Abstract
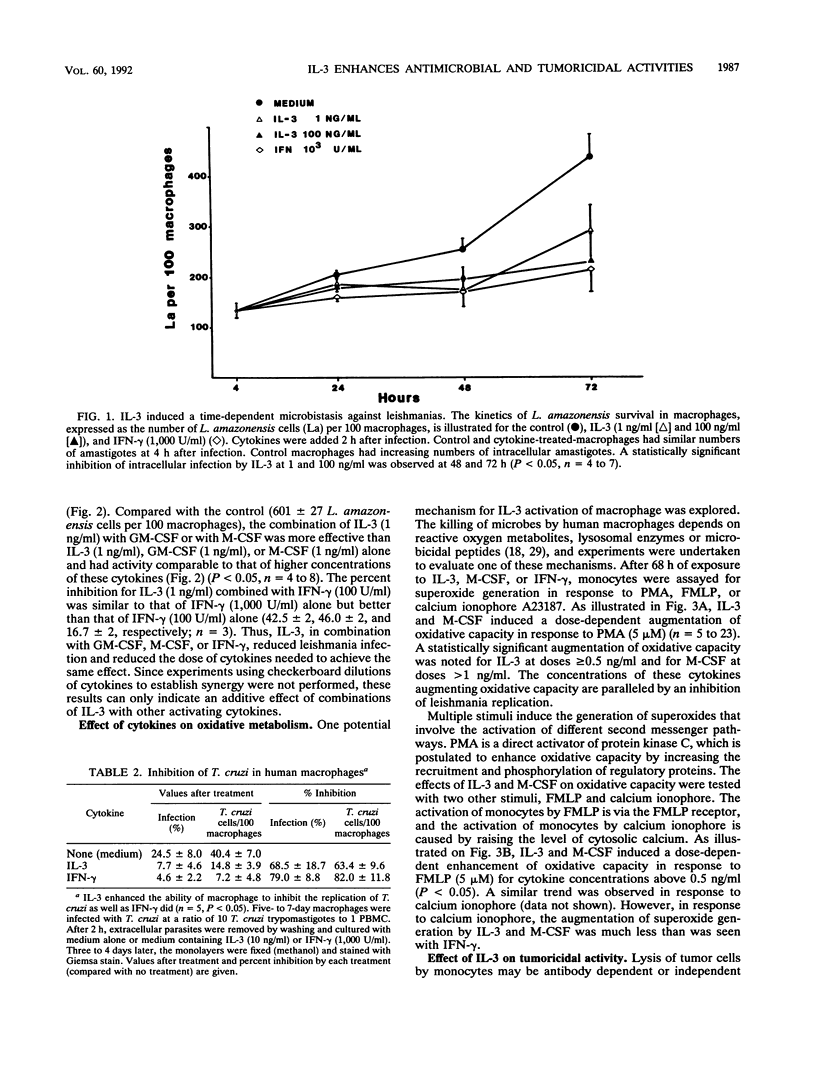
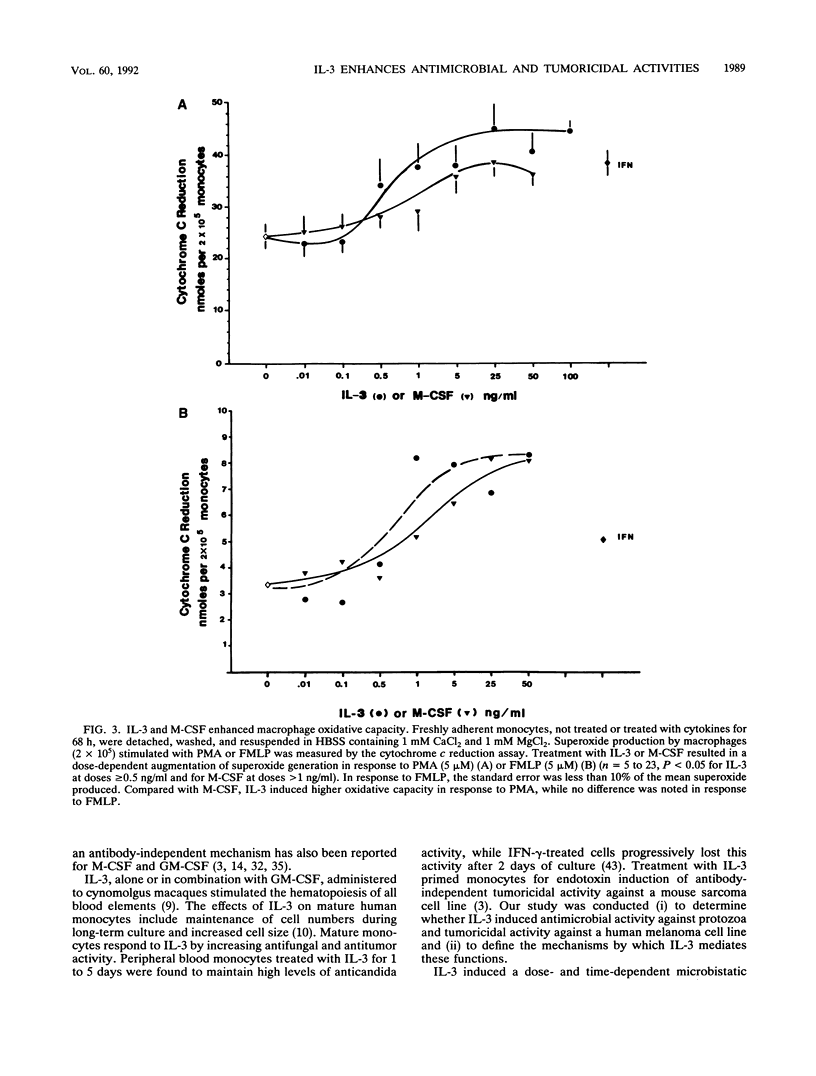
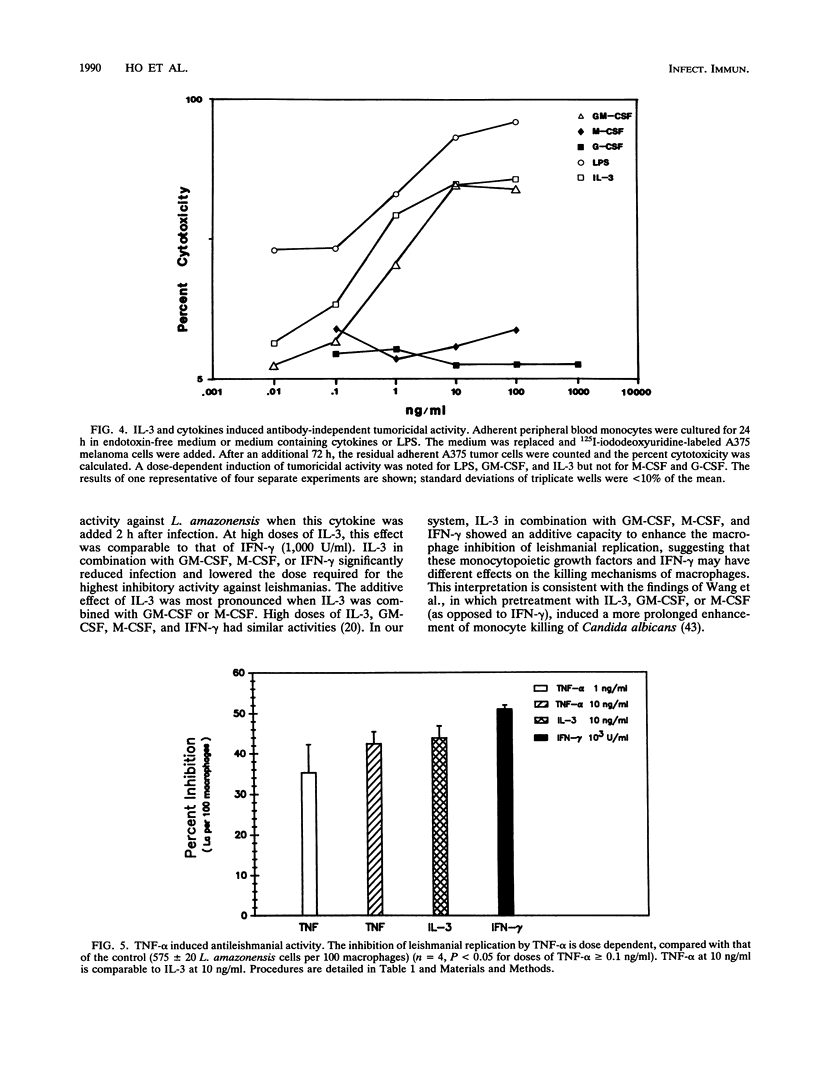
The ability of interleukin-3 (IL-3) to induce antimicrobial and tumoricidal activity was evaluated. Macrophages infected with two intracellular protozoa, Leishmania amazonensis or Trypanosoma cruzi, were treated with cytokines. IL-3 induced a dose-dependent enhancement of microbistasis against leishmanias, and the activity of IL-3 (100 ng/ml) was comparable to that of gamma interferon (IFN-gamma) (1,000 U/ml). In addition, IL-3 in combination with either granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage CSF (M-CSF) or with IFN-gamma reduced infection and lowered the required dose. IL-3 similarly activated macrophages to inhibit intracellular replication of T. cruzi. Furthermore, IL-3 induced antibody-independent tumoricidal activity against melanoma cells that was dose dependent and comparable to that of lipopolysaccharide and GM-CSF. The mechanisms by which IL-3 induced antimicrobial activity may involve at least the augmentation of oxidative capacity. IL-3, at concentrations of 0.5 ng/ml or greater, led to a significantly increased oxidative burst which paralleled the inhibition of protozoan replication. The enhancement of oxidative capacity by IL-3 (5 ng/ml or higher) was comparable to that of IFN-gamma. The induction of tumoricidal activity was associated with the production of tumor necrosis factor alpha (TNF-alpha), which in this system may feed back to enhance the macrophage inhibition of leishmanias, as demonstrated by neutralization of IL-3 activation by anti-TNF-alpha antibody. Thus, peripheral blood macrophages remain responsive to IL-3, as demonstrated by enhanced antimicrobial and tumoricidal activity. IL-3 may have potential clinical applications because of these properties and its effect on myelopoiesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Warren M. K., Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987 Dec 1;139(11):3703–3709. [PubMed] [Google Scholar]
- Bersani L., Colotta F., Mantovani A. Involvement of tumour necrosis factor in monocyte-mediated rapid killing of actinomycin D-pretreated WEHI 164 sarcoma cells. Immunology. 1986 Oct;59(2):323–325. [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Cantrell M. A., Anderson D., Cerretti D. P., Price V., McKereghan K., Tushinski R. J., Mochizuki D. Y., Larsen A., Grabstein K., Gillis S. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon J. Y., Blackwell J. M. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. I. Differences in sensitivity correlate with parasite-mediated removal of hydrogen peroxide. Parasitology. 1985 Oct;91(Pt 2):197–206. doi: 10.1017/s0031182000057309. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Donahue R. E., Seehra J., Metzger M., Lefebvre D., Rock B., Carbone S., Nathan D. G., Garnick M., Sehgal P. K., Laston D. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988 Sep 30;241(4874):1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Feinman R., Henriksen-DeStefano D., Tsujimoto M., Vilcek J. Tumor necrosis factor is an important mediator of tumor cell killing by human monocytes. J Immunol. 1987 Jan 15;138(2):635–640. [PubMed] [Google Scholar]
- Feng Z. Y., Louis J., Kindler V., Pedrazzini T., Eliason J. F., Behin R., Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988 Aug;18(8):1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988 Nov 15;948(2):151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Groopman J. E., Molina J. M., Scadden D. T. Hematopoietic growth factors. Biology and clinical applications. N Engl J Med. 1989 Nov 23;321(21):1449–1459. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. J Immunol. 1988 Sep 1;141(5):1516–1521. [PubMed] [Google Scholar]
- Ho J. L., He S. H., Rios M. J., Wick E. A. Interleukin-4 inhibits human macrophage activation by tumor necrosis factor, granulocyte-monocyte colony-stimulating factor, and interleukin-3 for antileishmanial activity and oxidative burst capacity. J Infect Dis. 1992 Feb;165(2):344–351. doi: 10.1093/infdis/165.2.344. [DOI] [PubMed] [Google Scholar]
- Ho J. L., Klempner M. S. Diminished activity of protein kinase C in tetanus toxin-treated macrophages and in the spinal cord of mice manifesting generalized tetanus intoxication. J Infect Dis. 1988 May;157(5):925–933. doi: 10.1093/infdis/157.5.925. [DOI] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Kleinerman E. S., Knowles R. D., Lachman L. B., Gutterman J. U. Effect of recombinant granulocyte/macrophage colony-stimulating factor on human monocyte activity in vitro and following intravenous administration. Cancer Res. 1988 May 1;48(9):2604–2609. [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- Liew F. Y., Parkinson C., Millott S., Severn A., Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Dyson P. G., To L. B., Elliott M. J., Milton S. E., Russell J. A., Juttner C. A., Yang Y. C., Clark S. C., Vadas M. A. Recombinant human interleukin-3 stimulation of hematopoiesis in humans: loss of responsiveness with differentiation in the neutrophilic myeloid series. Blood. 1988 Nov;72(5):1797–1804. [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Stimulation of macrophage tumoricidal activity by the growth and differentiation factor CSF-1. Cell Immunol. 1987 Apr 1;105(2):270–279. doi: 10.1016/0008-8749(87)90076-1. [DOI] [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson-Johannes A., Carlino J. A. Enhancement of human monocyte tumoricidal activity by recombinant M-CSF. J Immunol. 1988 Nov 15;141(10):3680–3686. [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 1985 Dec 6;230(4730):1171–1173. doi: 10.1126/science.3877981. [DOI] [PubMed] [Google Scholar]
- Styrt B., Klempner M. S. Inhibition of neutrophil oxidative metabolism by lysosomotropic weak bases. Blood. 1986 Feb;67(2):334–342. [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesio L., Blasi E., Thurman G. B., Talmadge J. E., Wiltrout R. H., Herberman R. B. Potent activation of mouse macrophages by recombinant interferon-gamma. Cancer Res. 1984 Oct;44(10):4465–4469. [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Temple P. A., Leary A. C., Witek-Giannotti J. S., Yang Y. C., Ciarletta A. B., Chung M., Murtha P., Kriz R., Kaufman R. J. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987 Mar 20;235(4795):1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


