Abstract
OBJECTIVE—Accumulating evidence suggests that energy-dense foods predispose to obesity and that such foods may also be associated with an increased risk of type 2 diabetes, but there is limited evidence. Our aim was to investigate whether there is an independent association between dietary energy density and incidence of diabetes.
RESEARCH DESIGN AND METHODS—The European Prospective Investigation of Cancer (EPIC)-Norfolk Cohort Study was a population-based prospective study of individuals aged 40–79 years at baseline. We calculated energy density for overall diet (all solids and drinks) using food frequency questionnaires. During 12 years of follow-up, we documented 725 new-onset cases of diabetes among 21,919 participants without diabetes, cancer, or cardiovascular disease at baseline.
RESULTS—Baseline energy density (adjusted for age, sex, and baseline BMI) was higher in those who developed type 2 diabetes (mean 3.08 kJ/g [95% CI 3.03–3.13]) than in those who remained nondiabetic (3.01 kJ/g [3.00–3.02]) (P = 0.012). Energy density was positively associated with incident diabetes (odds ratio 1.21 per unit increase [95% CI 1.06–1.38]) adjusted for known risk factors. There was a 60% higher risk of diabetes (1.60 [1.19–2.16]) in the highest quintile of energy density (range 3.55–7.97 kJ/g) compared with the lowest quintile (1.04–2.43 kJ/g) in adjusted analysis.
CONCLUSIONS—This is the first large population-based prospective study to report that an energy-dense diet may be associated with increased risk of development of diabetes, independent of baseline obesity. The potential public health impact of a low–energy-dense diet on reducing the risk of diabetes deserves further study.
Dietary energy density (DED) is defined as the amount of energy able to be metabolized per unit weight or volume of food (1). Thus, assuming energy expenditure is held constant, high energy density of a given volume of food consumed would lead to increased energy intake and weight gain, as demonstrated by both short-term and long-term intervention trials (reviewed by Yao and Roberts [1]). Energy-dense foods were also reported to be associated with body fatness in children: for instance, at age 7 years a 1 unit (1 kJ/g) rise in DED increased the odds of excess adiposity at 9 years by 36% (odds ratio [OR] 1.36 [95% CI 1.09–1.69]) (2). Mendoza et al. (3) also found that DED was independently associated with elevated fasting insulin and the metabolic syndrome in a cross-sectional setting. It is plausible that such foods may also be associated with an increased risk of type 2 diabetes, but there is limited evidence. In the Finnish Diabetes Prevention Study (DPS), overweight men and women with impaired glucose tolerance receiving standard care or intensive dietary and exercise counseling showed a positive, but not significant, association between energy density and the risk of developing diabetes after 3 years of follow-up (hazard ratio 1.74 [95% CI 0.89–3.37]) (4). To the best of our knowledge, this is the only published study regarding DED and the risk of diabetes. Therefore, the purpose of our study was to investigate the association of DED with new-onset diabetes in a population-based cohort study including both men and women, appropriately adjusted for a comprehensive range of lifestyle factors, social factors, and dietary factors.
RESEARCH DESIGN AND METHODS
The European Prospective Investigation of Cancer (EPIC)-Norfolk Cohort Study recruited a total of 25,639 volunteers, aged 40–79 years, from general practices in Norwich and surrounding towns in Norfolk between 1993 and 1997 and has been described in detail elsewhere (5). Briefly, it was a population-based cohort study for which participants completed a baseline health check, and follow-up constituted a postal questionnaire at 18 months, a second health check in 1998–2000, and a further postal questionnaire in 2002–2004. For the current analysis, we excluded participants with diagnosed prevalent diabetes, cancer, or cardiovascular disease at baseline because they may have altered their diet as a result of their condition. In addition, participants with a missing food frequency questionnaire (FFQ) or with >10 missing dietary items and participants in the top 0.5% and bottom 0.5% of the ratio of self-reported energy intake to basal metabolic rate (BMR) (6) were excluded from the analysis. After these exclusions, the analysis included 21,919 volunteers (9,781 men and 12,138 women) from the entire cohort. The study was approved by the local research ethics committee, and participants gave written informed consent.
Ascertainment of cases of type 2 diabetes
Multiple sources of case ascertainment for new-onset diabetes until the end of 2005 were as follows: self-report of doctor-diagnosed diabetes from the second health check or follow-up health and lifestyle questionnaires, self-report of diabetes-specific medication in either of the two follow-up questionnaires, or medication brought to the follow-up health check. In addition, external sources of information through record linkage included listing of any EPIC-Norfolk participant in the general practice diabetes register, local hospital diabetes register, hospital admissions data at that hospital screened for any diabetes-related admissions among study participants, and Office of National Statistics mortality data with coding for diabetes. Participants who gave a self-report of history of diabetes that could not be confirmed with any other sources of ascertainment were not included as confirmed cases of diabetes.
Assessment of diet
Information on diet was collected using a 130-item validated EPIC-FFQ at baseline (6,7). Participants were asked how often they had consumed a commonly used unit or portion size of each food on average during the previous year, choosing from nine possible frequency responses ranging from “never or less than once per month” to “more than six times per day.” Nutrient intakes (per day) were computed by multiplying the frequency response by the nutrient content of the specified portion size using the Compositional Analyses from Frequency Estimates (CAFE) program (6). Values for nutrients were derived from the U.K. food composition database McCance and Widdowson's The Composition of Foods and its supplements (6). The energy and weight of each food item were summed for all solid food and beverages (not including pure water consumption) to get the total energy intake (kilojoules per day) and the total weight of foods (grams per day). DED was calculated as the available dietary energy per unit weight of foods (kilojoules per gram):
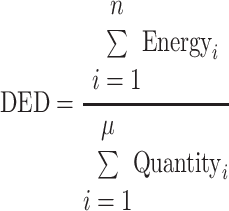 |
Assessment of nondietary factors
A detailed health and lifestyle questionnaire was completed at baseline (1993–1997). It included questions on demography, personal and family history of type 2 diabetes among first-degree relatives, smoking, physical activity, occupation, education, and medication (8). A menstrual/menopausal history was recorded in women, and those who had their last menstrual period ≥2 years previously were coded as postmenopausal. Physical activity level was assessed by a four-point physical activity index according to occupational and leisure-time physical activity (9). Smoking status was coded as never, former, or current. At the health check visit, a clinical examination was performed using a standard protocol as described previously (8). Anthropometric measurement included height (centimeters), weight (kilograms), and waist and hip circumference (centimeters). BMI was calculated as body weight in kilograms divided by the square of height in meters. A further postal questionnaire was sent in 2004 to collect self-reported body weight, and weight change was calculated as follow-up weight minus baseline weight.
Statistical analysis
Results are expressed as means ± SD and, for categorical variables, by frequency and percentage. Differences between men and women in baseline characteristics and DED were tested using t tests for continuous variables and by χ2 tests for categorical variables. The independent variable (DED) was defined both as a continuous variable and as a categorical variable with five categories (quintiles). Associations between DED and risk of developing type 2 diabetes were examined using two separate approaches. In the first approach, we constructed three logistic regression models: the first model was adjusted for age, sex, and baseline BMI; the second model was further adjusted for known risk factors for diabetes including lifestyle factors (family history of diabetes [yes or no], physical activity [inactive, moderate inactive, moderate active, or active], smoking [current, former, or never], and occupational status [professional, managerial and technical, skilled nonmanual, skilled manual, partly skilled, or unskilled] or education [no qualifications, O level, A level, or degree]); and the third model further included dietary factors (alcohol consumption [yes or no] and total energy intake [continuous]). The third model was further adjusted for the percentage of energy from dietary fat instead of total energy intake to examine whether the observed association was independent of fat intake. To explore the effect of central obesity, we also adjusted for baseline waist circumference in addition to baseline BMI or instead of baseline BMI. We also further adjusted model 3 for weight change between the baseline and follow-up postal questionnaire in 2004. The same analyses were repeated in men and women separately using sex-specific quintiles of DED. In women, the model was further adjusted for menopausal status and use of hormone replacement therapy. In the second approach, to reduce the confounding effect of total energy intake, we repeated the analysis using calorie-adjusted DED computed as the residuals from the regression model, with total energy intake as the independent variable and absolute DED as the dependent variable (10).
We also performed sensitivity analyses as follows. We attempted to identify plausible underreporters of total energy intake using two different published methods. One method used the Goldberg cutoff point of 1.35 for the ratio of reported energy intake to predicted BMR (11). BMR was estimated using published equations based on sex, age, weight, and height (12). The other method was based on the ratio of reported energy intake to estimated energy requirement (EER) (13), which takes into account each individual's BMR and physical activity level (14). In the current analysis, we adopted the range of the ratio of reported energy intake to EER between 0.8 and 1.2 as plausible energy intake reporters.
All statistical analyses were performed using STATA statistical software (version 9.2; Statacorp, College Station, TX).
RESULTS
During a median of 10.2 years of follow-up (range 7.6–12.8 years), we documented 725 new cases of type 2 diabetes among 21,919 participants. At baseline, participants who later developed diabetes during follow-up consumed a more energy-dense diet than those who did not develop diabetes (age-, sex-, and baseline BMI–adjusted mean DED 3.08 kJ/g [95% CI 3.03–3.13] vs. 3.01 kJ/g [3.00–3.02], P = 0.009). Case participants were less physically active (inactive 42.2 vs. 28.4% and active 16.0 vs. 19.1%, P < 0.001), more obese (BMI 29.7 vs. 26.2 kg/m2, waist circumference 99.4 vs. 87.5 cm, and obesity prevalence 40.4 vs. 13.8%, all P < 0.001), and more likely to have a positive family history of diabetes than those who did not develop diabetes. Table 1 shows the range of DEDs across the five DED quintiles. Waist circumference, total energy intake, and fat intake were significantly higher across categories of increasing DED. In contrast, BMI, prevalence of overweight and obesity, alcohol consumption, proportion of smokers, and occupational socioeconomic status were lower across increasing DED quintiles.
Table 1.
Baseline characteristics by quintile of DED: EPIC-Norfolk Study
| DED quintiles |
P value* | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| DED (kJ/g) | 1.04–2.43 | 2.43–2.78 | 2.78–3.12 | 3.12–3.55 | 3.55–7.97 | <0.001 |
| Age (years) | 57.2 ± 8.6 | 57.9 ± 9.1 | 58.5 ± 9.3 | 58.5 ± 9.5 | 58.6 ± 9.6 | 0.331 |
| Weight (kg) | 73.4 ± 13.4 | 73.0 ± 13.0 | 73.5 ± 13.0 | 73.4 ± 13.2 | 73.5 ± 12.9 | <0.001 |
| Height (cm) | 165.4 ± 8.8 | 166.0 ± 9.0 | 167.0 ± 9.2 | 167.7 ± 9.3 | 168.5 ± 9.1 | <0.001 |
| Waist (cm) | 87.0 ± 12.5 | 87.2 ± 12.4 | 88.1 ± 12.2 | 88.3 ± 12.2 | 88.8 ± 12.0 | <0.001 |
| BMI (kg/m2) | 26.8 ± 4.1 | 26.4 ± 3.9 | 26.3 ± 3.8 | 26.0 ± 3.8 | 25.8 ± 3.7 | <0.001 |
| Energy intake (kJ/day) | 6,493.0 ± 1,568.4 | 7,713.2 ± 1,720.7 | 8,526.5 ± 1,836.5 | 9,405.6 ± 2,099.5 | 10,904.8 ± 2,705.9 | <0.001 |
| Fat intake (g/day) | 50.0 ± 15.9 | 65.2 ± 18.2 | 75.9 ± 20.3 | 87.3 ± 23.9 | 106.6 ± 32.2 | <0.001 |
| Energy from fat (%) | 29.0 ± 5.8 | 31.8 ± 5.2 | 33.5 ± 5.2 | 34.9 ± 5.0 | 36.6 ± 5.2 | <0.001 |
| Alcohol (g/day) | 10.0 ± 15.7 | 9.9 ± 14.0 | 8.7 ± 12.6 | 7.9 ± 11.3 | 6.8 ± 9.8 | <0.001 |
| Occupational status | <0.001 | |||||
| Professional | 305 (7.1) | 330.00 (7.7) | 303 (7.0) | 292 (6.8) | 262 (6.1) | |
| Managerial and technical | 1,709 (39.7) | 1,646 (38.3) | 1,617 (37.5) | 1,540 (35.7) | 1,387 (32.3) | |
| Skilled nonmanual | 683 (15.9) | 730 (17.0) | 731 (17.0) | 683 (15.9) | 717 (16.7) | |
| Skilled manual | 943 (21.9) | 910 (21.2) | 944 (21.9) | 1,048 (24.3) | 1,115 (25.9) | |
| Partly skilled | 525 (12.2) | 547.00 (12.7) | 548 (12.7) | 578 (13.4) | 634 (14.7) | |
| Unskilled | 125 (2.9) | 128.00 (3.0) | 153 (3.6) | 153 (3.6) | 173 (4.0) | |
| Smoking | <0.001 | |||||
| Current | 610 (14.0) | 509 (11.7) | 470 (10.8) | 494 (11.4) | 484 (11.2) | |
| Former | 1,810 (41.6) | 1,785 (41.0) | 1,761 (40.5) | 1,835 (42.2) | 1,718 (39.7) | |
| Never | 1,935 (44.4) | 2,063 (47.4) | 2,115 (48.7) | 2,016 (46.4) | 2,131 (49.2) | |
| Physical activity | <0.001 | |||||
| Inactive | 1,235 (28.2) | 1,279 (29.2) | 1,272 (29.0) | 1,272 (29.0) | 1,276 (29.1) | |
| Moderately inactive | 1,313 (30.0) | 1,353 (30.9) | 1,290 (29.4) | 1,246 (28.4) | 1,142 (26.1) | |
| Moderately active | 1,026 (23.4) | 981 (22.4) | 1,032 (23.5) | 1,005 (22.9) | 1,029 (23.5) | |
| Active | 810 (18.5) | 771 (17.6) | 790 (18.0) | 861 (19.6) | 935 (21.3) | |
| Family history of diabetes (yes) | 552 (12.6) | 535 (12.2) | 566 (12.9) | 570 (13.0) | 522 (11.9) | 0.487 |
| Obesity status | <0.001 | |||||
| Normal | 1,561 (35.7) | 1,708 (39.1) | 1,711 (39.1) | 1,855 (42.4) | 1,867 (42.7) | |
| Overweight | 2,012 (46) | 1,946 (44.5) | 2,027 (46.3) | 1,922 (43.9) | 1,964 (44.9) | |
| Obesity | 787 (18.0) | 706 (16.2) | 628 (14.3) | 571 (13.1) | 510 (11.7) | |
Data are range, means ± SD, or n (%).
P values are from ANOVA for continuous variables and χ2 test for categorical variables.
There was an increased risk of diabetes associated with DED, as shown in Table 2. This was the case both for the continuous association per unit increase in energy density and for our examination of the population divided into five groups according to the quintiles of energy density. Compared with the lowest quintile of DED (range 1.04–2.43 kJ/g), there was a 60% higher risk of incident diabetes in the highest quintile of DED (range 3.55–7.97 kJ/g, OR 1.60 [95% CI 1.19–2.16]). Adjusting for percentage of energy from fat instead of total energy intake and/or baseline waist circumference instead of baseline BMI did not materially change the results (data not shown). Adjusting for weight change did not materially change the results (OR 1.23 per unit increase of DED [1.03–1.47] and 1.52 [1.03–2.24] in the highest quintile of DED compared with the lowest group, Ptrend = 0.021, in model 3 with additional adjustment for weight change). There was no significant interaction between DED and either BMI or waist circumference or between DED and sex on the risk of diabetes (all P > 0.86). The results were similar in men and women if we used sex-specific quintiles, even with further adjustment for menopausal status and hormone replacement therapy in women (data not shown).
Table 2.
Association between DED (continuous and quintiles) and risk of type 2 diabetes: EPIC-Norfolk study
| DED | P value | DED quintiles |
Ptrend | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| DED (kJ/g) | 1.04–7.97 | 1.04–2.43 | 2.43–2.78 | 2.78–3.12 | 3.12–3.55 | 3.55–7.97 | ||
| Men | 1.30–7.53 | 1.30–2.55 | 2.55–2.92 | 2.92–3.26 | 3.26–3.70 | 3.70–7.53 | ||
| Women | 1.04–7.97 | 1.04–2.35 | 2.35–2.67 | 2.67–3.00 | 3.00–3.42 | 3.42–7.97 | ||
| Incident cases | 725 | 135 | 140 | 138 | 143 | 169 | ||
| Model 1 | 1.12 (1.01–1.25) | 0.032 | 1.00 | 1.07 (0.83–1.37) | 1.05 (0.82–1.35) | 1.11 (0.87–1.43) | 1.34 (1.05–1.70) | 0.022 |
| Model 2 | 1.13 (1.01–1.26) | 0.028 | 1.00 | 1.04 (0.80–1.34) | 1.06 (0.82–1.36) | 1.10 (0.86–1.42) | 1.35 (1.06–1.73) | 0.016 |
| Model 3 | 1.20 (1.05–1.37) | 0.007 | 1.00 | 1.10 (0.85–1.42) | 1.15 (0.88–1.49) | 1.23 (0.93–1.61) | 1.58 (1.18–2.12) | 0.003 |
Data are range, n, or ORs (95% CI). Adjustments of covariates were performed using multiple regression analyses by cumulatively adding the following covariates into the model: model 1, age, sex, and baseline BMI; model 2, model 1 plus occupational status, smoking, physical activity, and family history of type 2 diabetes; and model 3, model 2 plus alcohol consumption and total energy intake.
In analyses using calorie-adjusted DED (see research design and methods), the association with the risk of clinically incident type 2 diabetes was unchanged. The DED was positively associated with an increased risk of diabetes in adjusted analyses (OR 1.21 [95% CI 1.06–1.38], model 3).
To address the issue of possible underreporting of self-reported dietary intake, we performed a sensitivity analysis in plausible energy reporters using two different methods of identifying underreporters. In the first method, which uses the Goldberg cutoff point (ratio of reported energy intake to BMR of 1.35), we identified 11,242 potential underreporters. In the second method, which takes into account the physical activity level of each individual, we identified 5,819 individuals outside of the range of the ratio of reported energy intake to EER of 0.8–1.2. Exclusion of underreporters using both methods did not materially change the results. (OR 1.23 [95% CI 1.004–1.519] for the ratio of reported energy intake to BMR ≥ 1.35 and 1.20 [1.02–1.42] for the ratio of reported energy intake to EER of 0.8–1.2).
To illustrate the intake pattern of an energy-diluted diet, we summarized the average intake of food groups by the quintile of DED (Table 3). Compared with the highest DED (energy-dense) quintile, participants in the lowest DED (energy-diluted) group consumed significantly more fresh fruit, more vegetables, less meat, less processed meat, less soft drinks, more alcoholic drinks, more non–energy-containing beverages, and a lower percentage of energy from fat.
Table 3.
Intake pattern of food groups by quintiles of DED at baseline: EPIC-Norfolk study
| DED quintiles |
P for trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Fresh fruit (g/day) | 279.6 ± 226.2 | 256.3 ± 195.4 | 232.2 ± 171.2 | 209.2 ± 154.0 | 186.5 ± 140.9 | <0.001 |
| Dried fruit (g/day) | 2.9 ± 8.1 | 3.2 ± 7.6 | 3.1 ± 7.4 | 3.3 ± 7.9 | 3.3 ± 9.6 | 0.171 |
| Vegetables (g/day) | 282.5 ± 151.7 | 283.2 ± 138.2 | 274.7 ± 125.7 | 262.6 ± 121.0 | 251.0 ± 121.1 | <0.001 |
| Meat (g/day) | 59.3 ± 34.7 | 66.0 ± 36.9 | 68.9 ± 38.4 | 71.4 ± 42.2 | 74.8 ± 48.1 | <0.001 |
| Processed meat (g/day) | 18.6 ± 15.8 | 22.2 ± 16.9 | 25.0 ± 19.7 | 27.7 ± 21.7 | 31.4 ± 26.6 | <0.001 |
| Vitamin C (FFQ) (mg/day) | 132.9 ± 69.3 | 131.0 ± 63.1 | 124.9 ± 56.4 | 119.6 ± 53.2 | 112.9 ± 50.7 | <0.001 |
| Non–energy-containing beverages (g/day) | 1,343.5 ± 394.6 | 1,172.9 ± 336.3 | 1,081.4 ± 328.6 | 1,003.7 ± 333.5 | 833.5 ± 380.7 | <0.001 |
| Energy-containing beverages (g/day) | 620.6 ± 371.2 | 620.5 ± 330.4 | 611.5 ± 300.1 | 593.5 ± 268.8 | 557.2 ± 245.6 | <0.001 |
| Soft drinks (g/day)* | 25.5 ± 79.6 | 31.9 ± 79.4 | 35.6 ± 79.3 | 38.6 ± 79.5 | 48.2 ± 82.0 | <0.001 |
| Alcoholic drinks (g/day) | 162.0 ± 321.8 | 148.8 ± 267.5 | 128.5 ± 227.0 | 112.7 ± 186.4 | 94.3 ± 148.9 | <0.001 |
| Total energy intake (kcal) | 1,539.0 ± 372.7 | 1,830.2 ± 408.9 | 2,024.2 ± 436.3 | 2,234.1 ± 499.0 | 2,592.1 ± 643.5 | <0.001 |
| Energy from carbohydrate (%) | 50.9 ± 7.5 | 50.2 ± 6.5 | 50.0 ± 6.4 | 49.9 ± 6.2 | 50.0 ± 6.3 | <0.001 |
| Energy from fat (%) | 29.0 ± 5.8 | 31.8 ± 5.2 | 33.5 ± 5.2 | 34.9 ± 5.0 | 36.6 ± 5.2 | <0.001 |
| Energy from protein (%) | 18.7 ± 3.2 | 17.3 ± 2.8 | 16.5 ± 2.6 | 15.7 ± 2.5 | 14.4 ± 2.6 | <0.001 |
Data are means ± SD. *Soft drinks were defined as fizzy soft drinks plus fruit squash or cordial.
CONCLUSIONS
In this prospective study, we found a positive association between DED and the development of incident diabetes independent of baseline BMI, total energy intake, fat intake, and lifestyle factors. Indeed, the risk was elevated by 60% in the highest DED group compared with the lowest DED category. This finding, if confirmed in other populations, will be of potential importance in understanding the etiology of type 2 diabetes and may have public health relevance in its prevention.
Our study adds new information regarding the association of dietary intake and incident diabetes. DED is one way to assess the nature of the overall diet rather than assessing individual nutrients or foods. Diets that are high in fat tend to be energy dense (15) because fat is the most energy-dense nutrient and fat content varies substantially in individual foods. In the Finnish DPS, compared with the low-fat/high-fiber group, the risk of developing diabetes increased by 89% in the high-fat/low-fiber group (4). Similarly, in the Health Professionals’ Study, which followed 42,504 men for 12 years, total fat intakes were associated with a higher risk of type 2 diabetes, but this association disappeared after adjustment for BMI (16). However, several factors other than fat intake also contribute to the energy density of the diet. The other important determinant of energy density is water, which has zero energy content and the proportion of which varies substantially among commonly consumed foods. Fiber also has the potential to influence DED because of its minimal energy content and the capacity to bind water, but fiber has a much smaller range of concentrations in common foods. Therefore, it is necessary to evaluate the nature of the overall diet using DED rather than the proportion of individual nutrients or foods.
The concept of energy density from the whole diet is appealing because it is simple both in terms of its calculation and in terms of its “utility” and understanding by the general public. This approach takes into account the complex interactions among nutrients and foods in the context of a free-living population. On the other hand, it cannot identify particular components responsible for an energy-dense diet and thus is less informative in terms of biological relations between individual dietary components and disease risk. However, DED is weighed energy density of individual components of the overall diet. Therefore, foods that have high energy per unit of weight contribute to a more energy-dense diet. Because the two most important determinants of DED are fat and water content, dry foods with high fat content are especially energy dense, whereas watery foods low in fat constitute an energy-diluted diet. Generally, in our study, less energy-dense diets consisted of more fresh fruit and vegetables, less meat and processed meat, more non–energy-containing beverages, and lower energy from fat.
There are also several other methods of calculating DED (17,18). Previous studies reported that different DEDs have different associations with health outcomes, i.e., BMI and body composition (19,20). The method we chose included all solid foods and all beverages (except water, which was not available in our study) because dietary habits influence every aspect of the diet, and adoption of a healthy diet should include not only the solid but also the liquid components, which have been reported to have an important role in the development of obesity and type 2 diabetes (21).
Our study is the first study, to our knowledge, to examine the association between DED and new-onset type 2 diabetes in a large population-based cohort setting of >21,000 individuals with long follow-up in both men and women. There was a nonsignificant positive association between DED and the risk of incident type 2 diabetes at 3 years of follow-up in the Finnish DPS, but that study was small, with a total sample size of 522, and limited to a high-risk group with overweight/obesity and impaired glucose tolerance (4). We report a significant and positive association between DED and new-onset diabetes in a low-risk, free-living general population of the EPIC-Norfolk study. Additional strengths of our study were that we examined the exposure variable of DED both as a continuous and as a categorical variable. We also found that energy-dense diets were predictive of diabetes independently of a comprehensive range of risk factors and confounders including baseline BMI and total energy intake. In addition, we accounted for the potential effect of underreporting, which is a major drawback of self-report dietary assessment (11,13,22). Our case ascertainment of diagnosed new-onset diabetes was thorough, with self-report information supplemented by external sources, i.e., general practice records, hospital records, and death certificates. Thus, a further strength of our study is that new cases of diabetes were identified through sources of data that did not depend on the follow-up participation rate.
Limitations of our study merit consideration. Dietary intake in our study was assessed by a semiquantitative FFQ with its associated limitations. For one thing, there is an issue of potential underreporting in self-report dietary intake. Our results, however, were robust with and without underreporters identified with two published methods taking into account individual characteristics. For another, the FFQ was designed to cover the most commonly consumed foods and represent the usual dietary pattern of each individual. Thus, foods not in the questionnaire were not included in the calculation of DED. We could only ascertain diagnosed incident cases of diabetes. The presence of any undiagnosed cases in the cohort will reduce the number of new cases and have the effect of attenuating any observed associations. Our population is predominantly of European-Caucasian origin (99.1%); thus, our findings cannot be considered equally valid in other groups. Our cohort, although representative of the general population with respect to clinical and anthropometric characteristics, may have included greater healthier lifestyle choices; for instance, smoking prevalence was lower (12%) than the national average (∼27% in 1998, Health Survey for England) (5). This would have led to a possible underestimation of the observed effect, and hence our strong positive association is noteworthy.
The mechanism for the association between energy density and type 2 diabetes is not yet fully understood. Humans tend to eat in a way that maintains a constant volume of food intake because stomach distension triggers afferent vagal signals of fullness (1). Therefore, consumption of foods with high energy density will result in excess energy intake because of the small volume of food in relation to its energy content. Foods that are high in energy density also tend to be more palatable, which is associated with increased food intake, the so-called “passive overeating,” which would probably result in overweight and body composition change (1,2,20), both of which have been reported to be important risk factors for the development of diabetes. However, in our study, DED was positively associated with increased total energy intake, as expected (Table 1), but the association between DED and risk of incident diabetes was independent of total energy intake, baseline BMI, and self-reported weight change. However, weight change data are only available until the follow-up questionnaire in 2004, whereas our end point (diabetes) ascertainment is still the end of 2005. Future studies are needed to elucidate the mechanism of association between high DED and development of type 2 diabetes, including an understanding of whether it is mediated by weight gain.
In summary, we have shown prospectively that higher DED at baseline predicts the risk of incident diabetes independently of baseline BMI, total energy intake, and other known risk factors. This finding has potential implications for preventing type 2 diabetes through adoption of a healthier lifestyle and merits further research, including confirmation in other studies.
Acknowledgments
This study was supported by the Medical Research Council Epidemiology unit and Ellison Medical Foundation-International Nutrition Foundation. The cohort of EPIC-Norfolk is supported by grant funding from the Cancer Research Campaign, the Medical Research Council, the Stroke Association, the British Heart Foundation, the Department of Health, the Europe Against Cancer Programme Commission of the European Union and the Ministry of Agriculture, Fisheries and Food.
We thank the staff of EPIC for their invaluable contributions, the general practitioners who allowed us to approach individuals on their lists, and the individuals of Norfolk who took part in this study.
Published ahead of print at http://care.diabetesjournals.org on 8 August 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Yao M, Roberts SB: Dietary energy density and weight regulation. Nutr Rev 59:247–258, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA: A prospective analysis of dietary energy density at age 5 and 7 years and fatness at 9 years among UK children. Int J Obes (Lond) 32:586–592, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Mendoza JA, Drewnowski A, Christakis DA: Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 30:974–979, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lindstrom J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, Tuomilehto J: High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 49:912–920, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham N: EPIC-Norfolk: study design and characteristics of the cohort: European Prospective Investigation of Cancer. Br J Cancer 80(Suppl. 1):95–103, 1999 [PubMed] [Google Scholar]
- 6.Welch AA, Luben R, Khaw KT, Bingham SA: The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J Hum Nutr Diet 18:99–116, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L, Khaw KT, Day NE: Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 26(Suppl. 1):S137–S151, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Harding AH, Day NE, Khaw KT, Bingham S, Luben R, Welsh A, Wareham NJ: Dietary fat and the risk of clinical type 2 diabetes: the European prospective investigation of Cancer-Norfolk study. Am J Epidemiol 159:73–82, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE: Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 6:407–413, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Willett W, Stampfer MJ: Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124:17–27, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM: Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 45:569–581, 1991 [PubMed] [Google Scholar]
- 12.Schofield WN: Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39(Suppl. 1):5–41, 1985 [PubMed] [Google Scholar]
- 13.Rennie KL, Coward A, Jebb SA: Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr 97:1169–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine of the National Academies: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC, National Academies Press, 2008
- 15.Diet, Nutrition and the Prevention of Chronic Diseases. World Health Org., 2003. (Tech. Rep. Ser., no. 916), p. i–149 [PubMed]
- 16.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB: Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 25:417–424, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Cox DN, Mela DJ: Determination of energy density of freely selected diets: methodological issues and implications. Int J Obes Relat Metab Disord 24:49–54, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ: Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr 135:273–278, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kant AK, Graubard BI: Energy density of diets reported by American adults: association with food group intake, nutrient intake, and body weight. Int J Obes (Lond) 29:950–956, 2005 [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey TA, Rennie KL, Kerr MA, Wallace JM, Hannon-Fletcher MP, Coward WA, Jebb SA, Livingstone MB: Energy density of the diet and change in body fatness from childhood to adolescence; is there a relation? Am J Clin Nutr 87:1230–1237, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB: Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292:927–934, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Voss S, Kroke A, Klipstein-Grobusch K, Boeing H: Is macronutrient composition of dietary intake data affected by underreporting? Results from the EPIC-Potsdam Study. European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 52:119–126, 1998 [DOI] [PubMed] [Google Scholar]


