Abstract

The enantioselective borane reduction of O-benzyloxime ethers to primary amines was studied under catalytic conditions using the spiroborate esters 5 – 10 derived from non-racemic 1,2-amino alcohols and ethylene glycol. Effective catalytic conditions were achieved using only 10% of catalyst 5 derived from diphenylvalinol in dioxane at 0 °C resulting in complete conversion to the corresponding primary amine in up to 99% ee.
The asymmetric reduction of oxime ethers with non-racemic chiral reducing agents represents an important synthetic route to enantiopure primary amines.1–5 Over the past two decades, oxazaborolidines have been developed as chirality transfer reagents for the reduction of the carbonyl and imine functionality.2 The borane-mediated catalytic reduction of ketones using 1,3,2-oxazaborolidines has been extensively investigated.2b These efforts have led to the synthesis of highly enantiopure alcohols using less than 10 mol % of catalyst. Applying this process to the reduction of the C=N provides direct access to non-racemic primary amines which are widely used as key intermediaries in the synthesis of pharmaceuticals, chiral auxiliaries and catalysts.1–4 For the borane-mediated reduction of oxime ethers, a stoichiometric amount of the oxazaborolidine is usually required to obtain high enantioselectivities.3,4 Fontaine et al.3k even employed 2.5 equiv of the diphenylvalinol-derived B-H oxazaborolidine to achieve complete reduction with high selectivity. Itsuno and coworkers3b reported the first catalytic borane-based reduction of acetophenone O-benzyl oxime. With 10 mol% of the (S)-diphenylvalinol oxazaborolidine, generated in situ, 52% ee was observed in the product (S)-1-phenylethanamine. This selectivity is much lower than the 93% ee obtained for this substrate employing 1 equiv of the catalyst.
In addition to their high cost air- and moisture sensitivity, B-H oxazaborolidines often contain impurities which diminish their effectiveness.2c–e This has led to the development of alternative catalytic systems for the reduction of oximes, but with only modest success.5 In the present study, a truly catalytic and highly enantioselective process for the borane-mediated asymmetric reduction of oxime ethers is reported for the first time.
Recently, we prepared stable enantiopure spiroborate esters5–10 derived from 1,2-amino alcohols, as new catalysts for the asymmetric reduction of ketones (Fig. 1).6 These spiroborates proved to be highly reactive and enantioselective catalysts for this process. Coupled with their operational convenience, we felt that they may provide effective catalysts for reduction of oxime ethers thereby providing a new, highly useful entry to non-racemic amines (Scheme 1).7
Figure 1.

Spiroborate esters derived from non-racemic 1,2-amino alcohols
Scheme 1.
Initially, we examined the reduction of (E)-benzyl oxime ether 1a (R = Bn) in toluene employing 50 mol % of spiroborate ester 5 and 2 equiv of BH3·DMS at 50 °C for 12 h followed by 3 h at 110 °C. The amine product 2a was isolated as its carbamate 4 in 75% yield and 93% ee. Seeking milder conditions, we discovered that the reduction could be conducted at 25 ºC in THF solvent and these conditions were used with varying amounts and sources of borane and 5 to determine optimal conditions for the reduction. These results are presented in Table 1.
Table 1.
Spiroborate ester 5-catalyzed reduction of 1a with different borane sources in THF at 25 ºC.
| entry | cat. 5(mol %) | borane reagents | time(h) | 2a , 3 (%)a | eeb |
|---|---|---|---|---|---|
| 1 | 50 | 1BH3·DMS | 48 | 0, 60 | 94 |
| 2 | 50 | 2BH3·DMS | 36 | 15 , 85 | 96 |
| 3 | 50 | 4BH3·DMS c | 12 | 0, 100 | 93 |
| 4 | 25 | 2BH3·DMS | 48 | 17, 58 | 93 |
| 5 | 25 | 6 BH3·DMS | 12 | 0, 85 | 89 |
| 6 | 20 | 2.4BH3·THF d | 36 | 9, 91 | 89 |
| 7 | 10 | 2.4BH3·THF d | 36 | 16, 44 | 86 |
| 8 | 10 | 4BH3·THFd | 36 | 0, 100 (75)e | 87 |
| 9 | 10 | 4BH3·DEA | 36 | 0, 0 | - |
The product ratio was determined by GC analysis.
The ee was determined by GC analysis using a Crompack Chirasil-Dex-CB column.
The oxime 1a in THF was added in 10 h, then 38 h stirring at 25 ºC;
he borane reagent was stabilized with <0.005 M N-isopropyl N-methyl tert-butylamine. The yield in parenthesis was obtained after purification of ethoxy carbamide.
The complete conversion of 1a to 3 under these conditions was achieved by increasing the amount of BH3·DMS to 4 equiv (Table 1, entries 1–3) and using 50% of spiroborate 5. The reduction of 1a occurred faster and with complete conversion to primary amine 3. Decreasing 5 to 25% and using 6 equiv of BH3·DMS, the conversion was partial (85%, entry 5). Hence, we decided to use BH3·THF stabilized by N-isopropyl N-methyl tert-butylamine, which has been demonstrated to be more stable and selective in carbonyl reductions.8 Indeed, with 20% catalyst and using 2.4 equiv BH3·THF a nearly quantitative conversion to 3 was observed (91%, entry 6). Remarkably, with only 10% of catalyst and 4 equiv of BH3·THF, the reduction of oxime 1a afforded the primary amine 3 quantitatively with only a slight decrease in ee (87%, entry 10). It must be mentioned that BH3·DEA was unreactive in this oxime reduction.9
A variety of solvents, temperature and borane sources were screened for the reduction of 1a under the previous optimized conditions.10 In general, ethereal solvents lead to higher ee’s of the isolated product 4 with dioxane giving the best results (90% ee). The reaction temperature was also varied to further optimize the reaction conditions. At 0 °C, the reaction requires longer reaction times for the complete conversion to 3, but the enantioselectivity is higher (96.5% ee). The BH3·THF, stabilized with NaBH4, affords 3, then treated with ClC(O)OEt produced 4 in 95% ee at 25 ºC in dioxane. Importantly, this reagent gives 96.5% ee at 0 ºC, the same result as for the amine-stabilized BH3·THF. The BH3·DMS reagent proved to be a less effective borane source for this reduction. Moreover, the selectivity does not change with the addition time of the oxime ether.
The optimized reaction conditions were extended to the other spiroborate esters indicated in Figure 1. At 0 °C, the reactivity of catalysts 6–9 was rather low. Therefore, the reaction temperature was changed to 25 ºC, except for catalyst 10, whose reactivity was examined at 0 ºC. Spiroborate ester 5 derived from diphenylvalinol shows superior enantioselectivity compared to these other systems.10
We further extended these studies to include the methyl and various substituted O-benzyl derivatives of acetophenone oxime (4-MeO-C6H4CH2; 4-CF3C6H4CH2; 2-NO2 C6H4CH2).10 While these all give excellent selectivities, the 4-CF3 substituted benzyl oxime gives 4 in 99% ee.
Because similar high selectivities were observed with all of the O-benzylated acetophenone oximes, the simple (E)-benzyl oxime derivatives 11 of representative aryl alkyl ketones were prepared by standard methods and submitted to the optimized reductive conditions (0.1 equiv of 5, dioxane, 25 and 0 °C). The product amines were isolated as their N-acetyl derivatives 12. In general, the process gives excellent enantioselectivity (83–99%) at 0 °C. These results are summarized in Table 2.
Table 2.
Asymmetric Reduction of Representative Oxime Benzyl Ethers with 0.1 equiv of Catalyst 5
| 11 | 12a | temp(°C) | yield(%)b | eec(%) |
|---|---|---|---|---|

a |

|
25 | 81 | 79 |
| 0 | 89 | 83 | ||

b |

|
25 | 68 | 98 |
| 0 | 71 | 99 | ||

c |

|
25 | 84 | 78 |
| 0 | 90 | 85 | ||

d |

|
25 | 88 | 85 |
| 0 | 92 | 98 | ||

e |

|
25 | 85 | 66 |
| 0 | 91 | 93 | ||

f |

|
25 | 71 | 88 |
| 0 | 77 | 94 | ||

g |
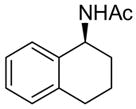
|
25 | 90 | 86 |
| 0 | 85 | 84 | ||

h |

|
25 | 68 | 89 |
| 0 | 77 | 97 |
The reactions were carried out using 1 equiv of oxime ether, 0.1 equiv of catalyst 5 and 4 equiv of borane stabilized with NaBH4 in dioxane for 36 h or until the conversion was 100%.
Purified by column chromatography.
The ee was determined using a Crompack Chirasil-Dex-CB GC column.
In summary, a highly efficient new borane-based catalytic process for the asymmetric synthesis of amines from oxime ethers has been discovered. The new process employs the easily prepared and stable spiroborate ester 5 as the chiral transfer agent. Employing simple procedures, this methodology provides a convenient entry to highly versatile and important non-racemic amines. Mechanistic studies and further applications of this new process are underway.
Supplementary Material
Experimental procedures, full characterization for all new compounds and 1H spectra and GC analysis for racemic and non-racemic acetamides. This material is free of charge via Internet at http://pubs.acs.org.
Acknowledgments
Financial support by the NIH through their MBRS (GM 08216) and IMBRE (NC P20 PR-016470) Grants is greatly appreciated. The NIH-IMBRE undergraduate support is also gratefully acknowledged.
References
- 1.(a) Johansson A. Contemp Org Synth. 1995;266:393–406. and ref. cited therein. [Google Scholar]; (b) Deloux L, Srebnik M. Chem Rev. 1993;93:763–784. [Google Scholar]
- 2.(a) Glushkov VA, Tolstikov AG. Russ Chem Rev (Engl Transl) 2004;73:581–608. and ref. cited therein. [Google Scholar]; (b) Cho BT. Tetrahedron. 2006;62:7621–7643. and ref. cited therein. [Google Scholar]; (c) Brown JM, Guy C, Lloyd-Jones GC, Layzell TP. Tetrahedron: Asymmetry. 1993;4:2151–2154. [Google Scholar]; (d) Lang A, Noth H, Schmidt M. Chem Ber. 1997;130:241–246. [Google Scholar]; (e) Mathre DJ, Thompson AS, Douglas AW, Carroll JD, Corley EG, Grabowski EJJ. J Org Chem. 1993;58:2880–2888. [Google Scholar]
- 3.(a) Itsuno S, Nakano M, Miyazaki K, Masuda H, Ito K. J Chem Soc Perkin Tran 1. 1985:2039–2044. [Google Scholar]; (b) Itsuno S, Sakurai Y, Ito K, Hirao A, Nakahama S. Bull Chem Soc Jpn. 1987;60:395–396. [Google Scholar]; (c) Itsuno S, Sakurai Y, Shimizu K, Ito K. J Chem Soc Perkin Trans 1. 1990:1859–1863. [Google Scholar]; (d) Bolm C, Felder M. Synlett. 1994:655–666. [Google Scholar]; (e) Cho BT, Ryu MH. Bull Korean Chem Soc. 1994;15:191–192. [Google Scholar]; (f) Lantos I, Flisak J, Liu L, Matsunoka R, Mendelson W, Stevenson D, Tubman K, Tucker L, Zhang WY, Adams J, Sorenson M, Garigipati R, Erhardt K, Ross S. J Org Chem. 1997;62:5385–5391. [Google Scholar]; (g) Demir AS. Pure Appl Chem. 1997;69:105–108. [Google Scholar]; (h) Inoue T, Sato D, Komura K, Itsuno S. Tetrahedron Lett. 1999;40:5379–5382. [Google Scholar]; (i) Itsuno S, Matsumoto T, Sato D, Inoue T. J Org Chem. 2000;65:5879–5881. doi: 10.1021/jo0007837. [DOI] [PubMed] [Google Scholar]; (j) Sailes HE, Watts JP, Whiting A. J Chem Soc Perkin Trans. 1;2000:3362–3374. [Google Scholar]; (k) Fontaine E, Namane C, Meneyrol J, Geslin M, Serva L, Roussey E, Tissandié S, Maftouh M, Roger P. Tetrahedron: Asymmetry. 2001;12:2185–2189. [Google Scholar]; (l) Krzeminski MP, Zaidlewicz M. Tetrahedron: Asymmetry. 2003;14:1463–1466. [Google Scholar]; (m) Sakito Y, Yoneyoshi Y, Suzukamo G. Tetrahedron Lett. 1988;29:223–224. [Google Scholar]
- 4.(a) Tillyer RD, Boudreau C, Tschaen D, Dolling U-H, Reider PJ. Tetrahedron Lett. 1995;36:4337–4340. [Google Scholar]; (b) Shimizu M, Kamei M, Fujisawa T. Tetrahedron Lett. 1995;36:8607–8610. [Google Scholar]; (c) Shimizu M, Tsukamoto K, Matsutani T, Fujisawa T. Tetrahedron. 1998;54:10265–10274. [Google Scholar]; (d) Masui M, Shioiri T. Tetrahedron Lett. 1998;39:5195–5198. [Google Scholar]
- 5.Chu YB, Shan ZX, Liu DJ, Sun NN. J Org Chem. 2006;71:3998–4001. doi: 10.1021/jo060123n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Stepanenko V, Ortiz-Marciales M, Correa W, De-Jesús M, Espinosa S, Ortiz L. Tetrahedron: Asymmetry. 2006;17:112–115. [Google Scholar]; (b) Ortiz-Marciales M, Stepanenko V, Correa W, De Jesús M, Espinosa S. 11/512,599. U S Patent Application. 2006 Aug. 30;
- 7.Ortiz-Marciales M, Huang X, Stepanenko V, De Jesús M, Merced FG. 60/841,147. Provisional U S Patent Application. 2006 Aug. 30;
- 8.See: Synthetic methods: Reduction, Aldrich Chemfiles. 2005;5:4.Josyula KVB, Potyen M, Gao P, Hewitt C. Patent application pending publication
- 9.Chung JYL, Cvetovich R, Amato J, McWilliams JC, Reamer R, DiMichele L. J Org Chem. 2005;70:3592–3601. doi: 10.1021/jo050178+. [DOI] [PubMed] [Google Scholar]
- 10.The details of the results obtained using different solvents, sources of borane, temperature, mode of addition, catalysts and oxime substituents are included in the Supporting Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, full characterization for all new compounds and 1H spectra and GC analysis for racemic and non-racemic acetamides. This material is free of charge via Internet at http://pubs.acs.org.



