Abstract
OBJECTIVE—The purpose of this study was to determine whether a strategy of aggressive cardiovascular risk management reduced the mortality associated with diabetic foot ulceration.
RESEARCH DESIGN AND METHODS—After an initial audit of outcomes demonstrating a high mortality rate in 404 diabetic foot ulcer patients with the first ulceration developing between 1995 and 1999, a new aggressive cardiovascular risk policy was introduced as standard practice at the Diabetic Foot Clinic, Royal Infirmary of Edinburgh, in 2001. In the first 3 years of this policy, 251 patients were screened and identified. The audit cycle was then closed by reauditing the 5-year mortality for this second group of foot ulcer patients in 2008.
RESULTS—Overall 5-year mortality was reduced from 48.0% in cohort 1 to 26.8% in cohort 2 (P < 0.001). Improvement in survival was seen for both neuroischemic patients (5-year mortality of 58% reduced to 36%; relative reduction 38%) and neuropathic patients (36% reduction to 19%; relative reduction 47%) (both P < 0.001). Patients were more likely to die if they were older at the time of ulceration or had type 2 diabetes, renal impairment, or preexisting cardiovascular disease or were already taking aspirin. Prior statin use, current smoker or ex-smoker status, blood pressure, A1C, and total cholesterol were not significantly different between survivors and those who died in the follow-up periods.
CONCLUSIONS—Diabetic foot ulcer patients have a high risk of death. Survival has improved over the past 13 years. The adoption of an aggressive cardiovascular risk management policy in diabetic foot ulcer clinics is recommended for these patients.
Lower limb amputation in diabetic patients is associated with significant excess mortality (1). Foot ulceration is also believed to be associated with increased deaths due to related cardiovascular disease (2,3). In addition, patients with foot ulceration often have advanced diabetes complications (4). Cardiovascular risk reduction has, over the last 10 years, become a major part of diabetes care, particularly for type 2 diabetic patients. However, it has only been since 2004 that the U.K. General Medical Services contract has made the treatment of cardiovascular risk a renumerated part of diabetes management in primary care (5). This initiative is driven by target A1C, blood pressure, and cholesterol levels rather than absolute or calculated risk as in the past and has increased prescribing of therapies aimed to reduce cardiovascular risk (6). Patients without established risk factors might not receive adequate treatment if their values are not above the target.
It is likely that the peripheral arterial disease and microvascular sclerosis associated with diabetic foot ulceration will reflect established arterial disease elsewhere in the body (3). Therefore, cardiovascular risk factor intervention might be expected to be effective in this setting. However, there are no studies of aggressive risk factor modification in patients with diabetic foot ulcer patients to determine whether such a strategy improves survival.
RESEARCH DESIGN AND METHODS
The specialist Diabetic Foot Clinic of the Royal Infirmary of Edinburgh was started, in its current form, in March 1995. As part of the structure of the clinic, separate notes, a database, and robust audit procedures were put in place. The healing rates and other outcome results of the first 4 years of care in the clinic have been published elsewhere (7), but the mortality after foot ulceration in this group of patients prompted this audit.
This audit was designed to determine whether a new policy of standard care, optimizing cardiovascular risk reduction, in an identified high-risk population would influence mortality. Two cohorts of patients were studied. Cohort 1 comprised patients referred with new ulceration between 1 March 1995 and 28 February 1999. Cohort 2 comprised patients referred between July 2001 and March 2004. All follow-up was until 31 July 2008.
In each group survival was measured from the time of the first ulceration to death even if the first ulcer occurred before referral to the clinic. Ulcer type was determined in the following manner. Patients with both pedal pulses present without a history of revascularization were deemed to be primarily neuropathic; patients with absent pulses or a prior history of revascularization were deemed to be neuroischemic.
When the mortality rate for cohort 1 was identified, the initial therapy history on first attendance to the clinic and all clinic notes and primary care referrals for this group were examined for drugs known to reduce cardiovascular risk. These were statin therapy, antiplatelet agents, ACE inhibitors, and β-blockers (8).
The care for cohort 2 was adapted from the results of the initial audit. All patients were screened for known cardiovascular risk factors including blood pressure and serum cholesterol. A1C, total cholesterol, and serum creatinine results were taken from the year of ulceration. The normal range for A1C in the Royal Infirmary of Edinburgh is up to 6.5%. When multiple values existed, the mean value in the year of first ulceration was used. Blood pressures in cohort 2 were obtained at the clinic during the cardiovascular risk assessment process. Sufficient blood pressure data for analysis of cohort 1 were not available.
A cardiovascular risk score for patients in cohort 2 was derived using the UK Prospective Diabetes Study (UKPDS) risk engine for primary prevention patients (9). Patients with known cardiovascular disease (ischemic heart disease or stroke) were deemed to be secondary prevention patients.
A detailed drug history was taken, and the following recommendations were made. All foot ulcer patients referred after 2001 were recommended to receive an antiplatelet agent (10,11), either 75 mg aspirin or 75 mg clopidogrel if they were aspirin intolerant. The only contraindications to antiplatelet therapy were known intolerance, a recent history of gastrointestinal bleeding, or unexplained anemia. Warfarin was a relative contraindication. Statin therapy was recommended for all patients without a history of statin intolerance and, if patients were already taking a statin, the dose was recommended to be optimized to pravastatin or simvastatin at a minimum dose of 40 mg (11–16).
ACE inhibitors or angiotensin receptor blockers were recommended for all patients with hypertension, previous cardiovascular disease, and/or microalbuminuria, unless there was known renovascular disease (11). Again, doses were maximized if possible. In addition, β-blockers were recommended for all patients with existing cardiovascular disease or in whom blood pressure was still uncontrolled despite ACE inhibition. This was normal practice in 2001 in line with the findings of the UKPDS study and the expected high levels of cardiovascular disease and, despite recent controversy, is still recommended for secondary prevention of cardiovascular events in the 2006 American Diabetes Association standards of diabetes care (11,17,18). Peripheral vascular disease was not an absolute contraindication for β-blockade. Letters were sent to the primary care team for every patient to inform them of the clinic policy, screening process, and the reasons underlying them, whether changes were thought to be required or not.
In 2004 and 2005 letters were sent to ensure that the first letter had been received and that the recommended therapy changes had been made. In addition, the causes of death for cohort 1 and any deaths up that point in cohort 2 were determined. The primary care physician for each patient was sent a letter asking what cause of death was registered on the death certificate. Death certificates are unreliable but are the best available information for cause of death in the absence of postmortem examinations (19). Any causes of deaths after 2005 have been sought prospectively.
Statistical analyses
All statistical analyses were performed using SPSS for Windows (version 11.0; SPSS, Chicago, IL). Between-group comparisons were performed using t tests and χ2 tests with the Yates correction, depending on the nature of the comparison and the distribution of the variables. Statistical significance was taken as two-tailed at the 0.05 level.
RESULTS
There were 404 foot ulceration patients in cohort 1 and 251 in cohort 2. Their characteristics are detailed in Table 1. There were no significant differences in the available data between the groups other than initial total cholesterol levels. Over the 13 years of follow-up, only six patients from cohort 1 have been completely lost to follow-up with no outcome data available because of a loss of case notes during a change in hospital location and a later change in hospital computer systems. At least three of these are known to have died, but the date and cause of death could not be determined as no primary care contact details were available. No patients were lost to follow up in cohort 2.
Table 1.
Demographic characteristics of the two cohorts
| Cohort 1 | Cohort 2 | |
|---|---|---|
| n | 404 | 251 |
| Sex (% male) | 62 | 66 |
| Type 2 diabetes (%) | 70 | 77 |
| Age at first ulcer (years) | 63.2 ± 13.8 | 61.9 ± 14.9 |
| Mean duration of diabetes (years) | 13.4 ± 11.2 | 13.8 ± 10.8 |
| Ischemic ulcers (%) | 52 | 48 |
| Previous cardiovascular disease (%) | 39 | 36 |
| Current smoker (%) | 24 | 24 |
| Systolic blood pressure (mmHg) | — | 139.1 ± 23.7 |
| Diastolic blood pressure (mmHg) | — | 81.7 ± 13.6 |
| A1C | 8.6 ± 1.6 | 8.4 ± 1.8 |
| Creatinine >130 μmol/l (%) | 22 | 19 |
| Total cholesterol (mmol/l) | 5.21 ± 1.01 | 4.77 ± 1.30* |
Data are means ± SD or %.
P < 0.05, cohort 1 versus cohort 2.
Major amputations have been performed on 11.3% of cohort 1 to date. Survival was not different between those with and without a major amputation (5-year mortality without amputation 48%, with amputation 47.8%, NS). Therefore, amputation was not used to subdivide the groups in subsequent analyses. Patients in cohort 1 with peripheral vascular disease had a higher 5-year mortality than those with mainly neuropathic ulcers (58 vs. 36%, P < 0.001).
Calculated 5-year cardiovascular mortality in cohort 2 was significantly lower than the actual mortality in cohort 2. Mortality for neuropathic patients was 7.5 versus 19% and for neuroischemic patients was 9.0 versus 36% (both P < 0.01).
Prescribing of cardiovascular risk–reducing drugs improved significantly by 2003 (Table 2). However, further changes were recommended for more than half the patients. Analysis of the actions by primary care teams after recommendations showed that these were acted on in ∼80% of occasions (P = 0.02). The final level of prescribing of statins and antiplatelet therapy increased to nearly 90% after a second letter was sent to follow up on the recommendations (Table 2).
Table 2.
Percent levels of prescribing for major cardiovascular and diabetes drug therapies on first clinic visit in each cohort
| Drug therapy | Cohort 1 | Cohort 2 initial | Cohort 2 after letter |
|---|---|---|---|
| Antiplatelet | 19 | 56* | 84† |
| Statin | 9.6 | 54* | 88† |
| ACE inhibitor | 8.9 | 45* | 55 |
| Angiotensin receptor blocker | 0 | 5* | 6 |
| β-Blocker | 7 | 26* | 35 |
| Diuretic | — | 46 | — |
| Insulin | — | 50 | — |
| Metformin | — | 36 | — |
| Sulfonylurea | — | 12 | — |
All P > 0.05, cohort 2 versus cohort 1.
P < 0.05 after follow-up letter versus on presentation to the clinic.
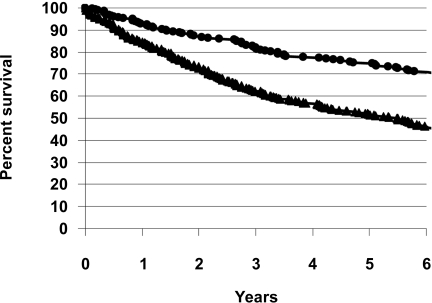
All 251 cohort 2 patients were followed to 4 years. The relative risk of death at 4 years was 49.4% lower in cohort 2. Overall mortality at 4 years in cohort 1 was 43.3% and in cohort 2 was 21.9% (P < 0.001). In cohort 2, 160 had an ulcer before 1 August 2003 and their survival was compared with the 5-year survival for cohort 1. Overall 5-year mortality was reduced from 48.0% in cohort 1 to 26.8% in cohort 2 (P < 0.001) (Fig. 1). Improvement in survival was seen for both neuroischemic patients (5-year mortality of 58% reduced to 36%) and neuropathic patients (mortality of 36% reduced to 19%) (both P < 0.001).
Figure 1.
Survival graphs for cohort 1 (▴) and cohort 2 (•). The 5-year survival of 52.0% in cohort 1 improved to 73.2% in cohort 2 (P < 0.001).
Patients in cohort 2 who died were older at the time of their first ulceration (70.6 ± 10.4 vs. 57.5 ± 15.0 years, P < 0.01) and were more likely to have type 2 diabetes (odds ratio [OR] 3.21 [95% CI 1.53–6.74], P < 0.01), renal impairment (creatinine >130 μmol/l) (3.04 [1.57–5.87]), preexisting cardiovascular disease (3.25 [1.87–5.67]), or already be taking aspirin (3.52 [1.93–6.42]). Similar results were seen in cohort 1. In both cohorts sex, prior statin use, current smoker or ex-smoker status, blood pressure (cohort 2 only), A1C, or total cholesterol were not significantly different between survivors and those who died in the follow-up periods.
The largest single recorded cause of death in both cohorts was ischemic heart disease. Ischemic heart disease was the recorded cause of death in 61% of cohort 1 and 65% of cohort 2 patients, with all vascular causes, including stroke, comprising 74 and 81% of deaths, respectively. Cancer (7%), chronic airway disease (6%), and end-stage renal failure (5%) were the next three most prevalent causes.
The patients in cohort 2 were on average 1.3 years younger at presentation (95% CI −3.5823 to 0.9823, P = 0.26). However, the patients in cohort 2 who died in the first 5 years after presentation (63 of 87 total deaths to date) were on average 3.5 years older at death than the cohort 1 patients who died in the same period (194 of 285 total deaths to date). Average age at death in cohort 2 was 73.9 ± 10.1 versus 1 70.4 ± 11.8 years (P = 0.025) in cohort 1.
CONCLUSIONS
This study has demonstrated improved survival for diabetic foot ulcer patients over the past 13 years. The marked improvement in mortality in our patients occurred at a time when greater attention was given to glycemic control, blood pressure, and lipid management after publication of the UKPDS studies and the main lipid studies of the 1990s (6,10–17,20). However, of the available data, only total cholesterol was significantly lower at the time of ulceration in these two groups. Given the higher levels of blood pressure therapy in cohort 2, it is likely that blood pressure would also be lower in this group. However, extrapolating from the major statin trials and the UKPDS blood pressure study, in which the relative risk reductions for cardiovascular events have been on the order of 25% and absolute overall mortality reductions in single figures, these differences alone are probably not enough to explain the difference in mortality between the groups (12–17). Even the recent study by Charlton et al. (6), which shows impressive relative reductions of ∼37% in early mortality from type 2 diabetes, reports overall absolute reductions in mortality of <2%. Other contemporaneous foot ulcer and amputation mortality studies still show mortality rates of ∼50% at 5 years in a similar diabetes and cardiovascular management climate (1–3). It is therefore likely that the introduction of the aggressive cardiovascular risk management policy has contributed to the improvement in mortality observed in these patients.
The levels of drug therapy prescribing in cohort 1 were derived from hospital case notes and referral letters and may be an underestimate of the true levels of prescribing, perhaps exaggerating the effect of the new treatment policy. However, they are in line with reported aspirin, statin, and ACE inhibitor use in type 2 diabetic patients in 1996 and 2004 (6,10). Despite the landmark studies of the 1990s there were still significant gaps in the prescription of proven cardiovascular risk–lowering medications in cohort 2. This finding is also in keeping with the gaps in prescribing in similar studies of therapies for cardiovascular risk reduction in diabetic patients (6,10,21,22). The gaps in prescribing might be partly explained because this group of patients was not widely recognized as being one with a proven high mortality outside of diabetic foot care centers nor one in which there was an evidence base for successful cardiovascular risk reduction. Even the most accurate cardiovascular risk modeling would have seriously underestimated mortality and, therefore, before universal treatment recommendations in type 2 diabetic patients only those with true secondary prevention needs would have been treated and then often incompletely (6,9,10). The eventual levels of statin and antiplatelet therapy are significantly higher than those achieved by national guidelines alone in other studies (6,10,21,22). This is likely to reflect the fact that the diabetes foot clinic, by focusing care for this high-risk group of patients and by regular review and follow-up, is in the best position to ensure that cardiovascular risk–reducing therapies are prescribed and used.
The significant benefits in mortality were seen despite blood pressure and total cholesterol measurements that would not have merited blanket treatment for cardiovascular risk according to Quality and Outcomes Framework or other target-driven guidelines (5). This result may be due to additional antiplatelet effects, ACE inhibition, and the noncholesterol-related benefits of statin treatment (11,23).
The association of older age but not duration of diabetes with risk of dying would be in keeping with the known higher mortality in type 2 diabetic patients (17,20). Preexisting vascular disease and secondary prevention measures explain the higher initial levels of aspirin use in those who died (6,10,11). Risk reduction therapy was similar between the dead and surviving patients after the changes imposed by the new prescribing policy. Smoking rates were high in both groups, and either the overall numbers in the study were too small to determine a difference in outcome or diabetes and other factors had a greater effect. The lack of a difference in A1C between survivors and those who died is unsurprising in a population of only 650 patients. In the UKPDS study, a difference of 0.9% in A1C did not significantly alter mortality (20).
The absence of an increase in mortality after major lower limb amputation is reassuring and has been reported previously (24). It is likely to be the underlying vascular disease in neuroischemic and neuropathic patients and not the procedure that influences outcomes. The decision to group all foot ulcer patients together was made on the basis of similar characteristics for the two cohorts and a high mortality for neuropathic ulcer patients in this study and in other prospective studies (25). The benefits of reduction of cardiovascular mortality were seen equally in both ulcer types, suggesting that even neuropathic diabetic patients have a degree of underlying macrovascular disease.
The relative risk of death within 5 years of foot ulceration was 48.5% lower in cohort 2 than in cohort 1. The size of the improvement in mortality and the observation that this improvement was also associated with older age at death suggest that cardiovascular risk reduction treatment does prolong survival in this population. The large effect seen in this population is in keeping with previous studies, which suggested that absolute risk reduction is greater in high-risk groups (12–16,22,23).
The findings of this study are based on actual outcomes from a clinic population and not a study population. The advantages of specialist multidisciplinary foot clinics in improving healing rates for diabetes foot ulceration and reducing amputation rates and hospital admissions have been described previously, and they are accepted as the best model of foot ulcer care (7). This study adds another justification for concentrating foot ulcer care in specialist centers. It suggests that specialist diabetes foot clinics should adopt a policy of aggressive cardiovascular risk management, with prescribing of secondary prevention therapies not just for those with previously known cardiovascular events or cholesterol or blood pressure levels above target but for all foot ulcer patients.
Acknowledgments
This work was sponsored in part by unrestricted research grants from sanofi-aventis and Bristol-Myers Squibb.
We thank Beth Robertson, podiatrist, for her work on data collection in the first stages of the audit.
Published ahead of print at http://care.diabetesjournals.org on 12 August 2008.
M.J.Y. has received honoraria for presentations on cardiovascular risk and diabetes from sanofi-aventis. L.E.R. and J.I.B. were paid in part from a grant from BMS and sanofi-aventis to screen diabetic foot patients for known cardiovascular risks.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Resnick HE, Carter EA, Lindsay R, Henly SJ, Ness FK, Welty TK, Lee ET, Howard BV: Relation of lower-extremity amputation to all-cause and cardiovascular disease mortality in American Indians: the Strong Heart Study. Diabetes Care 27:1286–1293, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Moulik PK, Mtonga R, Gill GV: Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 26:491–494, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ghanassia E, Villon L, Thuan dit Dieudonne J-F, Boegner C, Avignon A, Sultan A: Long-term outcome and disability of diabetic patients hospitalised for diabetic foot ulcers: a 6.5 year follow-up study. Diabetes Care 31:1288–1292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N: High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia 50:18–25, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Quality and outcomes framework: guidance—updated August 2004 [article online], 2004. Available from www.paymodernisation.scot.nhs.uk/gms/quality/docs/QualOutFrame0804.pdf. Accessed 30 June 2008
- 6.Charlton J, Latinovic R, Gulliford MC: Explaining the decline in early mortality in men and women with type 2 diabetes: population-based cohort study. Diabetes Care 31:1761–1766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick KG, Young MJ: A clinical audit of the Diabetic Foot Ulcer Clinic. Br J Podiatry 2:95–96, 1999 [Google Scholar]
- 8.MacDonald TM, Butler R, Newton RW, Morris AD: Which drugs benefit diabetic patients for secondary prevention of myocardial infarction? Diabet Med 15:282–289, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Guzder RN, Gatling W, Mullee MA, Mehta RL Byrne CD: Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed type 2 diabetes: results from a United Kingdom study. Diabet Med 22:554–562, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cull CA, Neill HAW, Holman RR. Changing aspirin use in patients with Type 2 diabetes in the UKPDS: Diabet Med 21:1368–1371, 2004 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association: Position statement: Standards of medical care in diabetes–2006. Diabetes Care 29:S4–S42, 2006 [PubMed] [Google Scholar]
- 12.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E: The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: the Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 335:1001–1009, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group: prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339:1349–1357, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, Davis BR, Cole TG, Pfeffer MA, Braunwald E: Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analysis in the Cholesterol and Recurrent Events (CARE) trial: the CARE Investigators. Circulation 98:2513–2519, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, Kjekshus J, Pyorala K: Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med 159:2661–2667, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361:2005–2016, 2003. 12814710 [Google Scholar]
- 17.UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317:703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 18.Freemantle N, Cleland J, Young P, Mason J, Harrison J: β Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 318:1730–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mühlhauser I, Sawicki PT, Blank M, Overmann H, Richter B, Berger M: Reliability of causes of death in persons with type I diabetes. Diabetologia 45:1490–1497, 2002 [DOI] [PubMed] [Google Scholar]
- 20.UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 21.Fonarow GC: Statin therapy after acute myocardial infarction: are we adequately treating high-risk patients? Curr Atheroscler Rep 4:99–106, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Woodward A, Bayley D, Overend L, Gill G: Antiplatelet drug use in a diabetic clinic. Q J Med 100:547–550, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Gupta S: Does aggressive statin therapy offer improved cholesterol-independent benefits compared to conventional statin treatment? Int J Cardiol 96:131–139, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH: Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 22:382–387, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Chammas NK, Hill RL, Foster AV, Edmonds ME: Is neuropathic ulceration the key to understanding increased mortality due to ischaemic heart disease in diabetic foot ulcer patients? A population approach using a proportionate model. J Int Med Res 30:553–559, 2002 [DOI] [PubMed] [Google Scholar]