Abstract
OBJECTIVE—We examined metabolic changes in the period immediately after the diagnosis of type 1 diabetes and in the period leading up to its diagnosis in Diabetes Prevention Trial–Type 1 (DPT-1) participants.
RESEARCH DESIGN AND METHODS—The study included oral insulin trial participants and parenteral insulin trial control subjects (n = 63) in whom diabetes was diagnosed by a 2-h diabetic oral glucose tolerance test (OGTT) that was confirmed by another diabetic OGTT within 3 months. Differences in glucose and C-peptide levels between the OGTTs were assessed.
RESULTS—Glucose levels increased at 90 (P = 0.006) and 120 min (P < 0.001) from the initial diabetic OGTT to the confirmatory diabetic OGTT (mean ± SD interval 5.5 ± 2.8 weeks). Peak C-peptide levels fell substantially between the OGTTs (median change −14.3%, P < 0.001). Among the 55 individuals whose last nondiabetic OGTT was ∼6 months before the initial diabetic OGTT, peak C-peptide levels decreased between these two OGTTs (median change −14.0%, P = 0.052). Among those same individuals the median change in peak C-peptide levels from the last normal OGTT to the confirmatory OGTT (interval 7.5 ± 1.3 months) was −23.8% (P < 0.001). Median rates of change in peak C-peptide levels were 0.00 ng · ml−1 · month−1 (P = 0.468, n = 36) from ∼12 to 6 months before diagnosis, −0.10 ng · ml−1 · month−1 (P = 0.059, n = 55) from 6 months before diagnosis to diagnosis, and −0.43 ng · ml−1 · month−1 (P = 0.002, n = 63) from the initial diabetic OGTT to the confirmatory diabetic OGTT.
CONCLUSIONS—It seems that postchallenge C-peptide levels begin to decrease appreciably in the 6 months before diagnosis and decrease even more rapidly within 3 months after diagnosis.
Evidence suggests that there is progressive metabolic dysfunction before and after the diagnosis of type 1 diabetes. A considerable number of individuals who develop type 1 diabetes appear to have a gradual metabolic deterioration (1–3) within 6 months of diagnosis, after which the deterioration becomes more rapid (4). After diagnosis, there also appears to be a progressive loss of insulin secretion (5–8). However, evidence for this loss has been derived from studies performed within a clinical context. Individuals were assessed after their diabetes was diagnosed by clinical presentation and after therapeutic measures were initiated. There are no studies that have followed changes in insulin secretion from before the diagnosis of type 1 diabetes to immediately after its diagnosis in humans. Such information would be highly useful for gauging how quickly interventions should be implemented to delay or prevent the loss of insulin secretion in type 1 diabetes. Interventions that are initiated before a substantial loss of insulin secretion occurs could be more efficacious.
The Diabetes Prevention Trial–Type 1 (DPT-1) provides unique data for examining insulin secretion in the early stages of type 1 diabetes (9,10). Oral glucose tolerance tests (OGTTs) were performed every 6 months for diagnostic surveillance, so that the diagnosis of type 1 diabetes would be captured very close to onset. Also, in participants who had OGTTs in the diabetic range, type 1 diabetes was confirmed with repeat OGTTs. These two features of the DPT-1 data were used to determine the rate and extent of metabolic deterioration that occurs in the perionset period of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Sixty-three participants of the parenteral and oral insulin DPT-1 trials whose diabetes was diagnosed with two consecutive diabetic 2-h OGTTs (initial and confirmatory) are included in the analyses. Those in the intervention arm of the parenteral insulin trial (n = 41) were excluded because the parenteral insulin was received (as per protocol) between the two diabetic OGTTs. Also excluded were those (n = 8) whose interval between the two OGTTs was greater than 3 months. The algorithm for determining risk in the DPT-1 has been described previously (9). The presence of islet cell autoantibodies was required for entry into both trials. Participants were considered to have a 5-year risk above 50% and be eligible for the parenteral insulin trial if either the first-phase insulin response on intravenous glucose tolerance testing was below a defined threshold and/or there were OGTT abnormalities. If those metabolic criteria were not present but insulin autoantibodies were positive, the 5-year risk was considered to be 26–50% and participants were eligible for the oral insulin trial. There was no overall treatment effect in either trial.
Procedures
Participants in the parenteral insulin trial intervention group received recombinant human ultralente insulin, whereas those in the oral insulin trial intervention group received recombinant human insulin crystals. OGTTs were performed at 6-month (±3 months) intervals in both trials. All study treatments were to be suspended for 3 days before the OGTT. The dose of oral glucose was 1.75 g/kg (maximum, 75 g carbohydrate). Samples were obtained for plasma glucose and C-peptide measurements in the fasting state and at 30, 60, 90, and 120 min. Insulin measurements were not obtained because there was concern over the formation of insulin autoantibodies. Individuals with glucose values in the diabetic range at a routine visit were asked to return for confirmation by an OGTT within 60 days (some returned beyond 60 days) unless an OGTT was clinically contraindicated. Participants were to continue the same study regimen they had been using before the initial diabetic OGTT. The age at the first of the diabetic OGTTs was considered the age at diagnosis. The thresholds for diabetes were fasting glucose values ≥126 mg/dl and/or 2-h glucose values ≥200 mg/dl.
Laboratory measures.
Plasma glucose levels were measured by the glucose oxidase method. C-peptide levels were measured by radioimmunoassay. The interassay coefficient of variation for the C-peptide assay was 6.9% in a reference pool with relatively high values and 7.8% in a reference pool with relatively low values. Fasting C-peptide values in the undetectable range (<0.2 ng/ml) were assigned a value of 0.1 ng/ml for the analyses.
Data analysis
The statistical significance of percent change against a null hypothesis of no change was assessed with signed-rank tests. Pearson correlations and linear regression were used to assess associations. Values for rates of change in peak C-peptide were obtained by dividing the difference in peak C-peptide values for an interval by the length of the interval. OGTT areas under the curve (AUCs) were calculated with the trapezoidal rule. Designated time intervals before diagnosis were within 3 months. SAS (version 9.1.3; SAS Institute, Cary, NC) was used for the analyses. All P values are two sided.
RESULTS
Sixty-three DPT-1 participants (51% female) are included in the analyses. All had a complete OGTT in the diabetic range that was confirmed by a second complete OGTT within an interval of 3 months. Of these, 31 were in the parenteral insulin trial and 32 were in the oral insulin trial (15 in the intervention group). The mean ± SD age at the first diabetic OGTT was 13.2 ± 6.9 years. The mean interval between the diabetic OGTTs was 5.5 ± 2.8 weeks.
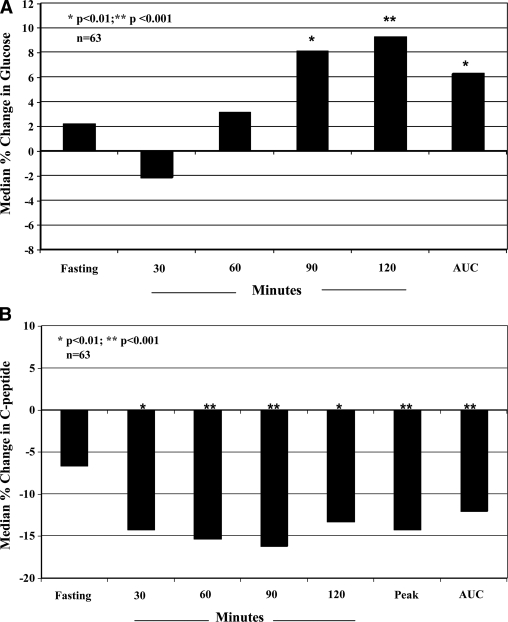
Table 1 shows glucose levels for the initial and confirmatory OGTTs. There was a tendency for glucose levels to increase between the first diabetic OGTT and the confirmatory diabetic OGTT with statistically significant increases at 90 (P = 0.006) and 120 min (P < 0.001) and for AUC glucose (P = 0.016). Figure 1A shows the corresponding percent changes.
Table 1.
Glucose values of initial and confirmatory diabetic OGTTs
| Glucose (mg/dl) |
P value | ||
|---|---|---|---|
| First OGTT | Confirmatory OGTT | ||
| Fasting | 106 (91, 115) | 107 (98, 119) | 0.117 |
| 30 min | 195 (168, 217) | 194 (170, 216) | 0.760 |
| 60 min | 241 (208, 267) | 254 (222, 283) | 0.089 |
| 90 min | 253 (234, 284) | 279 (238, 310) | 0.006 |
| 120 min | 246 (212, 280) | 283 (243, 332) | <0.001 |
| AUC (2-h)* | 25.6 (24.0, 28.2) | 27.5 (24.2, 31.1) | 0.016 |
Data are medians (25th, 75th percentiles). n = 63.
×10−3.
Figure 1.
A: Percent changes in glucose indexes after diagnosis. Shown are the medians for the percent changes of glucose indexes from the initial diabetic OGTT to the confirmatory diabetic OGTT. Glucose levels tended to increase, especially at the later time points of the OGTT. B: Percent changes in C-peptide indexes after diagnosis. Shown are the medians for the percent changes of C-peptide indexes from the initial diabetic OGTT to the confirmatory diabetic OGTT. With the exception of the fasting C-peptide, there was a >10% median decline for all of the indexes.
Table 2 shows the C-peptide levels for the initial and confirmatory OGTTs. There were significant declines in C-peptide levels at each postchallenge time point and for AUC and peak C-peptide values (P < 0.01 for all). Figure 1B shows the corresponding percent changes. The median percent change in peak C-peptide levels was −14.3% (P < 0.001). There was less of a decline in fasting C-peptide levels (−6.7%, P = 0.416). When the fasting C-peptide–to–fasting glucose and the AUC C-peptide–to–AUC glucose ratios were examined, percent changes were appreciable for both the former (−10.3%, P = 0.046) and the latter (−16.7%, P < 0.001).
Table 2.
C-peptide values of initial and confirmatory diabetic OGTTs
| C-peptide (ng/ml) |
P value | ||
|---|---|---|---|
| First OGTT | Confirmatory OGTT | ||
| Fasting | 1.5 (0.7, 2.1) | 1.2 (0.8, 1.7) | 0.054 |
| 30 min | 2.6 (1.9, 4.0) | 2.2 (1.6, 3.5) | 0.001 |
| 60 min | 3.1 (2.1, 4.5) | 2.7 (1.9, 3.8) | <0.001 |
| 90 min | 3.6 (2.3, 5.3) | 3.0 (2.1, 4.3) | 0.001 |
| 120 min | 3.5 (2.5, 5.5) | 3.2 (2.1, 5.0) | 0.004 |
| Peak | 3.8 (2.7, 5.9) | 3.2 (2.2, 5.0) | <0.001 |
| AUC (2-h) | 350 (249, 501) | 309 (212, 443) | <0.001 |
Data are medians (25th, 75th percentiles). n = 63.
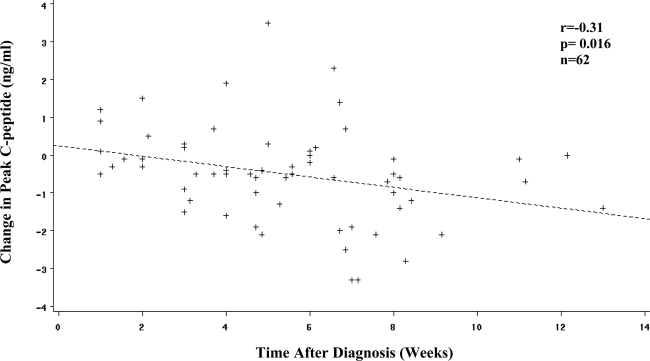
The change in AUC glucose values between the two diabetic OGTTs was positively associated with the length of the interval between them (r = 0.32, P = 0.011), whereas there was an inverse correlation of change of peak C-peptide levels with that interval (r = −0.31, P = 0.014). Thus, the fall in peak C-peptide levels increased with longer intervals. A scatter plot for the association of the change in peak C-peptide levels between the OGTTs and the interval between the diabetic OGTTs (with the removal of an outlier) is shown in Fig. 2. The correlation was almost identical (r = −0.31, P = 0.016) with the outlier excluded. With an allowance for the peak C-peptide levels from the first diabetic OGTT, the slope for the association of change in peak C-peptide levels with the interval between the diabetic OGTTs was −0.56 ng · ml−1 · month−1.
Figure 2.
Association between change in peak C-peptide and time after diagnosis. Shown is the scatter plot for the association between the change in peak C-peptide levels and the time after diagnosis. The amount of decline becomes more substantial with increasing time after diagnosis. (An outlier was removed with a change in peak C-peptide of −8.8 ng/ml and a time after diagnosis of 8.0 weeks [r = −0.31, P = 0.014 with the outlier included.]) When an allowance was made for the peak C-peptide at the first diabetic OGTT, the slope for the difference in peak C-peptide versus time after diagnosis was −0.56 ng · ml−1 · month−1.
Of the 63 individuals included in the analysis, 55 had an OGTT ∼6 months before the initial diabetic OGTT. The median percent change for the peak C-peptide in that interval was −14.0% (P = 0.052). The percent change in the peak C-peptide from the last nondiabetic OGTT to the second diabetic OGTT (mean ± SD interval 7.5 ± 1.3 months) was −23.8% (P < 0.001). The AUC C-peptide–to–AUC glucose percent change was even more marked (−45.7%, P < 0.001) in that interval.
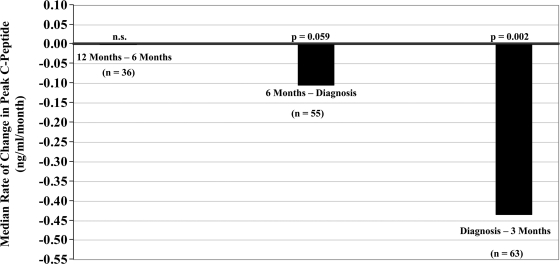
Figure 3 shows the median rates of change in peak C-peptide levels over intervals in the perionset period. The values were obtained by dividing the difference in peak C-peptide values for an interval by the length of the interval. There was minimal change (0.00 ng · ml−1 · month−1, P = 0.468, n = 36) in peak C-peptide from ∼12 to 6 months before diagnosis. There was a decline in peak C-peptide levels from 6 months before diagnosis to diagnosis (−0.10 ng · ml−1 · month−1, P = 0.059, n = 55) and an even greater rate of decline from diagnosis to within 3 months after diagnosis (−0.43 ng · ml−1 · month−1, P = 0.002, n = 63).
Figure 3.
Rates of change in peak C-peptide in the perionset period. Shown are the rates of change of peak C-peptide levels according to intervals before and after diagnosis. C-peptide levels changed minimally between ∼12 and 6 months before diagnosis. There was a decline in the 6 months before diagnosis that was more substantial in the period after diagnosis.
CONCLUSIONS
The data in this report show that, on average, C-peptide levels decreased substantially in the interval from diagnosis to 3 months after diagnosis. These changes occurred even with glucose levels still in a range associated with minimal or no symptoms.
We previously examined metabolic progression before diagnosis in DPT-1 participants (4). In that report, peak C-peptide levels were consistent during a period of ∼30 to 6 months before diagnosis, after which levels declined. This report extends observations to the postdiagnosis period and suggests that there is an acceleration of postchallenge C-peptide loss once glucose levels are in the diabetic range. The median decline of −23.8% in peak C-peptide levels from the last nondiabetic OGTT to the confirmatory OGTT indicates that there is a marked loss of insulin secretion in the perionset period. The extent to which this loss is reversible cannot be determined from the data.
Estimates for the rate of change of peak C-peptide levels in the postdiagnosis period were obtained in two ways. In one approach (Fig. 2) a regression analysis was used, whereas in the other approach (Fig. 3), the estimate was derived from an analysis based on rate of change calculated for each individual. The rate of decline was substantial with either approach.
Glucose levels seem to have been maintained relative to the decline in C-peptide levels after diagnosis. This suggests the possibility that compensatory mechanisms for glucose homeostasis are at play, such as an increase in insulin sensitivity. Because C-peptide levels are only indicative of insulin secretion, it is also possible that a slowing of insulin degradation could have contributed to the maintenance of glucose levels.
For calculations of the rate of change in peak C-peptide levels, it was assumed that the rate of change was constant throughout the interval. This assumption is of particular importance in the interval from 6 months before diagnosis to diagnosis, as one cannot discern from the data the pattern of C-peptide decline within that period. Thus, the rate of decrease in C-peptide may be more rapid closer to diagnosis and similar to the rate of decline in C-peptide after diagnosis. Also, it should be emphasized that the average change provides an overall picture; individual patterns of change vary considerably.
Participation in the DPT-1 trials could have influenced the findings. However, we excluded those receiving parenteral insulin from the analyses, and there was no overall effect from either insulin intervention. Knowledge of the results of the first diabetic OGTT could have resulted in lifestyle changes (11) or perhaps even have caused some to attempt to lower glucose levels with medication. Still, it is doubtful that such interventions would explain the large degree of C-peptide loss.
No prior studies have examined metabolic changes from before diagnosis to after diagnosis with OGTT surveillance. Also, no studies have assessed metabolic changes in individuals with newly diagnosed diabetes as close to the onset of type 1 diabetes. C-peptide levels appear to be much lower when type 1 diabetes is clinically diagnosed (12–14) than when it is diagnosed through OGTT surveillance. It is important to emphasize that of all individuals in whom type 1 diabetes was diagnosed in the DPT-1, 75% were asymptomatic (9). How our observations relate to the rate of decline of insulin secretion in symptomatic patients with clinically diagnosed diabetes is unknown. Studies of patients with clinically diagnosed diabetes suggest that there is a progressive loss of insulin secretion that can be decreased by effective glucose control (15,16).
The marked rate of decline of C-peptide levels in the perionset period provides a strong rationale for developing early interventions to prevent or delay the progression to type 1 diabetes. Moreover, the data suggest that postdiagnosis interventions should be developed for application as close to the diagnosis of type 1 diabetes as possible.
Published ahead of print at http://care.diabetesjournals.org on 23 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Greenbaum CJ, Cuthbertson D, Krischer JP, the Diabetes Prevention Trial of Type 1 Diabetes Study Group: Type 1 diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes 50:470–476, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Schatz D, Cuthbertson D, Atkinson M, Salzler MC, Winter W, Muir A, Silverstein J, Cook R, Maclaren N, She J, Greenbaum C, Krischer J: Preservation of C-peptide secretion in subjects at high risk of developing type 1 diabetes mellitus—a new surrogate measure of non-progression? Pediatr Diabetes 5:72–79, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS, the Diabetes Prevention Trial–Type 1 Study Group: Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes. Diabetes Care 30:38–42, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS, the Diabetes Prevention Trial–Type 1 Study Group. Patterns of metabolic progression to type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care 29:643–649, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Snorgaard O, Lassen LH, Binder C: Homogeneity in pattern of decline of β-cell function in IDDM: prospective study of 204 consecutive cases followed for 7.4 yr. Diabetes Care 15:1009–1013, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 53:250–264, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC: Insulin secretion in type 1 diabetes. Diabetes 53:426–433, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Sherry NA, Tsai EB, Herold KC: Natural history of β-cell function in type 1 diabetes. Diabetes 54 (Suppl. 2):S32–S39, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Prevention Trial–Type 1 Diabetes Study Group: Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346:1685–1691, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Trial–Type 1 Diabetes Study Group: Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care 28:1068–1076, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Johnson SB, Baughcum AE, Hood K, Rafkin-Mervis LE, Schatz DA, DPT-1 Study Group: Participant and parent experiences in the parenteral insulin arm of the Diabetes Prevention Trial for Type 1 Diabetes. Diabetes Care 30:2193–2198, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Faber OK, Binder C: β-Cell function and blood glucose control in insulin dependent diabetics within the first month of insulin treatment. Diabetologia 13:263–268, 1977 [DOI] [PubMed] [Google Scholar]
- 13.Komulainen J, Knip M, Lounamaa R, Vähäsalo P, Karjalainen J, Sabbah E, Åkerblom HK, Childhood Diabetes in Finland Study Group: Poor β-cell function after the clinical manifestation of type 1 diabetes in children initially positive for islet cell specific autoantibodies. Diabet Med 14:532–537, 1997 [DOI] [PubMed] [Google Scholar]
- 14.O'Leary LA, Dorman JS, LaPorte RE, Orchard TJ, Becker DJ, Kuller LH, Eberhardt MS, Cavender DE, Rabin BS, Drash AL: Familial and sporadic insulin-dependent diabetes: evidence for heterogeneous etiologies? Diabetes Res Clin Pract 14:183–190, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson J, Heding LG, Larsson Y, Leander E: C-peptide in juvenile diabetics beyond the postinitial remission period: relation to clinical manifestation at onset of diabetes, remission and diabetic control. Acta Paediatr Scand 66:177–184, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Shah SC, Malone JI, Simpson NE: A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes. N Engl J Med 350:550–555, 1989 [DOI] [PubMed] [Google Scholar]