Abstract
OBJECTIVE—We report the independent risk association of type 2 diabetic nephropathy with the z−2 allele of the 5′-(CA)n microsatellite and C-106T promoter polymorphisms of the aldose reductase gene (ALR2) using a case-control design. In this expanded cohort, we examined their predictive roles on new onset of cardiorenal complications using a prospective design.
RESEARCH DESIGN AND METHODS—In this 8-year prospective cohort of 1,074 type 2 diabetic patients (59% male, median age 61 years; disease duration 7 years) with an observation period of 8,592 person-years, none had clinical evidence of coronary heart disease (CHD) or chronic kidney disease at recruitment. The renal end point was defined as new onset of estimated glomerular filtration rate <60 ml/min per 1.72 m2 or hospitalizations with dialysis or death due to renal disease, and CHD was defined as hospitalizations with myocardial infarction, ischemic heart disease, or related deaths.
RESULTS—After controlling for baseline risk factors and use of medications, we found that the ALR2 z−2 allele of (CA)n microsatellite carriers had increased risk of renal (hazard ratio 1.53 [95% CI 1.14–2.05], P = 0.005) or combined cardiorenal (1.31 [1.01–1.72], P = 0.047) end points. Carriers of the ALR2 C-106T polymorphism also had increased risk of renal (1.54 [1.15–2.07], P = 0.004) and cardiorenal (1.49 [1.14–1.95], P = 0.004) end points. Compared with noncarriers, patients with two risk-conferring genotypes had a twofold increased risk of renal (2.41 [1.57–3.70], P < 0.001) and cardiorenal (1.94 [1.29–2.91], P = 0.002) end points.
CONCLUSIONS—In Chinese type 2 diabetic patients, genetic polymorphisms of ALR2 independently predicted new onset of renal and cardiorenal end points, with the latter being largely mediated through renal disease.
Cardiovascular diseases and end-stage renal disease (ESRD) are the main causes of mortality and morbidity in diabetic patients. The onset of chronic kidney disease (CKD) markedly increases the risk of cardiovascular disease as a result of further perturbation in the metabolic milieu and vascular homeostasis (1). In type 2 diabetes, control of hyperglycemia reduces proteinuria and the rate of decline in renal function (2). In type 1 diabetes, established renal lesions were reversed with restoration of normoglycemia after pancreatic transplantation (3). Aldose reductase (ALR2) converts glucose to sorbitol, especially under hyperglycemic condition. Accumulation of sorbitol can lead to increased oxidative and osmotic stresses, causing cataract, microvascular complications, and myocardial ischemic injury (4).
In a case-control cohort consisting of 92 Chinese type 2 diabetic patients without nephropathy despite long disease duration and 121 patients with both retinopathy and nephropathy, the z−2 allele of 5′-(CA)n microsatellite and promoter C-106T polymorphisms of the ALR2 gene independently conferred a risk of diabetic nephropathy (5). In this expanded consecutive cohort of 1,074 type 2 diabetic patients with documentation of risk factors, complications, and clinical outcomes, we examined the independent and additive effects of these two polymorphisms of the ALR2 gene on cardiorenal end points after an 8-year observational period.
RESEARCH DESIGN AND METHODS
Hong Kong Diabetes Registry
This registry was established in 1995 as part of a quality improvement program based on the European DIABCARE protocol and accompanied by a DNA bank donated by patients with written informed consent. Using this registry, we have reported the use of clinical and biochemical markers to predict cardiorenal complications (6–8). The study was approved by the Chinese University of Hong Kong Clinical Research Ethics Committee. The recruitment methods, definitions, and biochemical and genetic assays have been described previously (5,7).
Clinical and biochemical assessments and genotyping
After an 8-h overnight fast, patients were seen in the unit for measurement of BMI, waist circumference, and blood pressure after at least 5 min of rest. Hypertension was defined as blood pressure ≥140/90 mmHg and/or treatment with antihypertensive drugs. A family history of coronary heart disease (CHD) was defined as premature heart disease in first-degree relatives aged <60 years. Peripheral arterial disease was defined by a history of lower-extremity amputation, absent foot pulses confirmed by an ankle-to-brachial ratio <0.90 using Doppler ultrasound, or revascularization. Sensory neuropathy was defined by two of three features: abnormal sensation in the extremities, reduced vibration sense <6/8 (or <4/8 for those aged ≥65 years) using a graduated tuning fork, or abnormal sensation to monofilament on any part of the sole with a normal skin surface. An eye examination was performed by use of either fundus photography or direct viewing using an ophthalmoscope by ophthalmologists or trained medical personnel. Retinopathy was defined by typical retinal changes, including laser scars or a history of vitrectomy.
The albumin-to-creatinine ratio (ACR) was measured in a sterile, random, spot urine sample confirmed by a timed urinary collection (4 or 24 h). Microalbuminuria was defined as ACR ≥3.5 mg/mmol for women and ≥2.5 mg/mmol for men; macroalbuminuria was defined as ACR ≥25 mg/mmol. CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min per 1.72 m2 determined by the Chinese-modified Modification of Diet in Renal Disease equation: 186 × (SCR × 0.011)−1.154 × (age)−0.203 × (0.742 if female) × 1.233, where SCR is serum creatinine expressed as micromoles per liter (originally milligrams per deciliter converted to micromoles per liter) and 1.233 is the adjusting coefficient for Chinese (9).
The genetic assay and analysis, described previously (5), were applied to this expanded cohort, which contains the reported case-control cohort of 213 patients. Thirteen alleles of the 5′-(CA)n microsatellite were identified including z+12, z+10, z+8, z+6, z+4, z+2, z, z−2, z−4, z−6, z−12, z−14, and z−16, where z corresponded to 24 (CA) repeats. z+2, z, and z−2 accounted for 81.4% of total alleles. The genotypic frequencies were in Hardy-Weinberg equilibrium.
Clinical end points
In this cohort of 1,327 type 2 diabetic patients enrolled consecutively between 1995 and 1998, 8 had type 1 diabetes, 206 had CKD, and 39 had CHD defined as a history of myocardial infarction, revascularization, or typical chest pain with a positive stress test or abnormal coronary vasculature on imaging. These patients were excluded and, thus, 1,074 patients were included in the analysis.
The Hong Kong Hospital Authority is the governing body of all 48 publicly funded hospitals and clinics. Hospital discharge diagnoses, causes of death, and laboratory results for determination of eGFR were extracted from various Hong Kong Hospital Authority databases up to 30 July 2005. All discharge diagnoses were coded using the ICD-9. Causes of death were validated by records in the Hong Kong Death Registry.
The end point of CHD was defined as nonfatal myocardial infarction (code 410), ischemic heart disease (codes 411–414), or death due to CHD (codes 410–414). The renal end point was defined as 1) fatal and nonfatal diabetes with renal manifestations (code 250.4), CKD (code 585), or unspecified renal failure (code 586) (diagnosis 1–5); 2) dialysis (ICD-9 procedure code 39.95) or peritoneal dialysis (ICD-9 procedure code 54.98); or 3) first event of eGFR <60 ml/min per 1.73 m2 before the censor date. Follow-up time was calculated from the enrollment date to the date of the first cardiorenal event or death or 30 July 2005, whichever came first.
Statistical analysis
All data are expressed as median (interquartile range [IQR]) or as a percentage. Kruskal-Wallis and χ2 tests were used to compare continuous and categorical variables, including genotype and allele frequency as appropriate. Multivariable Cox proportional hazards regression was used to obtain the hazard ratio (HR) (95% CI) of baseline variables for prediction of CHD, renal, and composite cardiorenal end points. A proportional hazards assumption was checked using the Supremum test, which was implemented using the ASSESS statement in the SAS procedure PROC PHREG (Statistical Analysis System release 9.10; SAS Institute, Cary, NC). P < 0.05 was considered to violate the assumption. If indicated, stratified Cox regression was used to adjust for the violation of proportionality. The Kaplan-Meier estimator with a log-rank test for linear trend was used to examine the overall difference of survival functions stratified by the number of genotypes of interest. All analyses unless specified were performed using SPSS for Windows (release 13.0; SPSS, Chicago, IL). P < 0.05 (two-tailed) was considered to be significant.
Sample size estimation
To obtain a stable Cox regression model, 10 events per predictor are generally recommended. Based on our previous analysis, the incidence of CHD in Chinese type 2 diabetic patients was 9.28 (95% CI 8.31–10.24) per 1,000 person-years with seven predictors (age, male sex, disease duration, albuminuria, eGFR, smoking, and non-HDL cholesterol) (7). Using the lower limit of the CIs for estimation, a prospective cohort of 8,424 person-years [(70 ÷ 8.31) × 1,000] is required to develop a stable model to predict CHD. For ESRD, the event rate was 8.7 (7.8–9.6) per 1,000 person-years with hematocrit and ACR as predictors (8). A prospective cohort of 2,571 person-years is required to develop a stable model to predict ESRD. The total observation period of this cohort was 8,592 person-years, which gives sufficient power to compute a model using cardiorenal end points.
RESULTS
In this prospective cohort (n = 1,074, 59% men), the median (IQR) age was 61 (50–69) years with a disease duration of 7 (2–12) years. The total observational period was 8.4 (5.6–9.3) years with follow-up duration of 8,592 person-years. The annualized event rates for CHD, renal, and cardiorenal end points were 8.92 (95% CI 6.91–10.93), 25.98 (22.49–29.46), and 31.15 (27.30–35.00) per 1,000 person-years, respectively.
The clinical profile including use of medications among patients with 0, 1, 2, or ≥3 risk-conferring genotypes were similar at baseline. Compared with patients without the z−2 and T allele, those with either one or both of the risk-conferring alleles had a higher incidence of CHD, renal, and cardiorenal end points but similar death rates (Table 1). Patients who developed a renal end point had a higher frequency of z−2/x or z−2/z−2 genotype (47 vs. 37%, P = 0.006) and z−2 allele (24 vs. 18%, P = 0.008) than those without a renal end point. Patients who developed cardiorenal end points also had a higher frequency of the CT/TT genotype (44 vs. 35%, P = 0.008) and T allele (27 vs. 22%, P = 0.026) than those who did not (Table 2).
Table 1.
Baseline clinical and biochemical characteristics and drug usage in type 2 diabetic patients stratified by number of risk-conferring genotypes of ALR2 including the z−2 (CA)n and C-106T polymorphisms
| Baseline parameters | No risk genotypes | 1 risk genotype | 2 risk genotypes | P value |
|---|---|---|---|---|
| n | 399 | 532 | 143 | |
| Age (years) | 60 (51–68) | 61 (50–69) | 61 (51–68) | 0.954* |
| Sex (% male) | 57.4 | 59.2 | 62.9 | 0.509† |
| Duration of diabetes (years) | 7.5 (1.0–12.0) | 8.0 (2.0–12.0) | 7.0 (2.0–12.0) | 0.819* |
| Smoking status | 0.681† | |||
| Ex-smoker (%) | 17.3 | 13.9 | 16.2 | |
| Current smoker (%) | 12.6 | 13.2 | 14.1 | |
| BMI (kg/m2) | 24.2 (22.1–26.9) | 24.5 (22.2–26.6) | 21.4 (21.8–26.1) | 0.515* |
| Waist circumference (cm) | ||||
| Men | 88.0 (82.0–94.0) | 87.5 (81.0–93.0) | 88.0 (82.6–92.0) | 0.760* |
| Women | 82.0 (76.0–88.0) | 83.0 (77.0–89.0) | 82.0 (75.5–88.0) | 0.395* |
| Obesity (%)‡ | 57.4 | 57.4 | 57.3 | 1.000† |
| Systolic blood pressure (mmHg) | 135 (120–147) | 132 (119–146) | 133 (120–149) | 0.511* |
| Diastolic blood pressure (mmHg) | 79 (70–86) | 79 (70–85) | 79 (74–85) | 0.350* |
| Hypertension (blood pressure ≥140/90 mmHg or medication) | 45.6 | 49.3 | 46.9 | 0.520† |
| Family history of premature CVD (%) | 1.8 | 0.8 | 0.7 | 0.311† |
| Retinopathy (%) | 20.6 | 25.0 | 29.4 | 0.076† |
| Sensory neuropathy (%) | 19.3 | 23.3 | 23.1 | 0.315† |
| Peripheral arterial disease (%) | 5.0 | 8.6 | 7.7 | 0.101† |
| History of stroke (%) | 1.8 | 2.4 | 3.5 | 0.480† |
| A1C (%) | 7.4 (6.6–8.6) | 7.6 (6.7–8.9) | 7.5 (6.6–8.4) | 0.285 |
| Triglyceride (mmol/l) | 5.30 (4.60–6.10) | 5.30 (4.70–6.10) | 5.40 (4.50–6.10) | 0.962* |
| LDL cholesterol (mmol/l) | 3.30 (2.70–4.00) | 3.40 (2.80–4.00) | 3.30 (2.70–3.95) | 0.765* |
| HDL cholesterol (mmol/l) | 1.24 (1.03–1.54) | 1.24 (1.03–1.50) | 1.31 (1.10–1.57) | 0.110* |
| Spot urine ACR ratio (mg/mmol) | 1.29 (0.59–5.53) | 1.37 (0.64–8.79) | 1.97 (0.69–24.7) | 0.245* |
| Microalbuminuria (%) | 17.2 | 21.0 | 19.7 | 0.150† |
| Macroalbuminuria (%) | 17.9 | 18.9 | 25.4 | |
| Serum creatinine (μmol/l) | 71.0 (58.0–85.0) | 69.0 (59.0–84.0) | 68.0 (62.0–85.5) | 0.811* |
| Glomerular filtration rate (ml/min per 1.73m2) | 113.5 (99.0–134.7) | 115.7 (94.9–136.6) | 115.3 (93.3–136.4) | 0.918* |
| Use of medications at enrollment | ||||
| Insulin (%) | 12.3 | 15.2 | 19.6 | 0.096† |
| OAD (%) | 50.6 | 50.0 | 52.4 | 0.873† |
| Insulin and OAD (%) | 5.5 | 7.7 | 7.0 | 0.419† |
| Lipid-regulating drug (%) | 3.8 | 4.1 | 4.9 | 0.840† |
| ACEI/ARB (%) | 7.0 | 7.3 | 7.7 | 0.962† |
| Other antihypertensive drugs (%) | 17.0 | 21.1 | 22.4 | 0.219† |
| Clinical end points after 8-year follow-up | ||||
| CHD (%) | 4.8 | 9.0 | 5.6 | 0.032† |
| Renal end point (%) | 15.0 | 20.5 | 27.3 | 0.004† |
| Cardiorenal end point (%) | 17.0 | 25.4 | 28.7 | 0.002† |
| All-cause death (%) | 10.5 | 11.5 | 14.0 | 0.537† |
Data are median (IQR) or %.
Derived from Kruskal-Wallis test.
Derived from χ2 test.
Obesity was defined as BMI ≥25 kg/m2 or waist ≥80 cm in women or ≥90 cm in men. ACEI, ACE inhibitor; ARB, angiotensin II receptor blocker; CVD, cardiovascular disease; OAD, oral antidiabetic drug.
Table 2.
Genotype and allele frequencies of the z−2 allele of the 5′- (CA)n microsatellite and C-106T promoter polymorphisms in patients stratified by the development of cardiorenal end points in type 2 diabetes
|
ALR2 (CA)n polymorphism |
ALR2 C-107T polymorphism |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x/x | x/z−2 | z−2/z−2 | x | z−2 | CC | CT | TT | C | T | |
| Cardiac end point | ||||||||||
| No | 0.61 (611) | 0.33 (324) | 0.06 (64) | 0.77 (1,546) | 0.79 (118) | 0.64 (633) | 0.31 (312) | 0.05 (54) | 0.79 (1,578) | 0.74 (111) |
| Yes | 0.63 (47) | 0.32 (24) | 0.05 (4) | 0.23 (452) | 0.21 (32) | 0.52 (39) | 0.44 (33) | 0.04 (3) | 0.21 (420) | 0.26 (39) |
| P | 0.925 | 0.796* | 0.715 | 0.073 | 0.050† | 0.151 | ||||
| Renal end point | ||||||||||
| No | 0.63 (548) | 0.31 (266) | 0.06 (52) | 0.82 (1,362) | 0.18 (302) | 0.64 (553) | 0.31 (269) | 0.05 (44) | 0.81 (1,375) | 0.19 (314) |
| Yes | 0.53 (110) | 0.39 (82) | 0.08 (16) | 0.76 (370) | 0.24 (114) | 0.57 (119) | 0.37 (76) | 0.06 (13) | 0.78 (357) | 0.22 (102) |
| P | 0.022 | 0.006* | 0.008 | 0.204 | 0.075† | 0.081 | ||||
| Cardiorenal end point | ||||||||||
| No | 0.63 (522) | 0.31 (257) | 0.06 (51) | 0.78 (1,301) | 0.22 (363) | 0.65 (537) | 0.30 (249) | 0.05 (44) | 0.78 (1,323) | 0.22 (366) |
| Yes | 0.56 (136) | 0.37 (91) | 0.07 (17) | 0.74 (359) | 0.26 (125) | 0.55 (135) | 0.39 (96) | 0.05 (13) | 0.73 (337) | 0.27 (122) |
| P | 0.128 | 0.044* | 0.064 | 0.021 | 0.008† | 0.026 | ||||
| Study population | 0.61 (658) | 0.33 (348) | 0.06 (68) | 0.77 (1,664) | 0.23 (484) | 0.63 (672) | 0.32 (345) | 0.05 (57) | 0.79 (1,689) | 0.21 (459) |
Data are frequency (n).
P value refers to risk association based on the combined genotype frequency of x/z−2 and z−2/z−2 versus x/x genotype.
P value refers to risk association based on the combined genotype frequency of CT and TT versus CC genotype.
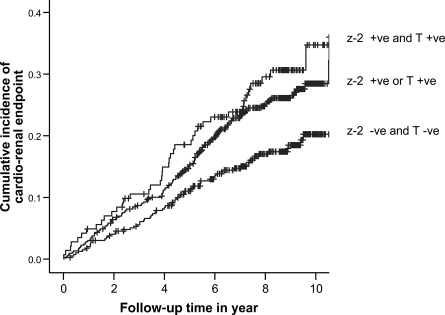
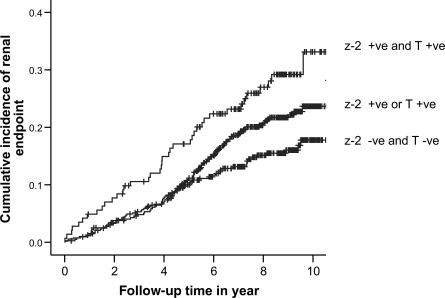
On multivariable analysis, the z−2 allele of −(CA)n and C-106T polymorphisms of ALR2 were selected as independent predictors for renal or cardiorenal end points along with age, male sex, and smoking status. The presence of two risk-conferring genotypes conferred a twofold increased risk of renal or cardiorenal end points (Table 3). Figures 1 and 2 show the cumulative effects of a number of risk-conferring genotypes on renal and cardiorenal end points.
Table 3.
HRs of predictors for cardiorenal end points in type 2 diabetes
| Predictors | CHD |
Renal end point* |
Cardiorenal end point |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Basic models† | ||||||
| Age (years) | 1.04 (1.02–1.06) | <0.001 | 1.03 (0.99–1.06) | 0.197 | 1.05 (1.04–1.07) | <0.001 |
| Sex (male) | 0.81 (0.44–1.49) | 0.498 | 0.91 (0.64–1.30) | 0.609 | 0.81 (0.58–1.13) | 0.208 |
| Smoking status | ||||||
| Ex-smoker | 1.72 (0.87–3.39) | 0.120 | 1.25 (0.82–1.90) | 0.293 | 1.22 (0.83–1.80) | 0.317 |
| Current smoker | 1.81 (0.88–3.72) | 0.108 | 1.46 (0.93–2.29) | 0.099 | 1.50 (1.00–2.25) | 0.053 |
| ALR2 z−2+/+or z−2+/− | 0.99 (0.62–1.59) | 0.972 | 1.52 (1.15–2.00) | 0.003 | 1.35 (1.05–1.75) | 0.020 |
| ALR2 CT/TT | 1.54 (0.98–2.42) | 0.064 | 1.36 (1.03–1.79) | 0.030 | 1.45 (1.12–1.87) | 0.004 |
| Risk associations with number of risk genotypes | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 1.94 (1.14–3.30) | 0.015 | 1.36 (0.99–1.87) | 0.056 | 1.53 (1.14–2.05) | 0.004 |
| 2 | 1.20 (0.52–2.75) | 0.665 | 2.07 (1.38–3.11) | <0.001 | 1.91 (1.29–2.82) | 0.001 |
| Adjusted models‡ | ||||||
| Age (years) | 1.02 (1.00–1.05) | 0.103 | 1.04 (1.00–1.09) | 0.064 | 1.03 (1.01–1.04) | 0.002 |
| Sex (male) | 0.79 (0.41–1.51) | 0.468 | 1.31 (0.88–1.86) | 0.180 | 1.08 (0.75–1.55) | 0.679 |
| Smoking status | ||||||
| Ex-smoker | 1.60 (0.79–3.23) | 0.191 | 1.14 (0.73–1.76) | 0.562 | 1.24 (0.83–1.87) | 0.297 |
| Current smoker | 1.55 (0.74–3.24) | 0.249 | 1.93 (1.20–3.11) | 0.007 | 1.83 (1.19–2.81) | 0.006 |
| ALR2 z−2+/+or z−2+/− | 1.06 (0.65–1.72) | 0.824 | 1.55 (1.15–2.08) | 0.004 | 1.31 (1.01–1.72) | 0.047 |
| ALR2 CT/TT | 1.44 (0.89–2.34) | 0.137 | 1.53 (1.13–2.06) | 0.005 | 1.49 (1.14–1.95) | 0.004 |
| Risk associations with number of risk genotypes | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 2.05 (1.15–3.63) | 0.014 | 1.37 (0.97–1.93) | 0.073 | 1.44 (1.05–1.97) | 0.023 |
| 2 | 1.18 (0.49–2.84) | 0.706 | 2.41 (1.57–3.70) | <0.001 | 1.94 (1.29–2.91) | 0.002 |
Stratified Cox regression model was used (stratified on quartiles of age).
Basic models are adjusted for age, sex, and smoking status.
Adjusted models are adjusted for age, sex, smoking status, duration of diabetes, BMI, systolic blood pressure, A1C, lipids (LDL cholesterol, HDL cholesterol, and triglycerides), log10(1 + ACR), and log10(eGFR). Use of log10(1 + ACR) other than log10(ACR) was to avoid calculation of log10(0).
Figure 1.
Cumulative incidence of renal end point stratified by the number of risk alleles of the z−2 allele of the 5′-(CA)n microsatellite and C-106T promoter polymorphisms of the ALR2 gene (Ptrend = 0.003, log-rank test) in type 2 diabetes. +ve, positive; -ve, negative.
Figure 2.
Cumulative incidence of cardiorenal end point stratified by the number of risk alleles of the z−2 allele of the 5′-(CA)n microsatellite and C-106T promoter polymorphisms of the ALR2 gene (Ptrend 0.002, log-rank test) in type 2 diabetes. +ve, positive; -ve, negative.
CONCLUSIONS
In this 8-year prospective analysis of 1,074 type 2 diabetic patients with a low prevalence of risk factors and complications and a mean A1C of 7.4% at enrollment, we confirmed our previous findings (5) regarding the independent and additive effects of the two genetic polymorphisms on the promoter region of ALR2 on renal and cardiorenal end points. These twofold increased risks were independent of other risk factors, notably A1C, triglycerides, albuminuria, renal function, smoking status, and treatments. Our results also lend support to ALR2 as a candidate gene located in chromosome 7, one of the most reproducible chromosomal regions for diabetic nephropathy (10). Conflicting results on previous risk association studies of ALR2 are probably due to differences in study design, patient selection, outcome measurements, and sample size. The long observation period and detailed documentation of clinical end points and confounders may contribute to our positive findings, which are supported by experimental studies regarding the adverse effects of glucotoxicity mediated through oxidative stress, glycation end products, cytokines, polyol, and growth factors (4). In this respect, activation of protein kinase C-δ and -ɛ in renal mesangial cells is dependent on activation of the polyol pathways in diabetic nephropathy (11).
The onset of CKD is associated with anemia, vascular dysfunction and calcification, metabolic acidosis, and inflammation, all of which are multipliers of CHD risk (1). Asian patients have a lower risk for CHD but a higher risk for stroke and ESRD than their Caucasian counterparts (12). In Chinese type 2 diabetic patients, anemia (13) and eGFR (14) are independent risk factors for CHD, suggesting that in populations in whom obesity and hypercholesterolemia are less prevalent (15), CKD may take on a more important role in determining CHD risk. Thus, given the intimate relationship between hyperglycemia and renal dysfunction and the fact that ALR2 can interact with other factors to promote atherosclerosis (16), the risk conferred by the ALR2 genotype on the cardiorenal end point, which was more powerful than that of age and smoking, was noteworthy, at least in Chinese populations. Our results remain robust after excluding the small number of patients in our previous case-control cohort (5) and adjustment for other confounders. Apart from these two genetic variants, which were not in linkage disequilibrium, we did not detect risk association with other microsatellite markers.
Optimal risk factor control reduces complication rates in type 1 (17) and type 2 (18) diabetes. Type 1 diabetic patients with one copy or more of the z−2 allele had a sevenfold increased risk of nephropathy than those without, with z−2 carriers having higher mRNA expression. A similar relationship was not observed in nondiabetic subjects, suggesting that hyperglycemia might modulate the risk for diabetic nephropathy through gene-environment interactions (19). Because multiple risk factors may influence progression of diabetic nephropathy, aggressive control of modifiable risk factors such as blood pressure, lipids, and glucose in patients carrying a risk-conferring genotype may be warranted although a randomized study or decision analysis is needed to test this hypothesis.
Several ALR2 inhibitors have been used with limited success in patients with diabetic nephropathy (20). In this light, despite optimization of therapies, type 2 diabetic patients with nephropathy have substantial residual risk of ESRD. In the Asian subgroup analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) Study, 10% of type 2 diabetic patients with nephropathy continued to develop ESRD on a yearly basis despite optimal risk factor control and inhibition of the renin-angiotensin system (21). Given the antiproteinuric effects of ALR2 inhibitors (20) and the fact that reduction in proteinuria predicts future risk of cardiorenal end points (21), our findings raise the possibility of using pathway-specific treatment to further reduce risk of complications.
Study limitations
Although ideally these results should be replicated in an independent cohort, we do not know of any similar prospective Asian cohorts to control for the confounder of ethnicity. There are inherent measurement errors of albuminuria and eGFR and with increasing use of medications to control risk factors, we have used death and hospitalizations as outcome measures. Although there are limitations with the use of ICD-9 codes and potential errors due to loss of patients who have emigrated, these numbers are expected to be small because of the heavily subsidized health care system in Hong Kong. Finally, the large body of experimental evidence and consistency of results in both case-control and prospective studies strongly favor the validity of our results.
In summary, our prospective data support the pivotal importance of the ALR2 pathway on renal complications that further accentuates CHD risk. Given the high rates of diabetes and nephropathy, especially in Asian populations, the use of biomarkers such as variants of the ALR2 genotypes, which affect 30% of the population, may identify high-risk subjects for intensive and targeted preventive therapy.
Acknowledgments
This study was supported by the Hong Kong Foundation for Research and Development in Diabetes established under the auspices of the Chinese University of Hong Kong and the Hong Kong Government Research Grant Committee.
Published ahead of print at http://care.diabetesjournals.org on 20 August 2008.
W.Y.-S. and Y.W. contributed equally to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108:2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63:225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339:69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Schrijvers BF, De Vriese AS, Flyvbjerg A: From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 25:971–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ng MCY, Lee SC, So WY, Tong CY, Cockram CS, Critchley JAJH, Chan JCN: Phenotypic heterogeneity associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care 26:2410–2415, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Yang X, So WY, Tong PC, Ma RC, Kong AP, Lam CW, Ho CS, Cockram CS, Ko GT, Chow CC, Wong VC, Chan JC: Development and validation of an all-cause mortality risk score in type 2 diabetes. Arch Intern Med 168:451–457, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Yang X, So WY, Kong AP, Ma RC, Ko GT, Ho CS, Lam CW, Cockram CS, Chan JC, Tong PC: Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol 101:596–601, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Yang XL, So WY, Kong AP, Ho CS, Lam CW, Ng MH, Lyu RR, Yin DD, Chow CC, Cockram CS, Tong PC, Chan JC: Modified end-stage renal disease risk score for Chinese type 2 diabetic patients: the Hong Kong Diabetes Registry. Diabetologia 50:1348–1350, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ma YC, Zuo L, Chen JH: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17:2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Imperatore G, Knowler WC, Nelson RG, Hanson RL: Genetics of diabetic nephropathy in the Pima Indians. Curr Diab Rep 1:275–281, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kapor-Drezgic J, Zhou X, Babazono T, Dlugosz JA, Hohman T, Whiteside C: Effect of high glucose on mesangial cell protein kinase C-δ and -ɛ is polyol pathway-dependent. J Am Soc Nephrol 10:1193–1203, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Morrish NJ, Wang S, Stevens LK, Fuller JH, Keen H: Mortality and causes of death in the WHO Multinational Survey of Vascular Diseases in Diabetes. Diabetologia 44:S14–S21, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Tong PCY, Kong APS, So WY, Ng MHL, Yang XL, Ozaki R, Ma RCY, Lam CWK, Ho CS, Chow CC, Cockram CS, Chan JCN: Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes mellitus. Diabetes Care 29:2439–2444, 2006 [DOI] [PubMed] [Google Scholar]
- 14.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, Ng V, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC: Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 29:2046–2052, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bhatt D, Steg P, Ohman E, Hirsch A, Ikeda Y, Mas J, Goto S, Liau C, Richard A, Rother J, Wilson P, the REACH Registry Investigators: International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 11:180–189, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ: Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest 115:2434–2443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358:580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Shah VO, Scavini M, Nikolic J, Sun Y, Vai S, Griffith JK, Dorin RI, Stidley C, Yacoub M, Vander Jagt DL, Eaton RP, Zager PG: Z−2 microsatellite allele is linked to increased expression of the aldose reductase gene in diabetic nephropathy. J Clin Endocrinol Metab 83:2886–2891, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Iso K, Tada H, Kuboki K, Inokuchi T: Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in type 2 diabetic patients. J Diabetes Complications 15:241–244, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Chan JCN, Wat NMS, So WY, Lam KSL, Chua CT, Wong TS, Morad Z, Dickson TZ, Hille D, Zhang Z, Cooper ME, Shahinfar S, Brenner BM, Kurokawa KA, the Asian RENAAL Study Investigators: RAAS blockade and renal disease in type 2 diabetic patients: an Asian perspective from the RENAAL Study. Diabetes Care 27:874–879, 2004 [DOI] [PubMed] [Google Scholar]