPS injection (Fig. 1B). As expected, LPS induced a progressive and sustained decrease in body weight in all treated mice compared to saline-treated controls [treatment: F(1,40)=86.29, p<0.001; time: F(3,40)=10.74, p<0.001; treatment × time: F(3,40)=32.39; p<0.001].
Fig 1.
(A) Temporal evolution of changes in body weight in response to i.p. saline or LPS. (B) Time course of lung IDO activity in response to LPS. Mice were injected i.p. with either saline or LPS (830 μg/kg) and IDO activity was assessed by the KYN/TRP ratio in lungs collected 2, 6, 12 or 24 h after injection. Data represent the means ± SEM (n= 6).The * symbol represented statistical differences between saline and LPS groups, **p<0.01; ***p<0.001. Bars labeled with different letters (a, b or c) are significantly different from each other (p<0.05). Note that the basal body weight did not differ between the two experimental groups (global mean and SEM).
While lung IDO activity remained very low over-time in control saline-treated mice, it progressively increased in LPS-treated mice to reach a peak at 24 h [treatment: F(1,37)=38.48, p<0.001; time: F(3,37)=20.10, p<0.001; treatment × time: F(3,37)=18.40, p<0.001] (Fig. 1B). The present time course of lung IDO activation is similar to that observed in the brain under identical experimental conditions (Lestage et al., 2002). Since lung IDO activation is associated with stimulation of brain IDO activity (Fujigaki et al., 2001; Moreau et al., 2005; Moreau et al., 2008), these results, together with body weight loss, confirm the full efficacy of LPS in the present study.
3.2. Effect of peripheral LPS challenge on the time course of plasma cytokine production
The increase in plasma cytokine levels observed in response to peripheral LPS results from the activation of both peritoneal and tissue macrophages, including pulmonary macrophages (Dantzer, 2006). Therefore, this measure provides an accurate indication of the entire systemic peripheral cytokine response to LPS that can be relayed to the brain to induce the production of brain cytokines by microglial and macrophage-like cells (Dantzer, 2006). In the present study, it was important to determine how plasma TNFα, IL-1β, IL-6 and IFNγ production paralleled brain induction of cytokines and IDO expression. Basal plasma levels of cytokines were undetectable or very low at all time points. As expected, i.p. LPS injection induced a rapid increase in plasma IL-1β [F(1,28)=7.91, p<0.01], TNFα [F(1,28)=16.16, p<0.001] and IL-6 levels [F(1,28)=139.78, p<0.001] (Fig. 2A-B-C), that peaked 2 h post-treatment. Then, the plasma levels of each cytokine progressively declined, returning to basal levels with different time courses for each cytokine. Plasma TNFα concentrations normalized by 6 h post-LPS [time: F(3,28)=16.44, p<0.001; treatment × time: F(3,28)=16.33, p<0.001] (Fig. 2B), whereas plasma levels of IL-1β remained elevated 6 h after treatment [time: F(3,28)=5.59, p<0.01; treatment × time: F(3,28)=6.09, p<0.01] (Fig. 2A). The longest increase was observed for IL-6 since its plasma levels were still significantly elevated 12 h after LPS [time: F(3,28)=25.87, p<0.001; treatment × time: F(3,28)=24.31, p<0.001] (Fig. 2C). On the contrary, plasma IFNγ levels increased progressively from 2 h post-LPS to reach a delayed peak of production 12 h later [treatment: F(1,28)=8.64, p<0.01; time: F(3,28)=3.42, p<0.05; treatment × time: F(3,28)=3.10, p<0.05] (Fig. 2D).
Fig 2.
Time course of IL-1β (A), TNFβ (B), IL-6 (C), and IFNγ (D) concentrations measured in plasma collected 2, 6, 12, or 24 h after LPS (830 μg/kg) or saline injection. Bars represented the means ± SEM (n= 6). The * symbol represented statistical differences between saline and LPS groups, *p<0.05; **p<0.01; ***p<0.001. Bars labeled with different letters (a, b or c) are significantly different from each other (p<0.05).
3.3. Differential effect of peripheral LPS challenge on the time course of IDO and cytokine expression in the hippocampus and hypothalamus
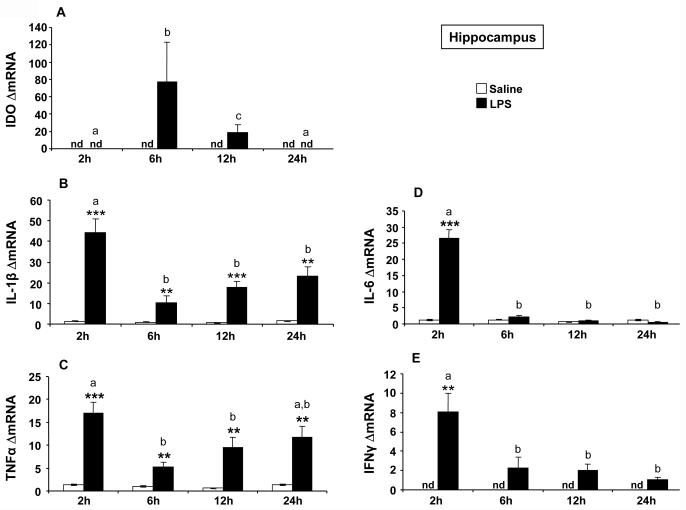
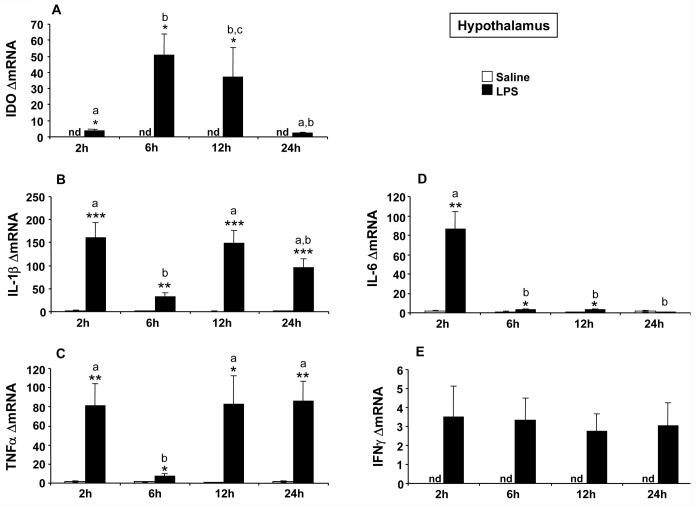
A positive association between increased IDO expression and activity has been reported in vitro (Fujigaki et al., 2001) and after peripheral parasite infection (Fujigaki et al., 2002; Silva et al., 2002). These findings point to IDO mRNA expression as a good indicator of IDO enzymatic activity. Similarly, we observed that the increase of brain IDO activity induced by peripheral LPS was preceded by an enhanced transcription of IDO mRNA (Godbout et al., 2007; O'Connor et al., 2008). This was done, however, at the whole brain level. Therefore, we have carefully delineated these results in a time course of LPS-induced mRNA expression of cytokines and IDO in the specific brain structures represented by the hippocampus and hypothalamus. These brain structures had already been shown to exhibit a marked increase in cytokine expression shortly after LPS (Castanon et al., 2004; Laye et al., 1994), although the possibility of long-term effects had not yet been explored. In addition, these two brain areas show a time-dependent differential pattern of cellular activation after LPS, as assessed by neuroanatomical analysis of FosB/deltaFosB immunostaining (Frenois et al., 2007). While IDO mRNA was undetectable in the hippocampus and hypothalamus of saline-treated mice, its expression was markedly increased by LPS in the hippocampus [F(1,37)=8.89, p<0.001] (Fig. 3A) and hypothalamus [F(1,38)=16.14, p<0.001] (Fig. 4A) with a differential time course according to each brain structure. IDO mRNA peaked at 6 h post-LPS in both the hippocampus [time: F(3,37)=4.23, p<0.05; time × treatment: F(3,37)=4.29, p<0.05] and hypothalamus [time: F(3,38)=3.63, p<0.05; treatment × time: F(3,38)=3.59, p<0.05], but this stimulation was protracted up to 12 h post-LPS only in the hypothalamus.
Fig 3.
Effect of LPS on mRNA expression of IDO (A), IL-1β (B), TNFα (C), IL-6 (D) and IFNγ (E) in the hippocampus. Mice were injected i.p. with saline or LPS (830 μg/kg) and hippocampus were collected 2, 6, 12 or 24 h after injection. Data represented the means ± SEM (n= 6). The * symbol represented statistical differences between saline and LPS groups, *p<0.05; **p<0.01; ***p<0.001. Bars labeled with different letters (a, b or c) are significantly different from each other (p<0.05). nd = no detectable.
Fig 4.
Effect of LPS on mRNA expression of IDO (A), IL-1β (B), TNFα (C), IL-6 (D) and IFNγ (E) in the hypothalamus. Mice were injected i.p. with saline or LPS (830 μg/kg) and hypothalamus were collected 2, 6, 12 or 24 h after injection. Data represented the means ± SEM (n= 6). The * symbol represented statistical differences between saline and LPS groups, *p<0.05; **p<0.01; ***p<0.001. Bars labeled with different letters (a, b or c) are significantly different from each other (p<0.05). nd = no detectable.
In both the hippocampus (Fig. 3) and hypothalamus (Fig. 4), basal expression of cytokine mRNAs was either undetectable (as in the case of IFNγ) or very low (as in the case of TNFα, IL-1β and IL-6). The temporal patterns of the cytokine response to LPS differed according to the cytokine and brain area. In the hippocampus, LPS induced the same temporal profile of expression for IL-1β [treatment: F(1,38)=91.80, p<0.001; time: F(3,38)=9.76, p<0.001; treatment × time: F(3,38)=9.30, p<0.001] and TNfα mRNAs [F(1,38)=87.37, p<0.001; time: F(3,38)=5.82, p<0.01; treatment × time: F(3,38)=4.99, p<0.01] with a marked increase of expression at 2 h post-treatment in LPS-treated mice compared to controls (Fig. 3B-C). Then, expression of both cytokines remained significantly elevated up to 24 h after LPS although to a slightly smaller extent than at the 2 h time-point. In the hypothalamus, LPS induced a marked biphasic profile of expression for both IL-1β [treatment: F(1,38)=88.74, p<0.001; time: F(3,38)=6.55, p<0.01; treatment × time: F(3,38)=6.44, p<0.01] and TNFα mRNA (treatment: F(1,38)=36.42, p<0.001; time: F(3,38)=3.22, p<0.05; treatment × time: F(3,38)=3.22, p<0.05] (Fig. 4B-C). IL-1β and TNFα mRNA expression was significantly increased in LPS-treated mice compared to controls from 2 to 24 h post-treatment, but this increase was less pronounced at 6 h post-LPS than at the other time-points (p<0.01). In contrast, the expression of IL-6 mRNA was increased only 2 h after LPS injection in both the hippocampus [treatment: F(1,38)=86.80, p<0.001; time: F(3,38)=86.76, p<0.001; treatment × time: F(3,38)=84.54, p<0.001; Fig. 3D] and hypothalamus [treatment: F(1,38)=21.08, p<0.001; time: F(3,38)=20.01, p<0.001; treatment × time: F(3,38)=19.22, p<0.001; Fig. 4D]. The expression of IFNγ mRNA in response to LPS varied according to the brain structure. In the hippocampus, LPS induced a significant increase of IFNγ mRNA expression [treatment: F(1,37)=16.69, p<0.001], with a marked enhancement of this expression at 2 h post-treatment, followed by a rapid return to baseline levels thereafter [time: F(3,37)=9.77, p<0.001; treatment × time: F(3,37)=7.57, p<0.001] (Fig. 3E). On the contrary, LPS-induced IFNγ mRNA expression observed in the hypothalamus was much weaker although significant [treatment: F(1,38)=6.69, p<0.05] and showed no variation over time (Fig. 4E). Taken together, these results suggest the possibility of distinct mechanisms of IDO activation according to the brain structure. In the hippocampus, the induction of IDO mRNA that was observed 6 h after LPS followed IFNγ mRNA expression with a time course that was compatible with a mediating implication of IFNγ, as shown at the periphery. On the other hand, the lack of clear and time-dependent induction of IFNγ mRNA expression by LPS in the hypothalamus that contrasted with the marked biphasic induction of IL-1β and TNFα, was not in favor of IFNγ as the main mediator of IDO induction in this last brain structure.
4. Discussion
Although LPS-induced IDO activity in peripheral immune cell lines has been extensively studied in vitro, the mechanisms underlying in vivo activation of peripheral, and overall brain IDO by peripheral stimulation of innate immune cells are still poorly understood. Furthermore, although it is widely accepted that IFNγ is the major inducer of IDO at the periphery, its potential expression in the brain, as well as its implication in the in vivo activation of brain IDO, have not been clearly demonstrated so far. The main significance of the present study is that it allows, for the first time, a direct comparison of the temporal changes in induction of cytokines and IDO by LPS not only at the periphery but also in two key brain areas for the control of behavioral and physiological responses to infection, namely the hypothalamus and hippocampus (Dantzer, 2006; Frenois et al., 2007). By monitoring how cytokines parallel IDO expression, we have obtained preliminary evidence that the in vivo mechanisms of LPS-induced brain IDO mRNA expression could vary in the hippocampus and hypothalamus especially in terms of the respective importance of the different cytokines.
In agreement with previous data obtained after peripheral parasite or mycobacterial infections (Fujigaki et al., 2002; Moreau et al., 2005; Silva et al., 2002) and after i.p. injections of IFNγ (Saito et al., 1991; Saito et al., 1992), the present results show that a maximal increase of plasma IFNγ precedes the peak of lung IDO activation induced by LPS. These results, together with the expected changes in plasma concentrations of IL-1β, TNFα and IL-6, confirm therefore that LPS activates the peripheral innate immune system and they are fully compatible with a key mediating role of IFNγ in IDO stimulation at the periphery. Detection of a significant increase of lung IDO activity in response to LPS in the present study also indirectly indicate a likely stimulation of brain IDO activity, which would be coherent with the positive correlation previously reported between IDO activation in the lung and brain (Fujigaki et al., 2001; Moreau et al., 2005; Moreau et al., 2008). However, the delayed production of plasma IFNγ compared to the earlier induction of brain IDO does not argue for a role of plasma IFNγ in mediating LPS-induced activation of brain IDO expression. Although recent in vitro data indicate that LPS-induced IDO activation can be mediated in human THP-1 cells by an IFNγ-independent mechanism involving synergistic effect of IL-1β, TNFα and IL-6 (Fujigaki et al., 2006), the time course of plasma release of these cytokines in the present study argues against the possibility of their direct involvement in lung IDO induction observed 24 h after LPS administration. These in vivo results diverge from in vitro data showing that IDO activity can be up-regulated in IFNγ-treated monocyte/macrophage cells by various proinflammatory mediators such as LPS, IL-1β or TNFα (Currier et al., 2000; Hu et al., 1995). However, different results have also been obtained in vivo. Based on experiments assessing lung and brain IDO activity in TNFα-knockout mice 24 h after LPS treatment, it has been proposed that both IFNγ-dependent and independent mechanisms could coexist to increase IDO activity (Fujigaki et al., 2001). Although it is difficult to directly compare this last study with the present one since they were performed in different strains of mice (C57BL/6J vs. CD1) and with different sources of LPS (from Salmonella vs. E. coli), it cannot be totally excluded that IFNγ-independent mechanisms do not participate in the in vivo stimulation of both lung and brain IDO activity by LPS in our experimental conditions.
Brain IDO induction has been previously reported in experiments performed with very potent infectious micro-organisms such as Toxoplasma gondii or malaria parasites (Fujigaki et al., 2002; Hansen et al., 2000). However, the severe and even lethal pathological alterations that are commonly associated with these infections (Neill and Hunt, 1992; Silva et al., 2002) strongly compromise the study of the mechanisms of brain IDO activation in these rather extreme experimental conditions. In the present experiments, we have made use of LPS to induce a mild and transient stimulation of the peripheral innate immune system. In these conditions, we were able to confirm that lung IDO activation is associated with brain IDO activation (Lestage et al., 2002). Moreover, activation of brain IDO mRNA expression was associated with increased IDO enzymatic activity, which is compatible with the existence of a causative link between both phenomenon, as already shown in vitro in LPS-treated THP-1 cells (Fujigaki et al., 2001) and in vivo after parasite infection (Fujigaki et al., 2002; Silva et al., 2002). Because brain IDO activity was found to be increased 24 h after LPS treatment (Lestage et al., 2002), we investigated IDO and proinflammatory cytokine expression in the hippocampus and hypothalamus up to 24 h post-treatment. Brain cytokine expression had already been studied repeatedly during the first hours following peripheral LPS administration (Laye et al., 1994; Pitossi et al., 1997). However, there was, to our knowledge, no study of the long-lasting effects of LPS on brain cytokine mRNA expression. Despite the fact that the present study was restricted to cytokine transcripts, LPS-induced changes in cytokine mRNAs are likely to be reflected in changes of protein levels (Koenig, 1991; van Dam et al., 1998). Therefore, the simultaneous assessment of IDO and cytokine mRNA expression is an important first step in assessing the potential role of each cytokine in the mediation of LPS effect on brain IDO activity.
The results of the present study reveal a differential regional and temporal pattern of brain IDO mRNA expression after LPS, with a protracted induction in the hypothalamus compared to the hippocampus. Concomitantly, cytokines can be classified in three distinct categories (IL-6, IL-1β and TNFα, IFNγ) according to their respective temporal and structure-dependent profile of expression. Firstly, both the hippocampus and hypothalamus display a marked but transient expression of IL-6 mRNA 2 h after LPS treatment, as previously described (Laye et al., 1994; Pitossi et al., 1997). We have recently shown in aged mice that exacerbation of LPS-induced plasma IL-6 production was associated with protracted brain IDO induction (Godbout et al., 2007). The IDO promoter region contains numerous regulatory elements including gamma activation sequences (GAS) which bind the signal transducer and activator of transcription-1 (STAT-1) (Sotero-Esteva et al., 2000). Since both IL-6 and IFNγ act on target cells via the activation of JAK/STAT signaling pathway, IL-6 as well as IFNγ can therefore potentially participate in IDO activation. However, several in vitro and in vivo studies report a very inefficient STAT-1 induction upon normal IL-6 stimulation, compared to the induction of its main target STAT-3 (Haan et al., 2005; Sanz et al., 2008). Moreover, anti-IL-6 neutralizing antibody had no effect on LPS-mediated IDO induction in vitro (Fujigaki et al., 2001). Therefore, although IL-6 might play a role in LPS-induced IDO activation particularly in chronic inflammatory states such as aging or obesity that are characterized by a marked exacerbation of IL-6 production (Amar et al., 2007; Godbout et al., 2005; Mito et al., 2000), this cytokine is probably not crucial for LPS-induced activation of brain IDO in healthy young adult mice.
In the present study, IL-1β and TNFα expression was strongly increased in both the hippocampus and hypothalamus up to 24 h after LPS treatment. There were, however, differences in the time course and intensity of this induction between both brain areas. This could be accounted for by differences in the density of microglial and/or macrophage-like cells between both brain areas. However, there are no clear data in favor of such a possibility (Lawson et al., 1990; Savchenko et al., 2000). In addition, such an explanation does not fit with the differential results observed for IL-1β and TNFα on one hand and IFNγ and IDO on the other hand, despite the fact that these last two factors are also expressed in microglia (Alberati-Giani and Cesura, 1998; De Simone et al., 1998; Kwidzinski et al., 2005). There were, however, slight differences in the time course and intensity of this induction between both brain areas. In the hippocampus, the expression of these cytokines increased at 2 h post-treatment, declined at 6 h from the initial high stimulation observed at 2 h and then tended to progressively increase again up to 24 h post-treatment. In the hypothalamus, both cytokine transcripts clearly displayed a marked biphasic profile of expression with a first peak of induction occurring 2 h after LPS and a delayed increase 12-24 h later. These results extend our previous observations of regional differences in expression of cytokine transcripts over the first hours post-LPS injection (Castanon et al., 2004; Laye et al., 1994). Moreover, they are consistent with other studies that also report a biphasic profile of expression for IκB mRNA, as a marker of LPS or IL-1β-induced NFκB signaling pathway activation (Nadjar et al., 2003; Quan et al., 1997). The authors report that the first wave of activation was restricted to the level of the blood brain barrier and circumventricular organs, whereas the second activation reaches intra-parenchymal cells. Therefore, LPS could induce an early IL-1β and TNFα mRNA expression in endothelial cells or cells associated with the brain vasculature that is at the origin of sickness behavior (Nadjar et al., 2005). The second wave of activation of brain cytokine signaling occurs from 12 h after treatment and is probably due to progressive activation of intra-parenchymal cells. This secondary wave of activation could be responsible for the delayed depressive-like behavior induced by LPS (Frenois et al., 2007).
Interestingly, recent pharmacological data show that these delayed behavioral alterations induced by LPS are mediated by IDO activation, since blockade of the expression and activity of this enzyme by the anti-inflammatory tetracycline derivative minocycline abrogated the development of depressive-like behavior (O'Connor et al., 2008). Moreover, minocycline concomitantly abrogated LPS-induced brain IL-1β and TNFα expression (O'Connor et al., 2008), indicating that both cytokines probably participate in LPS-induced brain expression of IDO. However, other results indicate that abrogation of TNF-α production by minocycline is responsible for the anti-inflammatory effect of this compound ((Kwon et al., 2008; Zhao et al., 2007). Moreover it has been shown in vitro that blockade of TNFα, but not IL-1β, with selective neutralizing antibodies reduced LPS-induced IDO mRNA expression in THP-1 cell line (Fujigaki et al., 2001). Although the respective role of TNFα and IL-1β in the induction of IDO expression by LPS cannot be definitively determined from the present set of studies, our data strongly support the potential participation of central TNFα rather than IL-1β in this phenomenon, at least in the hypothalamus.
IFNγ expression has been extensively studied in vitro and several studies report the presence of IFNγ in different brain cells including endothelial cells (Wei et al., 2000), astrocytes and microglia (De Simone et al., 1998). Moreover, LPS up-regulates in vitro IFNγ mRNA expression in microglial cells, but has no effect in astrocytes cultures (De Simone et al., 1998). However, few studies have directly examined the possible in vivo expression of brain IFNγ in response to peripheral immune stimulation. Pitossi et al (1997) were able to detect IFNγ mRNA in some brain areas, but only shortly after LPS and at levels much lower than that of the other proinflammatory cytokines, as found in the present study. In previous experiments, we reported a later induction of IFNγ mRNA expression, but at the whole brain level (O'Connor et al., 2008). The present results show that among the proinflammatory cytokines under study, IFNγ was the only one of which the expression profile clearly differed between the hypothalamus and the hippocampus, not only by the magnitude of the response, but more importantly by its time-course, as it was also the case for IDO. Whereas, the marginal expression of IFNγ mRNA detected in the hypothalamus appeared totally time-independent, a significant increase in its expression was observed in the hippocampus 2 h after LPS, i.e. just before the induction of IDO expression, and both IFNγ and IDO expression normalized thereafter. An important limitation of the present study is that we only measured mRNA but not protein levels of cytokines and IDO in the brain. In order to test the intriguing possibility that IFNγ is the main mediator of LPS-induced IDO expression in the hippocampus whereas TNFα is responsible for LPS-induced IDO expression in the hypothalamus, it will be necessary to confirm that the observed changes in transcript levels translate in changes in protein levels and that regional blockade of the relevant cytokine has differential effects in the hippocampus as compared to the hypothalamus.
Although the physiological significance of such structure-dependent differences in the mechanisms of IDO activation by LPS still needs to be elucidated, they are consistent with recent data showing that the hypothalamus and hippocampus also show after LPS a time-dependent differential pattern of cellular activation, as assessed by the neuroanatomical analysis of c-fos and FosB/ΔfosB immunostaining (Frenois et al., 2007). Interestingly, such a functional dissociation was related to LPS-induced sickness and depressive-like behaviors (Frenois et al., 2007). Similarly, the possible role of the present spatio-temporal differences of the mechanisms of brain IDO activation in the distinction between sickness behavior and depressive-like behavior remain to be determined. In conclusion, the present findings constitute a first important step towards the identification of new pharmacological strategies aimed at treating mood alterations associated with inflammatory states related to impairment of tryptophan metabolism.
Acknowledgments
This study was funded by INRA, CNRS, Région Aquitaine, the French Ministry of Research (ACI “Neurosciences Intégratives et Computationnelles” to NC) and National Institutes of Health (NIH) to KWK (MH-51569 and AG-029573) and RD (R01 MH-71349 and MH-079829).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberati-Giani D, Cesura AM. Expression of the kynurenine enzymes in macrophages and microglial cells: regulation by immune modulators. Amino Acids. 1998;14:251–255. doi: 10.1007/BF01345271. [DOI] [PubMed] [Google Scholar]
- Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12:588–594. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- Brown RR, Lee CM, Kohler PC, Hank JA, Storer BE, Sondel PM. Altered tryptophan and neopterin metabolism in cancer patients treated with recombinant interleukin 2. Cancer Res. 1989;49:4941–4944. [PubMed] [Google Scholar]
- Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Carlin JM, Borden EC, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- Castanon N, Medina C, Mormede C, Dantzer R. Chronic administration of tianeptine balances lipopolysaccharide-induced expression of cytokines in the spleen and hypothalamus of rats. Psychoneuroendocrinology. 2004;29:778–790. doi: 10.1016/S0306-4530(03)00142-2. [DOI] [PubMed] [Google Scholar]
- Currier AR, Ziegler MH, Riley MM, Babcock TA, Telbis VP, Carlin JM. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced antichlamydial indoleamine dioxygenase activity independently. J Interferon Cytokine Res. 2000;20:369–376. doi: 10.1089/107999000312306. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Castanon N, Lestage J, Moreau M, Capuron L. Inflammation, sickness behaviour and depression. In: Steptoe A, editor. Depression and Physical Illness. Cambridge University Press; 2006. [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Levi G, Aloisi F. Interferon gamma gene expression in rat central nervous system glial cells. Cytokine. 1998;10:418–422. doi: 10.1006/cyto.1997.0314. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51:406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Sekikawa K, Tone S, Takikawa O, Fujii H, Wada H, Noma A, Seishima M. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–786. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S, Bartfai T. Induction of tumor necrosis factor-alpha mRNA in the brain after peripheral endotoxin treatment: comparison with interleukin-1 family and interleukin-6. Brain Res. 1993;624:291–294. doi: 10.1016/0006-8993(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, Connor JO, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging Exacerbates Depressive-like Behavior in Mice in Response to Activation of the Peripheral Innate Immune System. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301649. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Haan S, Keller JF, Behrmann I, Heinrich PC, Haan C. Multiple reasons for an inefficient STAT1 response upon IL-6-type cytokine stimulation. Cell Signal. 2005;17:1542–1550. doi: 10.1016/j.cellsig.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Driussi C, Turner V, Takikawa O, Hunt NH. Tissue distribution of indoleamine 2,3-dioxygenase in normal and malaria-infected tissue. Redox Rep. 2000;5:112–115. doi: 10.1179/135100000101535384. [DOI] [PubMed] [Google Scholar]
- Hu B, Hissong BD, Carlin JM. Interleukin-1 enhances indoleamine 2,3-dioxygenase activity by increasing specific mRNA expression in human mononuclear phagocytes. J Interferon Cytokine Res. 1995;15:617–624. doi: 10.1089/jir.1995.15.617. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Koenig JI. Presence of cytokines in the hypothalamic-pituitary-axis. Prog Neuroendocrineimmunol. 1991;4:143–153. [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- Kwon SJ, Ahn TB, Yoon MY, Jeon BS. BV-2 stimulation by lactacystin results in a strong inflammatory reaction and apoptotic neuronal death in SH-SY5Y cells. Brain Res. 2008;1205:116–121. doi: 10.1016/j.brainres.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism. 2000;49:1295–1300. doi: 10.1053/meta.2000.9523. [DOI] [PubMed] [Google Scholar]
- Moreau M, André C, O'Connor JC, Dumich SA, Woods JA, Kelley KK, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.04.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Bluthe RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Laye S, Tridon V, Dantzer R, Amedee T, Parnet P. Nuclear factor kappaB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. J Neurochem. 2003;87:1024–1036. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- Neill AL, Hunt NH. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992;105(Pt 2):165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Kim L, Herkenham M. Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc Natl Acad Sci U S A. 1997;94:10985–10990. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Hale PT, Carlin JM. NF-kappa B activation contributes to indoleamine dioxygenase transcriptional synergy induced by IFN-gamma and tumor necrosis factor-alpha. Cytokine. 2006;35:53–61. doi: 10.1016/j.cyto.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23:413–421. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Markey SP, Heyes MP. Chronic effects of gamma-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res. 1991;546:151–154. doi: 10.1016/0006-8993(91)91171-v. [DOI] [PubMed] [Google Scholar]
- Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- Saito K, Seishima M, Noma A, Suyama K, Markey SP, Heyes MP. 4-chloro-3-hydroxyanthranilate attenuate quinolinic acid accumulation in brain following transient cerebral ischemia in the gerbil. Adv Exp Med Biol. 1996;398:407–411. doi: 10.1007/978-1-4613-0381-7_62. [DOI] [PubMed] [Google Scholar]
- Sanz E, Hofer MJ, Unzeta M, Campbell IL. Minimal role for STAT1 in interleukin-6 signaling and actions in the murine brain. Glia. 2008;56:190–199. doi: 10.1002/glia.20602. [DOI] [PubMed] [Google Scholar]
- Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96:195–203. doi: 10.1016/s0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotero-Esteva WD, Wolfe D, Ferris M, Taylor MW. An indoleamine 2,3-dioxygenase-negative mutant is defective in stat1 DNA binding: differential response to IFN-gamma and IFN-alpha. J Interferon Cytokine Res. 2000;20:623–632. doi: 10.1089/107999000414790. [DOI] [PubMed] [Google Scholar]
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- van Dam AM, Poole S, Schultzberg M, Zavala F, Tilders FJ. Effects of peripheral administration of LPS on the expression of immunoreactive interleukin-1 alpha, beta, and receptor antagonist in rat brain. Ann N Y Acad Sci. 1998;840:128–138. doi: 10.1111/j.1749-6632.1998.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Wei YP, Kita M, Shinmura K, Yan XQ, Fukuyama R, Fushiki S, Imanishi J. Expression of IFN-gamma in cerebrovascular endothelial cells from aged mice. J Interferon Cytokine Res. 2000;20:403–409. doi: 10.1089/107999000312342. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]