Abstract
OBJECTIVE—Debate remains as to whether short- or long-term glycemic instability confers a risk of microvascular complications in addition to that predicted by mean glycemia alone. In this study, we analyzed data from the Diabetes Control and Complications Trial (DCCT) to assess the effect of A1C variability on the risk of retinopathy and nephropathy in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS—A1C was collected quarterly during the DCCT in 1,441 individuals. The mean A1C and the SD of A1C variability after stabilization of glycemia (from 6 months onwards) were compared with the risk of retinopathy and nephropathy with adjustments for age, sex, disease duration, treatment group, and baseline A1C.
RESULTS—Multivariate Cox regression showed that the variability in A1C added to mean A1C in predicting the risk of development or progression of both retinopathy (hazard ratio 2.26 for every 1% increase in A1C SD [95% CI 1.63–3.14], P < 0.0001) and nephropathy (1.80 [1.37–2.42], P < 0.0001), with the relationship a feature in conventionally treated patients in particular.
CONCLUSIONS—This study has shown that variability in A1C adds to the mean value in predicting microvascular complications in type 1 diabetes. Thus, in contrast to analyses of DCCT data investigating the effect of short-term glucose instability on complication risk, longer-term fluctuations in glycemia seem to contribute to the development of retinopathy and nephropathy in type 1 diabetes.
The effect that glycemic variability may have on the risk of development of microvascular complications of diabetes remains controversial (1). Studies such as the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes and the UK Prospective Diabetes Study (UKPDS) in type 2 diabetes have left little doubt that the risk of microvascular complications rises exponentially as mean blood glucose (assessed using A1C) increases (2–5). However, with regard to glycemic variability, the clinical evidence has not been consistent. For example, in the DCCT the rate of complications at a given value of A1C was apparently higher in the conventionally treated patients than in those treated intensively (3), leading to the suggestion that this result may be a consequence of larger glycemic excursions in the former group of patients as they received fewer injections of insulin per day (6). Nonetheless, further analyses of the DCCT dataset has shown that within-day glucose variability, using seven-point laboratory measured glucose profiles, had no additional influence on the risk of micro- or macrovascular complication risk beyond that predicted by the mean glucose value alone (7–9). A more recent reanalysis of the A1C data by the DCCT group has shown that the original differences between treatment groups was probably an artifact of model assumptions originally used and that no discrepancies in microvascular risk at the same A1C actually existed (10). Indeed, it has subsequently been suggested that the increased complication risk in conventionally treated patients was simply because their blood glucose values were higher compared with those of intensively treated patients at the same A1C (11).
It is also currently unknown whether short-term (within-day) variability may have a different influence on complications compared with longer-term (day-to-day or week-to-week) glucose fluctuations. Certainly, data from the Pittsburgh Epidemiology Study showed that A1C variability seemed to be an additional risk factor for the development of macrovascular complications (12).
It is fundamental to managing diabetes for a clinician to know whether a patient with glucose instability has a higher risk of microvascular complications than one without, especially because many of the recent pharmacological advances have focused on reducing glucose variability (as well as mean glucose) largely by targeting postprandial hyperglycemia. Therefore, in this current study we have analyzed the publicly available DCCT dataset to investigate the potential for A1C variability to have an influence on microvascular complications.
RESEARCH DESIGN AND METHODS
We used the publicly accessible datasets collected by the DCCT, which were stored in SAS format (http://www.gcrc.umn.edu). The DCCT was a 9-year follow-up study of 1,441 participants with type 1 diabetes comparing the effect of intensive versus conventional blood glucose management on the development of the microvascular complications of diabetes. At randomization, patients were stratified into one of two cohorts. The primary prevention cohort (n = 726) had no evidence of retinopathy by fundus photography and a urinary albumin excretion rate (AER) <40 mg/24 h (28 μg/min). The secondary prevention cohort (n = 715) had only minimal retinopathy, and an AER <200 mg/24 h (140 μg/min). The study participants were randomly assigned into intensive (n = 711) and conventional (n = 730) treatment groups.
Definition of events
Severity of retinopathy was determined by the 25-point Early Diabetic Retinopathy Treatment Study (EDRTS) interim score (2). The development and progression of sustained retinopathy was defined as a change from baseline of ≥3 units on the EDRTS score on any two successive annual evaluations. During the 9 years of follow-up, 242 individuals developed sustained retinopathy, 67 of whom were in the intensive treatment group. Nephropathy was defined as an increase in AER ≥40 mg/24 h (28 μg/min) on any annual evaluation providing that the baseline AER was <40 mg/dl (28 μg/min). The mean age was 27 years (range 13–39 years). Just over half (n = 761, 52.8%) were men. Average BMI was 23.4 kg/m2; <2% had a BMI >30 kg/m2. Nearly all participants were Caucasian. The median disease duration was 4 years. Approximately one-fifth declared themselves as current smokers.
A1C measurement and statistical methods
A1C was measured quarterly in both treatment groups. In this analysis, we only used data from 6 months into the trial because the subjects in the intensively treated group were undergoing a period of rapidly changing glycemic control before reaching an A1C nadir at 6 months. The relationship between A1C and the development of diabetes complications was assessed by Cox regression from which hazard ratios (HRs) and 95% CIs were calculated. The Cox regression model is semiparametric in the sense that no assumption concerning event-free survival times is necessary; it is based on the assumption that the effect of a risk factor, expressed as an HR, is constant over time. The assumption of proportionality of the Cox model covariates was tested by plotting Schoenfeld residuals. All Cox regression models were adjusted for the following baseline covariates: age (years), sex, disease duration (years), randomization treatment (conventional versus intensive), prevention cohort (primary versus secondary), and A1C at the study eligibility stage.
Three models were constructed. The first looked solely at the influence of the updated mean A1C, from 6 months onwards, on the risk of subsequent microvascular complications, whereas the second model also included the variability in A1C throughout the period expressed as SDs of A1C over all visits. As the number of visits an individual patient had could also influence this SD (such that few visits would make the SD apparently greater than many visits), the SD value was also divided by √[n/(n − 1)] to adjust for this possibility. The third model expressed A1C variability as an updated time-dependent SD (3).
Patients in the conventionally treated group who became pregnant were unblinded to their A1C values and treated with a goal of near-normal glycemia, before returning to the conventional treatment protocol after delivery (13). To ensure that our results were not influenced by these 86 individuals, a separate analysis that excluded these patients was performed.
There has been considerable interest in calculating coefficients of determination (r2) in models other than those for least-squares regression. Many researchers have looked at developing an r2 equivalent for the Cox proportional hazards model. However, the difficulty lies in how to take censoring into account. Because there is no consensus on how best to measure r2 for the Cox model (14), we have not pursued it here.
The GLIM4 and SPSS statistical computer packages were used to analyze the data. An arbitrary level of 5% statistical significance (two-tailed) was assumed.
RESULTS
A1C variability was greater in the conventionally treated patients than in those treated intensively (A1C SD 0.86 vs. 0.59). Variability was also closely related to the mean A1C of all patients (r = 0.55), being especially so in intensively treated patients compared with conventionally treated patients (r = 0.71 vs. r = 0.32).
Table 1 shows the relationship between the updated mean A1C and both retinopathy and nephropathy risk in the intensively treated group, conventionally treated group, and both groups combined (model 1). Models 2 and 3 show the effect of including A1C variability (either as the SD over the entire study duration or as an updated SD) within the same model. In each case in which mean A1C was initially predictive of complications, A1C SD either added to (or explained) the risk indicated by the mean value of A1C alone. Expressing SD after dividing by √[n/(n − 1)] made no difference as to the effect of A1C variability on complication risk. In addition, limiting the analysis to only those conventionally treated patients who had not become pregnant during the DCCT did not alter the findings. For example, in model 2, the HR for SD among nonpregnant conventionally treated patients was 2.2 (95% CI 1.4–3.4).
Table 1.
Influence of updated mean A1C alone (model 1), inclusive of mean A1C SD (model 2), or updated mean A1C (model 3) on complication risk
| Intensive group HR (95% CI) | P value | Conventional group HR (95% CI) | P value | Combined HR (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Retinopathy | ||||||
| Mean A1C | 1.20 (1.04–1.39) | 0.016 | 1.24 (1.12–1.36) | <0.0001 | 1.22 (1.12–1.32) | <0.0001 |
| Nephropathy | ||||||
| Mean A1C | 1.06 (0.96–1.17) | 0.24 | 1.21 (1.10–1.33) | <0.0001 | 1.13 (1.05–1.22) | 0.001 |
| Model 2 | ||||||
| Retinopathy | ||||||
| Mean A1C | 1.15 (0.95–1.40) | 0.16 | 1.18 (1.07–1.29) | 0.001 | 1.17 (1.08–1.28) | <0.0001 |
| A1C SD | 2.53 (1.36–4.68) | 0.003 | 2.11 (1.42–3.20) | <0.0001 | 2.26 (1.63–3.14) | <0.0001 |
| Nephropathy | ||||||
| Mean A1C | 1.052 (0.89–1.23) | 0.54 | 1.15 (1.04–1.27) | 0.005 | 1.11 (1.02–1.21) | 0.009 |
| A1C SD | 1.51 (0.88–2.60) | 0.14 | 2.30 (1.57–3.37) | <0.0001 | 1.80 (1.37–2.42) | <0.0001 |
| Model 3 | ||||||
| Retinopathy | ||||||
| Mean A1C | 1.09 (0.97–1.23) | 0.17 | 1.18 (1.07–1.31) | 0.001 | 1.14 (1.05–1.24) | 0.002 |
| Updated A1C SD | 3.22 (1.95–5.32) | <0.0001 | 1.70 (1.14–2.52) | 0.009 | 2.11 (1.54–2.89) | <0.0001 |
| Nephropathy | ||||||
| Mean A1C | 1.03 (0.94–1.13) | 0.52 | 1.15 (1.04–1.27) | 0.004 | 1.08 (1.01–1.16) | 0.027 |
| Updated A1C SD | 1.91 (1.21–3.10) | 0.005 | 1.94 (1.34–2.89) | <0.0001 | 1.86 (1.41–2.47) | <0.0001 |
Models were adjusted for age, sex, duration, intervention group (when groups were combined), and A1C at eligibility: A1C time dependent and blood glucose time dependent. Models used were from 6 months onward.
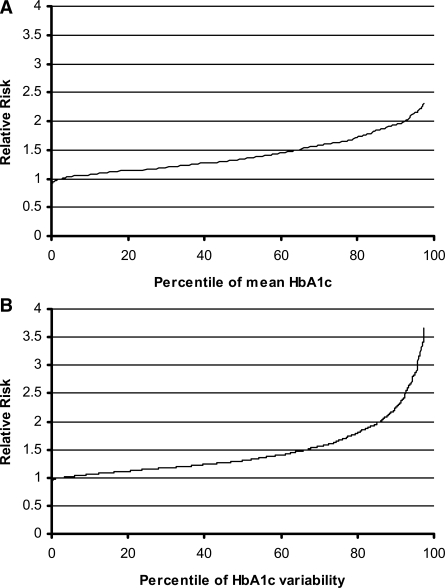
Figure 1A shows the relative risk of retinopathy progression using both treatment groups combined in model 2 (Table 1) when applied to DCCT patients in the range of 0–97.5th centile of mean A1C after adjustment for A1C SD, using the 2.5th centile as a reference. Figure 1B shows the same patients over the same range of A1C SD after adjustment for mean A1C.
Figure 1.
Relative risk of retinopathy progression over the range of patients within the 0–97.5th centile of mean A1C after adjusting for A1C SD, using the 2.5th centile as a reference (A) and for A1C SD after adjustment for mean A1C (B).
CONCLUSIONS
This analysis of the DCCT data has shown that in patients with type 1 diabetes, increasing variability in A1C adds to the risk of microvascular complications over and above that predicted by the mean A1C value alone. This finding was present in the DCCT cohort overall and was also a feature of both treatment groups individually when the mean A1C alone was initially predictive. The effect was most pronounced among those patients who were in the conventionally treated group, presumably because the event rate, the range of variability, and the spread of variability at any given mean A1C was much larger than those for patients in the intensively treated patients.
The magnitude of the effect of A1C variability is marked, such that a 1% absolute increase in A1C SD results in at least a doubling in retinopathy and an 80% increase in nephropathy risk using either of our models. As shown in Fig. 1B, put into the context of individuals participating in the DCCT, it means that a patient in the 97.5th centile of A1C variability (SD 1.87%) has more than three times the retinopathy risk and more than twice the nephropathy risk of a patient in the 2.5th centile (SD 0.25%) for a given A1C. The findings are in contrast to those of a previous analysis of the DCCT data, which suggested that A1C variability had little effect on complication risk (3), but probably included all patient visits, including those before A1C stabilized at 6 months in intensively treated patients.
There are several possible reasons for the current findings. One is that medium- to long-term fluctuation in blood glucose truly is an additional risk for the development of microvascular complications. This fluctuation is in contrast to within-day glucose variability, which showed little influence on retinopathy or nephropathy risk in the same patients in the DCCT (7,8). The latter finding was surprising because numerous studies have shown that short-term glycemic excursions lead to an overproduction of reactive oxygen species in cell cultures (15) as well as in patients with type 2 diabetes (16) (though not confirmed in type 1 [17]). There are also data on the effect of longer-term changes in glycemia on free radical production (18) as well as both clinical and laboratory evidence that periods of sustained hyperglycemia are “remembered” and so place patients at higher subsequent long-term risk of complications (19,20). In this regard, the detrimental effect of A1C variability may be mediated through the same mechanism underlying that of the “metabolic memory” phenomenon (20,21).
An alternative possible explanation for our data relates to the fact that the risk of microvascular complications seems to rise exponentially, rather than linearly, as A1C rises (2,3). Thus, although patients who have more variable A1C values will be spending the same time above and below their mean value as another with comparatively stable A1C, their average risk will be higher because their periods of sustained glycemia far above their mean will be placing them at an especially high complication risk, which will more than cancel out any reduction in risk resulting from them also having equal periods far below their mean. If true, it would be expected that this effect would be exaggerated as the individual's mean A1C was higher, which may be one reason why the influence of A1C variability seems greater in conventionally treated patients.
Another possible reason linking A1C fluctuations with complication risk is the consistent observation that improving glycemic control can lead to a short-term worsening in retinopathy (22,23) before subsequently resulting in a net long-term improvement (23): the “normoglycemic reentry phenomenon.” Indeed, the potential for early retinopathy worsening was one of the main concerns of improving glucose control when the DCCT was conceived. The mechanism for this paradoxical deterioration is not fully known but is thought to involve changes in ocular blood flow and increased IGF-1 concentrations (24) consequent on improved glycemic control. Whatever the cause, the cyclical improvements in glycemic control found in patients with more variable A1C could result in a cyclical worsening of retinopathy, which is in addition to that predicted by the mean A1C value alone. This excess risk, of course, could also be compounded by the intervening periods of worsening control. In opposition to this hypothesis is the fact that the early worsening of nephropathy after glycemic improvement is not well recognized (with the possible exception of patients in the DCCT who became pregnant) (13), although this may be more a reflection of retinal changes becoming apparent before those of urinary albumin.
These explanations may partially reconcile why short-term (within-day) glucose variability in the same patients has been found to have little effect on microvascular risk (7,8), whereas longer-term instability in the form of A1C has been found here to be much more predictive. Alternatively, it is possible that A1C is more sensitive for detecting the effect of glucose changes than daily glucose profiles, although the same DCCT data have shown the mean glucose, rather than glucose variability, has the major influence on the A1C value (25).
There do not seem to be many limitations in this study related to the size and completeness of the dataset, which is a reflection of the rigorous protocol of the DCCT and of the dedication of the patients involved. In sum total, the 1,441 individuals had 31,260 A1C measurements performed from 6 months into the study on which to base our conclusions. It must also not be forgotten that the study benefited by being performed before other potential confounding factors such as antihypertensive and lipid-lowering agents came in to common use. Nevertheless, in interpreting these data, it should also be remembered that A1C in the DCCT as a whole explained no more than 23% of the risk of retinopathy progression for the entire cohort, with the risk reduction associated with intensive treatment rather than conventional treatment A1C values being just a fraction of this percentage (3).
In summary, this study has shown that longer-term fluctuations in glycemia seem to independently relate to the development of retinopathy and nephropathy in type 1 diabetes. Thus, sole measurement of mean glucose or mean A1C may not be the best predictor of complication risk.
Published ahead of print at http://care.diabetesjournals.org on 23 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Monnier L, Colette C: Glycemic variability: should we and can we prevent it? Diabetes Care 31:150–154, 2008 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group: The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44:968–983, 1995 [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M, Hirsch IB: Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 295:1707–1708, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Service FJ, O'Brien PC: The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 44:1215–1220, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kilpatrick ES, Rigby AS, Atkin SL: The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 29:1486–1490, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick ES, Rigby AS, Atkin SL: Mean blood glucose compared with HbA1c in the prediction of cardiovascular disease in type 1. Diabetologia 51:365–371, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN: The effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisited. Diabetes 57:995–1001, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick ES, Rigby AS, Atkin SL: Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin Chem 53:897–901, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Prince C, Becker D, Costacou T, Miller R, Orchard T: Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia 50:2280–2288, 2007 [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group: Effect of pregnancy on microvascular complications in the Diabetes Control and Complications Trial. Diabetes Care 23:1084–1091, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schemper M, Henderson R: Predictive accuracy and explained variation in Cox regression. Biometrics 56:249–255, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A: Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52:2795–2804, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol J-P, Colette C: Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Wentholt I, Kulik W, Michels R, Hoekstra J, DeVries J: Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia 51:183–190, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C: In vivo formation of 8-iso-prostaglandin F2α and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99:224–229, 1999 [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group: Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287:2563–2569, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihnat M, Thorpe J, Ceriello A: Hypothesis: the ‘metabolic memory,’ the new challenge of diabetes. Diabet Med 24:582–586, 2007 [DOI] [PubMed] [Google Scholar]
- 22.The Kroc Collaborative Study Group: Blood glucose control and the evolution of diabetic-retinopathy and albuminuria: a preliminary multicenter trial. N Engl J Med 311:365–372, 1984 [DOI] [PubMed] [Google Scholar]
- 23.The Diabetes Control and Complications Trial Research Group: Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol 116:874–886, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Smith LEH, Kopchick JJ, Chen W, Knapp J, Kinose F, Daley D, Foley E, Smith RG, Schaeffer JM: Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 276:1706–1709, 1997 [DOI] [PubMed] [Google Scholar]
- 25.McCarter RJ, Hempe JM, Chalew SA: Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 29:352–355, 2006 [DOI] [PubMed] [Google Scholar]