Abstract
OBJECTIVE—The purpose of this study was to assess the efficacy of an insulin priming dose with a continuous insulin infusion versus two continuous infusions without a priming dose.
RESEARCH DESIGN AND METHODS—This prospective randomized protocol used three insulin therapy methods: 1) load group using a priming dose of 0.07 units of regular insulin per kg body weight followed by a dose of 0.07 unit · kg−1 · h−1 i.v. in 12 patients with diabetic ketoacidosis (DKA); 2) no load group using an infusion of regular insulin of 0.07 unit · kg body weight−1 · h−1 without a loading dose in 12 patients with DKA, and 3) twice no load group using an infusion of regular insulin of 0.14 · kg−1 · h−1 without a loading dose in 13 patients with DKA. Outcome was based on the effects of insulin therapy on biochemical and hormonal changes during treatment and recovery of DKA.
RESULTS—The load group reached a peak in free insulin value (460 μU/ml) within 5 min and plateaued at 88 μU/ml in 60 min. The twice no load group reached a peak (200 μU/ml) at 45 min. The no load group reached a peak (60 μU/ml) in 60–120 min. Five patients in the no load group required supplemental insulin doses to decrease initial glucose levels by 10%; patients in the twice no load and load groups did not. Except for these differences, times to reach glucose ≤250 mg/dl, pH ≥7.3, and HCO3− ≥15 mEq/l did not differ significantly among the three groups.
CONCLUSIONS—A priming dose in low-dose insulin therapy in patients with DKA is unnecessary if an adequate dose of regular insulin of 0.14 unit · kg body weight−1 · h−1 (about 10 units/h in a 70-kg patient) is given.
Although positive therapeutic responses to low-dose insulin therapy have been established in adult patients with diabetic ketoacidosis (DKA) (1–8), none of these studies and guidelines for the treatment of DKA, including the American Diabetes Association (ADA) Consensus and Position Statements, has ever assessed or addressed the use of a continuous insulin infusion without a loading dose of insulin (9). In the current study, we used a dose of 0.14 unit · kg−1 · h−1 without a loading dose instead of the recommended 0.1 unit · kg−1 · h−1 with a loading dose. This insulin regimen was chosen because one study in a pediatric population used a dosing regimen of 0.1 unit · kg−1 · h−1 without a loading dose that resulted in a total plasma insulin level of 50–60 μU/ml (10). This level proved to be too low for optimal suppression of hepatic glucose output and optimal glucose uptake (11). In addition, bolus doses of insulin may result in hypokalemia as well as other undesirable effects (12), especially when used in a routine hospital setting.
The efficacy of low-dose insulin without a priming dose has not been established in a prospective randomized study (13). Thus, the following questions have remained unanswered in the treatment of DKA: 1) Is an insulin bolus needed before a continuous insulin infusion? 2) What is the optimal insulin infusion rate if a bolus dose is not used? and 3) What is the dose response of continuous insulin infusion used alone in regard to decremental changes in glucose, metabolic parameters, cortisol, and free fatty acids (FFAs)? Therefore, we evaluated responses to three low-dose insulin regimens in 37 consecutive patients with DKA in a prospective randomized fashion to address these issues. Changes in plasma free insulin, serum potassium levels, and outcome recovery measures were assessed using a higher infusion dose (0.14 unit · kg−1 · h−1 without a bolus dose) compared with a lower infusion dose (0.07 unit · kg−1 · h−1) with and without a loading dose (0.07 unit/kg) of insulin.
RESEARCH DESIGN AND METHODS
Our criteria for the diagnosis of DKA included plasma glucose >250 mg/dl, HCO3− <15 mEq/l, pH <7.3, and moderate ketones in the urine or blood by the nitroprusside method. A total of 37 patients were prospectively randomly assigned to three groups as follows: 1) the load group consisted of 12 patients who received a loading dose of insulin at 0.07 unit/kg body weight followed by an infusion of 0.07 unit · kg body weight−1 · h−1; 2) the no load group consisted of 12 patients who received only an intravenous infusion of insulin at a dose of 0.07 unit · kg body weight−1 · h−1 without a loading dose; or 3) the twice no load group consisted of 13 patients who received an infusion of insulin at a dose of 0.14 unit · kg body weight−1 · h−1 without a bolus dose. Responses to therapy were based on the time to achieve a glucose level of ≤250 mg/dl, bicarbonate of ≥15 mEq/l, and pH ≥7.3. All other modes of therapy (hydrating solution, KCl, and HCO3−) were similar in the three groups and followed the recommendations of the first ADA position statement (6). All patients were seen in the emergency department of the Regional Medical Center at Memphis. Upon arrival, history was obtained and a physical examination was performed. Urine ketones (by nitroprusside) plus glucose by fingerstick and blood gas measurements were obtained immediately. Once a suspected diagnosis of DKA was made, the initial laboratory blood samples were obtained for basal metabolic parameters and complete blood count with differential; urinalysis was performed for blood and urine ketones by both the nitroprusside and enzymatic methods. Patients then received 1 liter of 0.9% NaCl per hour until laboratory results were available. After laboratory confirmation, the intravenous fluids were adjusted, depending on the glucose and corrected serum sodium value, to ½ N saline or dextrose with 1 N or ½ N saline. With confirmation of the diagnosis of DKA and an acceptable potassium level (>3.3 mEq/l), the patients were then transferred to the Clinical Research Center (CRC) of the University of Tennessee Bowld Hospital after the research study was described to the patients or their next of kin and the consent form, which was approved by the University of Tennessee Health Science Center of Memphis Institutional Review Board, was signed. On arrival at the CRC, the treatment and care of patients was managed by the DKA team, including the medical housestaff on rotation for the service, following an established protocol with consultation of the staff endocrinologist. Hourly blood glucose measurements and blood samples for free insulin were obtained at 0, 5, 15, 30, 45, 60, 120, 240, and 480 min. Blood for measurement of other hormones and ketone bodies, blood gases, and metabolic profile; urinalysis for ketones; and other parameters were obtained. Random assignment of patients to different insulin regimens was done after arrival at the CRC. Random assignment was not based on sex or ethnicity. Because a priming dose of insulin adds an additional risk of hypokalemia, potassium levels were assessed and corrected on an hourly basis for the first few hours of therapy until glucose reached the predetermined goal of <250 mg/dl (i.e., ∼4 h) and every 2 h thereafter.
Laboratory procedures
Laboratory studies included complete blood cell count, blood gas measurements, and routine blood chemistry values measured by standard assays in the clinical chemistry laboratory. In addition, plasma free insulin, C-peptide, β-hydroxybutyrate, acetoacetate, pyruvate, lactate, cortisol, and FFAs were assayed. Glucose was measured by a glucose oxidase method using a Beckman glucose analyzer. Plasma free insulin and C-peptide were measured after polyethylene glycol precipitation by a double-antibody radioimmunoassay method (14). Cortisol, FFAs, and ketone bodies were determined by previously described methods (2,15).
Data analysis
Overall mean comparisons for means and slopes for the three groups (load, no load, and twice no load) were made with one-way ANOVA using the Clinfo and SAS (SAS Institute, Cary, NC) software packages. These were followed by pairwise comparisons of averages and regression estimates of the slopes by observing the overlap/nonoverlap of the 95% confidence limits for all of the estimates (means and slopes). If any 95% confidence limits for an estimate overlapped, then no statistical significance was declared at P = 0.05. If there was no overlap due to variability, then statistical significance was declared at P < 0.05. This significance and nonsignificance is denoted by superscript letters (as in Table 4). Thus, groups having superscripts in common are not significant at P = 0.05, whereas those having no superscripts in common are significant at P < 0.05.
Table 4.
Slope estimates for clinical and hormonal responses to therapy over the first 8 h
| Parameter | Load group |
No load group |
Twice no load group |
|||
|---|---|---|---|---|---|---|
| Initial value* | Estimate of slope† | Initial value* | Estimate of slope† | Initial value* | Estimate of slope† | |
| Glucose (mg/dl) | 578.1 ± 63.7 | −86.49a | 459.2 ± 38.2 | −52.22b | 521.9 ± 47.0 | −77.67a |
| Venous pH | 7.1 ± 0.03 | 0.029a,b | 7.13 ± 0.03 | 0.025a | 7.04 ± 0.02 | 0.042b |
| Ketones (mmol/l) | 12.98 ± 1.14 | −1.92a | 10.33 ± 1.3 | −0.93b | 13.17 ± 1.47 | −1.23c |
| FFAs (mmol/l) | 1.92 ± 0.18 | 0.34a | 1.83 ± 0.23 | −0.21b | 2.14 ± 0.10 | −0.26a,b |
| Serum bicarbonate (mEq/l) | 6.7 ± 0.7 | 0.863a | 5.9 ± 0.8 | 0.551a | 4.9 ± 0.5 | 0.779a |
| Cortisol (μg/dl) | 71.8 ± 11.7 | −7.09a | 54.3 ± 10.5 | −4.40a | 82.7 ± 12.2 | −7.73a |
Data are means ± SEM.
No significant differences noted in initial values.
In comparisons involving load, no load, and twice no load treatment groups, those slope estimates with the same superscript are not significantly different at P = 0.05.
RESULTS
Demographic and initial clinical data
Table 1 shows the baseline characteristics of patients in the three treatment groups, with the following findings: 1) none of the patients were obese, 2) the majority of the patients were African Americans and were receiving insulin therapy, 3) omission of insulin (47%) and infection (33%) were the major precipitating factors for DKA, and 4) within the load group there were twice as many men as women, within the no load group there were three times as many women as men, and within the twice no load group men and women were equally distributed. However, in all of these groups, the majority of subjects were African American.
Table 1.
Demographics and clinical data of 37 patients with DKA with three intravenous insulin protocols
| Load | No load | Twice no load | |
|---|---|---|---|
| n | 12 | 12 | 13 |
| Age (years) | 28.6 ± 2.3 (19–44) | 37.3 ± 4.2 (20–66) | 31.8 ± 2.8 (17–50) |
| Sex | |||
| Female | 4 | 9 | 6 |
| Male | 8 | 3 | 7 |
| Race | |||
| Caucasian | 4 | 0 | 4 |
| African American | 8 | 12 | 9 |
| BMI (kg/m2) | 22.1 ± 1.6 | 24.6 ± 2.6 | 22.1 ± 1.3 |
| Duration of diabetes (years) | 8.5 ± 1.7 | 5.6 ± 2.0 | 8.0 ± 2.5 |
| Treatment | |||
| None | 2 | 5 | 2 |
| Diet | 2 | ||
| Insulin | 10 | 7 | 9 |
| Precipitating factor | |||
| Lack of insulin | 6 | 6 | 5 |
| Infection | 3 | 4 | 5 |
| Other | 3 | 2 | 3 |
| Blood pressure | |||
| Systolic (mmHg) | 125 ± 8 | 127 ± 8 | 128 ± 5 |
| Diastolic (mmHg) | 66 ± 3 | 81 ± 4 | 82 ± 4 |
| Pulse (beats/min) | 109 ± 3 | 108 ± 5 | 113 ± 6 |
| Respiratory rate (per min) | 27 ± 1.0 | 24 ± 1.0 | 29 ± 1.0 |
| Mental status | |||
| Alert | 9 | 5 | 5 |
| Drowsy | 3 | 5 | 7 |
| Stupor | 1 | 1 | |
| Comatose | 1 |
Data are means ± SEM, means ± SEM (range), or n.
Biochemical data on admission and after recovery
Table 2 shows biochemical data on admission and at complete recovery from DKA in the three groups. Although there were no statistically significant differences among any of the parameters in the groups on admission, cortisol values are lower in the no load group than in the other two groups. It is of interest that all the subjects in this group are African Americans, the majority are women, and the free insulin level in this group is also about twice that of the other two groups. However, this counter-regulatory hormone, similar to that in previous reports (5,16,17), is still twofold higher than the baseline value. All measured parameters reached their near-normal values at recovery, with total ketone bodies reaching ∼3.5 mmol/l. Of interest was the FFA level, which at recovery was about twice the normal value but consistent with elevated levels of FFA in diabetic patients without DKA (16). The mean anion gap at recovery was <12.
Table 2.
Biochemical and hormonal data at baseline and recovery of patients with DKA receiving three insulin protocols
| Load |
No load |
Twice no load |
||||
|---|---|---|---|---|---|---|
| Baseline | Recovery | Baseline | Recovery | Baseline | Recovery | |
| Sodium (mEq/l) | 131 ± 2 | 137 ± 1.1 | 137 ± 3 | 136 ± 3.2 | 131 ± 1 | 136 ± 1.8 |
| Potassium (mEq/l) | 5.4 ± 0.3 | 4.0 ± 0.14 | 4.8 ± 0.2 | 3.8 ± 0.14 | 5.5 ± 0.3 | 3.8 ± 0.09 |
| Bicarbonate (mEq/l) | 6.7 ± 0.7 | 17.8 ± 1.0 | 5.9 ± 0.8 | 16.2 ± 0.43 | 4.9 ± 0.5 | 16.0 ± 0.35 |
| Glucose (mg/dl) | 578 ± 64 | 162 ± 12 | 459 ± 38 | 133 ± 15 | 522 ± 47 | 110 ± 9 |
| Blood urea nitrogen (mg/dl) | 29 ± 6 | 16.3 ± 3.8 | 21 ± 4 | 16.3 ± 4.2 | 22 ± 2 | 17.2 ± 2.9 |
| Anion gap (mEq/l) | 27 ± 1 | 11.3 ± 1.8 | 24 ± 2 | 7.8 ± 1.7 | 27 ± 2 | 10.3 ± 1.7 |
| pH | 7.12 ± 0.03 | 7.3 ± 0.01 | 7.15 ± 00.03 | 7.31 ± 0.01 | 7.07 ± 0.02 | 7.32 ± 0.01 |
| β-Hydroxybutyrate (mmol/l) | 10.5 ± 1.1 | 2.4 ± 0.5 | 8.8 ± 0.9 | 2.7 ± 0.6 | 10.5 ± 1.2 | 2.5 ± 0.4 |
| Acetoacetate (mmol/l) | 2.5 ± 0.3 | 1.06 ± 0.19 | 2.6 ± 0.3 | 1.20 ± 0.34 | 2.4 ± 0.4 | 0.90 ± 0.20 |
| Total ketones (mmol/l) | 13.0 ± 1.1 | 3.5 ± 0.7 | 11.4 ± 1.0 | 3.9 ± 0.9 | 13.2 ± 1.5 | 3.5 ± 0.5 |
| Pyruvate (mg/dl) | 0.6 ± 0.1 | 0.34 ± 0.06 | 0.7 ± 0.3 | 0.35 ± 0.08 | 0.9 ± 0.2 | 0.65 ± 0.09* |
| Lactate (mg/dl) | 18.3 ± 3.8 | 7.9 ± 1.3 | 13.7 ± 1.6 | 8.3 ± 0.8 | 19.5 ± 2.3 | 10.7 ± 2.1 |
| Free fatty acid (mmol/l) | 1.92 ± 0.18 | 0.74 ± 0.09 | 1.83 ± 0.23 | 1.00 ± 0.17 | 2.14 ± 0.10 | 1.92 ± 0.10 |
| Free insulin (μU/ml) | 4.17 ± 1.82 | ND | 6.66 ± 1.76 | ND | 3.69 ± 1.69 | ND |
| C-peptide (pmol/ml) | 0.08 ± 0.07 | ND | 0.14 ± 0.1 | ND | 0.11 ± 0.05 | ND |
| Cortisol (μg/dl) | 72 ± 12 | 27.1 ± 4.7 | 54 ± 11 | 26.9 ± 9.6 | 83 ± 12 | 33.2 ± 5.8 |
Data are means ± SEM.
P < 0.05, compared with the other groups. ND, not done.
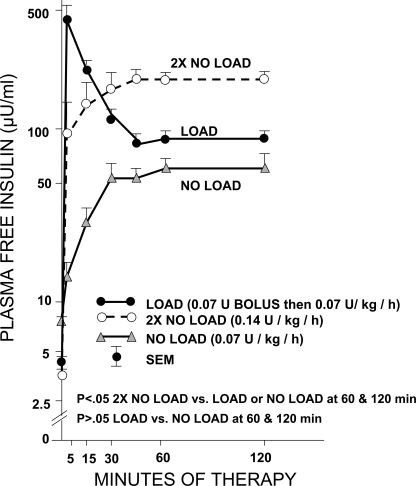
Figure 1 demonstrates the kinetics of intravenous insulin after infusion in the three arms. This figure shows that infusion of insulin of 0.07 (no load) or 0.14 unit · kg−1 · h−1 (twice no load) without an initial bolus resulted in peaks of 66 and 202 μU/ml, respectively, whereas the use of an intravenous bolus of 0.07 unit/kg followed by a 0.07 unit · kg−1 · h−1 infusion resulted in peak insulin of ∼460 μU/ml at 5 min, which reached a plateau level of ∼86 μU/ml in 30 min. It is also of interest that plateau levels of insulin for both the load and no load groups are not significantly different from 60 to 120 min. However, the twice no load group (0.14 unit · kg−1 · h−1, which is ∼10 units/h of insulin in a 70-kg patient) maintained higher levels of insulin during 30–120 min of infusion than those of the load or no load group.
Figure 1.
Kinetics of three doses of low-dose insulin in patients with DKA.
Effect of various doses of insulin on recovery parameters
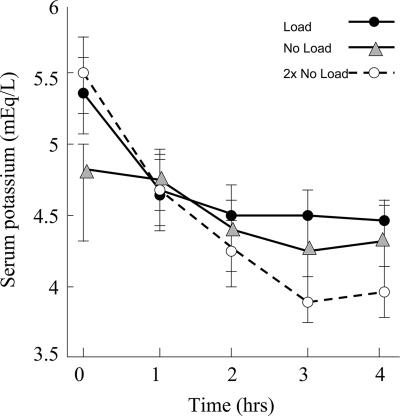
Table 3 demonstrates the outcome recovery data for DKA, depicting the time that predetermined values were reached for glucose, HCO3−, and pH. The results suggested no significant differences in these values for the three doses of insulin, but 5 of 12 patients in the no load group required additional insulin for control of their blood glucose. The length of hospitalization was not significantly different among the groups, there were no complications in any of the groups, and no deaths occurred in this cohort. No hypokalemia was noted in any treatment group. The potassium nadirs were 4.3, 4.2, and 3.7 mEq/l in the load, no load, and twice no load groups, respectively (Fig. 2).
Table 3.
Outcome data for three doses of intravenous insulin in DKA
| Load | No load | Twice no load | |
|---|---|---|---|
| n | 12 | 12 | 13 |
| Hours to glucose control (≤250 mg/dl) | 4.33 ± 0.66 | 4.67 ± 0.83 | 3.69 ± 0.46 |
| Hours to bicarbonate control (≥15 mEq/l) | 10.3 ± 1.9 | 13.8 ± 2.8 | 9.62 ± 1.1 |
| Hours to pH control (≥7.3) | 8.33 ± 1.88 | 8.08 ± 1.74 | 8.46 ± 1.11 |
| Hours to recovery | 11.1 ± 1.7 | 13.8 ± 2.8 | 10.2 ± 1.1 |
| Insulin dose to recovery (units) | 48.2 ± 9.5 | 81.3 ± 18.5 | 80.0 ± 9.5 |
| Intravenous fluid given (ml) | |||
| First 8 h | 3,740 ± 410 | 4,210 ± 440 | 3,570 ± 250 |
| First 24 h | 7,170 ± 600 | 7,950 ± 490 | 7,510 ± 300 |
| Complications | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 |
| Days in hospital | 5.9 ± 0.7 | 4.7 ± 0.6 | 6.9 ± 0.7 |
Data are means ± SEM.
Figure 2.
Changes in hourly serum potassium with three low-dose insulin regimens in the treatment of DKA.
Table 4 shows estimates of the slopes for glucose, venous pH, ketones, FFAs, bicarbonate, and cortisol during the first 8 h of therapy in the three insulin therapy groups. The response of serum bicarbonate and cortisol showed no differences among the treatment groups. However, the slopes for glucose, venous pH, ketones, and FFAs varied among treatment groups.
CONCLUSIONS
Although numerous studies have effectively used low-dose insulin in the treatment of DKA (3,9,18), there are no data from a prospective randomized study evaluating an insulin bolus (0.07 U/kg) compared with no bolus in conjunction with an infusion of low-dose insulin (0.07 vs. 0.14 unit · kg−1 · h−1). Previous studies have demonstrated that although 0.1 unit/h of insulin in patients with DKA can effectively inhibit lipolysis, this dose will only suppress hepatic gluconeogenesis by 50% (11).
We had previously shown that an intermediate dose of insulin of 0.1 unit · kg body weight−1 · h−1 (equivalent of 7 units/h of insulin in a 70-kg patient) by intravenous infusion was much more effective than a dose by the subcutaneous or intramuscular route (4). In these studies we used a priming dose. In addition, in a selected case report we have used as little as 6 units/h for a patient with modest DKA with further maintenance of euglycemia by 1–2 units/h (14). However, a bolus dose of insulin preceded the low-dose infusion (19). Furthermore, a priming dose has not been used for pediatric patients with DKA (10) and has not been recommended in the pediatric age-group in the latest ADA consensus report (12). Deleterious outcomes with the use of bolus insulin in this group may include hypokalemia as well as possible cerebral edema (12).
In summary, our study suggests that the use of a bolus or priming dose of insulin is not necessary when an adequate continuous insulin infusion such as 0.14 unit · kg−1 · h−1 (or 10 units/h in a 70-kg patient) is used. However, a dose of 0.07 unit · kg−1 · h−1 without priming is not adequate to obtain desired changes in glucose without supplemental doses of insulin.
Acknowledgments
This study was supported in part by U.S. Public Health Service Grant RR00211 (to the General Clinical Research Center) and by Grant DK07405 and Training Grant AM070884 from the National Institutes of Health.
The assistance of Drs. Dale Childress and James Peyton in assessment of some of the data during their training is greatly appreciated. The authors appreciate the secretarial assistance of Brenda Scott on this manuscript.
Published ahead of print at http://care.diabetesjournals.org on 11 August 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Alberti KGMM, Hockaday TDR, Turner RC: Small doses of intramuscular insulin in the treatment of diabetic “coma.” Lancet 5:515–522, 1973 [DOI] [PubMed] [Google Scholar]
- 2.Kitabchi AE, Ayyagari V, Guerra SM: The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med 84:633–638, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Wagner A, Risse A, Brill HL, Wienhausen-Wilke V, Rottmann M, Sondern K, Angelkort B: Therapy of severe diabetic ketoacidosis: zero-mortality under very-low-dose insulin application. Diabetes Care 22:674–677, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Fisher JN, Shahshahani MN, Kitabchi AE: Diabetic ketoacidosis: low-dose insulin therapy by various routes. N Engl J Med 297:238–247, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM: Management of hyperglycemic crises in patients with diabetes. Diabetes Care 24:131–153, 2001 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Position Paper: Hyperglycemic crisis in patients with diabetes mellitus. Diabetes Care 19:S82–S90, 2001 [Google Scholar]
- 7.Morris LR, Murphy MB, Kitabchi AE: Bicarbonate therapy in severe diabetic ketoacidosis. Ann Intern Med 105:836–840, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Sacks HS, Shahshahani MN, Kitabchi AE, Fisher JN, Young RT: Similar responsiveness of diabetic ketoacidosis to low-dose insulin by intramuscular injection and albumin-free infusion. Ann Intern Med 90:36–42, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA: Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29:2739–2748, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Burghen GA, Etteldorf JN, Fisher JN, Kitabchi AE: Comparison of high-dose to low-dose insulin by continuous intravenous infusion in the treatment of diabetic ketoacidosis in children. Diabetes Care 3:15–20, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Luzi L, Barrett EJ, Groop LC, Ferrannini E, De Fronzo RA: Metabolic effects of low-dose insulin therapy on glucose metabolism in diabetic ketoacidosis. Diabetes 37:1470–1477, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Wolfsdorf J, Glaser N, Sperling M: Diabetic ketoacidosis in Infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 29:1150–1159, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kitabchi AE, Umpierrez G, Fisher JN, Murphy MB, Stentz F: Thirty years of personal experience in 12 hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab 92:1541–1552, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzuya H, Blix PM, Horwitz DL, Steiner DF, and Rubenstein AH: Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes 26:22–29, 1977 [DOI] [PubMed] [Google Scholar]
- 15.Lawson V, Young R, Kitabchi AE: Maturity-onset diabetes of the young: an illustrative case for control of diabetes and hormonal normalization with dietary management. Diabetes Care 4:108–112, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Stentz FB, Umpierrez GE, Cuervo R Kitabchi AE: Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 53:2079–2086, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Schade DS, Eaton RP: The temporal relationship between endogenously secreted stress hormones and metabolic decompensation in diabetic man. J Clin Endocrinol Metab 50:131–136, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Fisher JN, Murphy MB, Rumbak MJ: Diabetic ketoacidosis and the hyperglycemic hyperosmolar nonketotic state. In Joslin's Diabetes Mellitus. 13th ed. Kahn CR, Weir GC, Eds. Baltimore, Williams & Wilkins 1994, p. 738–770
- 19.Kitabchi AE: Low-dose insulin therapy in diabetic ketoacidosis: fact or fiction. In Diabetes Metabolism Reviews. DeFronzo R, Ed. New York, John Wiley & Sons, 1989, p. 337–363 [DOI] [PubMed]