Abstract
OBJECTIVE—To determine the impact of islet transplantation (ITx) on hypoglycemia awareness in patients with unstable type 1 diabetes and its relation to islet function.
RESEARCH DESIGN AND METHODS—A total of 31 ITx recipients were studied. Hypoglycemia unawareness was assessed using the Clarke hypoglycemic score (0 = no hypoglycemia; ≥4 = hypoglycemia unawareness). Subjects were grouped based on graft function: off-insulin (n = 8), graft dysfunction (on-insulin and stimulated C-peptide ≥0.3 ng/ml, n = 13), and graft failure (stimulated C-peptide <0.3 ng/ml, n = 10, evaluated 11.5 ± 14.5 months after graft failure).
RESULTS—The hypoglycemia score improved after ITx when compared with baseline values (before vs. after: 5.29 ± 1.51 vs. 1.35 ± 1.92, P < 0.001). This result was sustained even after patient stratification based on islet function (pre vs. post off-insulin: 5.63 ± 2.00 vs. no hypoglycemia reported; graft dysfunction: 5.31 ± 1.49 vs. 1.15 ± 1.63, P < 0.001; and graft failure: 5.00 ± 1.16 vs. 2.70 ± 2.26, P = 0.014).
CONCLUSIONS—The improved metabolic control achieved with ITx can restore hypoglycemia awareness in patients with type 1 diabetes, persisting even after islet graft failure.
Occurrence of hypoglycemia is the major limitation of intensive control aimed at A1C normalization in patients with type 1 diabetes. Frequent hypoglycemic episodes are particularly common in subjects with unstable type 1 diabetes and can lead to hypoglycemia unawareness. Although islet transplantation (ITx) prevents severe hypoglycemia (1) and restores some counterregulatory hormone secretion (2), data showing its effects on restitution of hypoglycemia awareness are not conclusive (2,3).
The aim of this study was to determine if the optimal metabolic control achieved by ITx can restore hypoglycemia awareness and whether or not these effects persist after islet graft failure.
RESEARCH DESIGN AND METHODS
A retrospective cohort study was conducted on 31 type 1 diabetic recipients of ITx (ITx alone, n = 25; islet after kidney, n = 6) between 2001 and 2007. Procedures were performed as described (1) under protocols approved by the University of Miami Health Research Ethics Board, and informed consent was obtained from each subject.
Hypoglycemia unawareness was assessed using the Clarke hypoglycemic score (minimum = 0; maximum = 7; no hypoglycemia = 0; hypoglycemia unawareness ≥4) (4) twice, including pre-ITx and at the most recent follow-up (47.2 ± 21.3 months after first ITx). Subjects were grouped based on graft function: off-insulin (n = 8), graft dysfunction (restarted insulin and stimulated C-peptide ≥0.3 ng/ml, n = 13), and graft failure (stimulated C-peptide <0.3 ng/ml; n = 10, evaluated 11.5 ± 14.5 months after graft failure).
Plasma glucose (hexokinase method) and C-peptide (double antibody radioimmunoassay) were measured during a mixed-meal test. A1C (high-performance liquid chromatography [HPLC]; Bio-Rad, Richmond, CA; normal values: 4.2–6.1%) was determined before ITx and at the most recent follow-up.
Proportions were compared with the χ2 test. The paired t test was used to compare variables pre- and post-ITx, and one-way ANOVA was used to compare the post-ITx hypoglycemic score among groups. P values (two-tailed) <0.05 and <0.016 (Bonferroni's correction for differences among subgroups) were considered significant.
RESULTS
Patient age at baseline was 43.8 ± 8.7 years, and diabetes duration was 29.3 ± 11.8 years. Subjects were white, and 13 (42%) were males. A total of 13 subjects (42%) had hypertension, and 11 (35%) had dyslipidemia. Diabetes complications at baseline included retinopathy (71%; n = 22; 14 proliferative), neuropathy (45%; n = 14; 4 autonomic, 7 peripheral, and 3 both), and nephropathy (29%; n = 9; 3 microalbuminuria and 6 with a kidney transplant 9.1 ± 6.3 years old).
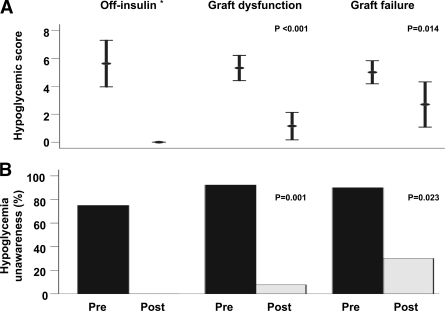
Mean hypoglycemic score pre-ITx was 5.29 ± 1.51 and was inversely correlated with pre-ITx glycemic control, as measured by A1C (r = −0.370, P = 0.040). A decrease in hypoglycemic score was observed post-ITx (1.35 ± 1.92, P < 0.001). Similarly, there was a reduction in the proportion of patients with hypoglycemia unawareness (pre- vs. post-ITx: 87 vs. 13%, P < 0.001) and an increase in glycemic threshold that resulted in symptoms (pre- vs. post-ITx: 41.4 ± 17.6 vs. 58.4 ± 10.3 mg/dl, P = 0.001). Results were sustained even after the patient's stratification based on islet function (pre- vs. post-ITx off insulin: 5.63 ± 2.00 vs. no hypoglycemia reported; graft dysfunction: 5.31 ± 1.49 vs. 1.15 ± 1.63, P < 0.001; and graft failure: 5.00 ± 1.16 vs. 2.70 ± 2.26, P = 0.014) (Fig. 1); however, an increase in post-ITx hypoglycemic score was observed as patients lost graft function (P = 0.007).
Figure 1.
Hypoglycemic score (Clarke score; 4) (A) and proportion of subjects with hypoglycemia unawareness (hypoglycemic score ≥4) (B) pre- and post-ITx, according to islet function. P = 0.007 for comparison of posttransplant hypoglycemic score between off-insulin and graft failure groups. *P value is not applicable, since no hypoglycemia was reported posttransplant.
A1C improved in off-insulin (6.8 ± 0.8 vs. 6.0 ± 0.6, P = 0.038) and graft dysfunction (7.5 ± 1.2 vs. 6.7 ± 0.5, P = 0.022) subjects, and no further deterioration was observed after graft failure (7.0 ± 0.9 vs. 7.4 ± 0.9, P = 0.318), suggesting that recovery of awareness was a consequence of glycemic stability rather than metabolic deterioration associated with a less strict therapy.
Additionally, a lower hypoglycemic score correlated with better β-cell function (r = −0.440, P = 0.013, and r = −0.496, P < 0.001, post-ITx fasting and stimulated C-peptide, respectively). Follow-up was similar for all groups (49.3 ± 20.3 vs. 42.7 ± 22.2 vs. 51.5 ± 21.9 months, P = 0.602), and no clinical or laboratory factors were associated with hypoglycemia unawareness post-ITx, including diabetes duration and presence of neuropathy (data not shown).
CONCLUSIONS
In this sample of unstable type 1 diabetic patients, glucose stabilization after ITx was associated with restoration of hypoglycemia awareness. Interestingly, improvements in hypoglycemia awareness were sustained even after graft failure.
Restoration of hypoglycemia awareness can be achieved with medical treatment or pancreatic transplantation. Medical treatment is driven by hypoglycemia avoidance but can lead to metabolic deterioration if it is necessary to lower the insulin dose (5). Pancreatic transplantation reestablishes normal glucose homeostasis (6), but the surgical risks are a limitation.
No hypoglycemia has been reported after ITx in patients achieving insulin independence (1). After developing graft dysfunction and reintroducing exogenous insulin, hypoglycemic episodes may resume, although with a much lower frequency than pre-ITx. Based on these observations, it is conceivable that ITx recipients regain adrenergic symptoms. Indeed, an improvement in hypoglycemia awareness after ITx was suggested by a small-sample short-term clamp study (2), although the literature is not consistent (3).
To the best of our knowledge, this is the first report of restoration of hypoglycemia awareness after ITx, which includes more than a few patients and has a longer follow-up, and we have demonstrated statistically significant benefits even after graft failure No hypoglycemia was reported by off-insulin subjects; the recovery of hypoglycemia awareness found in patients with graft dysfunction was predictable and was probably related to tight glycemic control due to ITx and prevention of hypoglycemia. In graft failure patients, avoidance of hypoglycemia during the early post-ITx period possibly led to increased awareness, which was maintained after loss of graft function. Hypoglycemia unawareness relapse was observed in this group, even though the score remained in the normal range.
The retrospective design of this study did not allow for more frequent evaluations and better description of patient symptoms that would have enhanced the study. Despite this limitation we were able to identify the restoration of hypoglycemia awareness.
In conclusion, the stable metabolic control achieved by ITx restores hypoglycemia awareness in type 1 diabetic subjects. These benefits were accomplished without metabolic deterioration, independent of diabetes duration or presence of autonomic neuropathy, and persisted beyond the duration of islet graft function.
Acknowledgments
This study was supported by the National Institutes of Health (NIH)/National Center for Research Resources (NCRR) (U42 RR016603, M01RR16587), Juvenile Diabetes Research Foundation International (JDRFI) (4-2000-946, 4-2004-361), NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5 R01 DK55347, 5 R01 DK056953), the State of Florida, and the Diabetes Research Institute Foundation (Hollywood, FL). CB.L. was the recipient of a scholarship from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq).
We thank Dr. Norma Kenyon for her suggestions in editing this manuscript.
Published ahead of print at http://care.diabetesjournals.org on 12 August 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R: Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 5:2037–2046, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Rickels MR, Schutta MH, Mueller R, Kapoor S, Markmann JF, Naji A, Teff KL: Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J Clin Endocrinol Metab 92:873–879, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP: Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes 51:3428–3434, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W: Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 18:517–522, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Liu D, McManus RM, Ryan EA: Improved counter-regulatory hormonal and symptomatic responses to hypoglycemia in patients with insulin-dependent diabetes mellitus after 3 months of less strict glycemic control. Clin Invest Med 19:71–82, 1996 [PubMed] [Google Scholar]
- 6.Kendall DM, Rooney DP, Smets YF, Salazar Bolding L, Robertson RP: Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long-standing type I diabetes and autonomic neuropathy. Diabetes 46:249–257, 1997 [DOI] [PubMed] [Google Scholar]