Abstract
Craniofacial tissue engineering promises the regeneration or de novo formation of dental, oral, and craniofacial structures lost to congenital anomalies, trauma, and diseases. Virtually all craniofacial structures are derivatives of mesenchymal cells. Mesenchymal stem cells are the offspring of mesenchymal cells following asymmetrical division, and reside in various craniofacial structures in the adult. Cells with characteristics of adult stem cells have been isolated from the dental pulp, the deciduous tooth, and the periodontium. Several craniofacial structures—such as the mandibular condyle, calvarial bone, cranial suture, and subcutaneous adipose tissue—have been engineered from mesenchymal stem cells, growth factor, and/or gene therapy approaches. As a departure from the reliance of current clinical practice on durable materials such as amalgam, composites, and metallic alloys, biological therapies utilize mesenchymal stem cells, delivered or internally recruited, to generate craniofacial structures in temporary scaffolding biomaterials. Craniofacial tissue engineering is likely to be realized in the foreseeable future, and represents an opportunity that dentistry cannot afford to miss.
Keywords: stem cells, tissue engineering, biomaterials, wound healing, regenerative medicine
(1) INTRODUCTION
The majority of craniofacial structures derive from mesenchymal cells (MCs). During development, MCs originating from the neural crest are known to migrate, differentiate, and subsequently participate in the morphogenesis of virtually all craniofacial structures, such as cartilage, bone, ligaments, cranial sutures, musculature, tendons, the periodontium, and the teeth. Once migrated, MCs work synergistically with mesodermal cells in the morphogenesis of craniofacial structures. Both mesenchymal cells and mesodermal cells are derivatives of embryonic stem cells, a few hundred cells of the inner cell mass of the blastocyst.
Mesenchymal cells undergo asymmetric division, with one offspring cell differentiating toward an end-stage cell, while the other replicates into an offspring mesenchymal cell. Residual offspring of mesenchymal cells, upon the completion of morphogenesis, continue to reside in various craniofacial tissues, and retain their status as stem cells. After birth, mesenchymal cells are called ‘mesenchymal stem cells’ (MSCs). In the adult, MSCs maintain physiologically necessary tissue turnover and, upon injury or disease, differentiate to launch tissue regeneration.
MSCs have been experimentally differentiated into all mesenchymal or connective tissue lineages (Caplan, 1991; Pittenger et al., 1999) and, in many cases, have been used to engineer the craniofacial structures that their prenatal predecessors, mesenchymal cells, are capable of generating during development. The capacity of MSCs in the de novo formation and/or regeneration of craniofacial structures is too natural an endeavor to make one wonder why this potential has not been exploited substantially up until the past decade. As it turns out, our ability to grow human craniofacial tissues and organs is by no means a small task. Engineering craniofacial structures from stem cells was an insurmountable effort until advances from several seemingly unrelated disciplines—such as cell and molecular biology, polymer chemistry, molecular genetics, materials science, robotics, and mechanical engineering—converged into the self-assembling field of tissue engineering (Nerem, 1992; Langer and Vacanti, 1993). To engineer a functional biological structure, cells must be instructed to differentiate and receive positional cues, and to synthesize the appropriate extracellular matrix molecules in the overall shape and dimensions of the diseased or missing tissues/organs. Biomimetic scaffolds are frequently needed to enable cell growth and differentiation to occur in an environment that has been previously unfamiliar to either biologists or engineers. Craniofacial structures offer complex and, in many cases, unique challenges when they are being engineered.
Large-scale tissue-engineering research, craniofacial or otherwise, began to take place in the early 1990s and has grown exponentially ever since. The first overview of craniofacial tissue engineering appeared within the past few years and focused on primarily tissue-engineering principles (Hollinger and Winn, 1999; Alsberg et al., 2001). As outlined below, substantial advances have been made in craniofacial tissue engineering:
Stem cells have been isolated from several craniofacial tissues, with ongoing effort to purify and apply them in the tissue engineering of craniofacial structures.
Several prototypes of the human-shaped temporomandibular joint condyle have been engineered with integrated cartilage and bone layers from a single population of mesenchymal stem cells.
Various elements of the periodontium, including the periodontal ligament and cementum, have been engineered via cell-based or non-cell-based approaches.
Craniofacial bone has been engineered from stem cells, growth factors, and/or biomaterials. A cranial suture-like structure has been engineered from cell- and growth-factor-based approaches.
Adipose tissue has been engineered in vivo from mesenchymal stem cells, with potential applications in facial plastic and reconstructive surgeries.
The tissue-engineered craniofacial structures to date are undoubtedly prototypes that warrant further development and refinements, but nonetheless were not even available a few years ago (Hollinger and Winn, 1999; Alsberg et al., 2001). Despite formidable challenges, this review offers the optimistic view that functional craniofacial tissues and organs can be grown via in vitro and/or in vivo approaches for ultimate therapeutic applications. This review is designed not only to serve as a timely and comprehensive synthesis of our current knowledge of craniofacial tissue engineering, but also to identify immediate challenges in this dynamic field. Craniofacial tissue engineering, like tissue engineering in general, is an interdisciplinary field by nature. A review such as this cannot possibly include some of the necessary technical details of those more individualized fields that have converged to form the foundation of craniofacial tissue engineering. The reader is referred to several recent in-depth reviews in the individual fields of stem cell biology, biomaterials, cell and molecular biology and genetics, and regenerative medicine (Drury and Mooney, 2003; Alhadlaq and Mao, 2004; Patel and Mikos, 2004; Atala, 2005: Gregory et al., 2005; Hollister, 2005; Kyba, 2005; Mao, 2005a; Rahaman and Mao, 2005; Shi et al., 2005).
(2) MESENCHYMAL STEM CELLS AND CRANIOFACIAL-DERIVED STEM CELLS
A stem cell is self-renewable and capable of differentiating into at least two distinctive cell types (Parker et al., 2004). These two properties must both be satisfied for a cell to be defined as a stem cell. Self-renewal denotes that undifferentiated daughter cells are a precise replica and can further replicate many generations without losing their original characteristics (Caplan, 1991; Alhadlaq and Mao, 2004). Cells of an immortalized cell line can replicate many generations, but are generally incapable of multi-lineage differentiation. Thus, cell lines are not stem cells. Multi-lineage differentiation refers to the capacity of a single population of stem cells to differentiate into at least two distinctively different cell types (Caplan, 1991; Parker et al., 2004). For example, a single population of MSCs can differentiate into both osteoblasts and chondrocytes. Pre-osteoblasts, a term frequently used in bone and dental literature, can differentiate into osteoblasts, but are incapable of differentiating into other mesenchymal lineages, such as chondrocytes or adipocytes, at least not without undergoing de-differentiation back toward MSCs (Caplan, 1991). Thus, pre-osteoblasts are not stem cells. It is convenient and often necessary to define a progenitor cell as one that is between a stem cell and an end-stage cell, although there are likely many unnamed cell differentiation stages between a stem cell and a progenitor cell, and then between a progenitor cell and an end-stage cell. For example, a pre-osteoblast is a progenitor cell between an MSC and an osteoblast or osteocyte.
(2.1) Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are self-renewable and can differentiate into all cell lineages that form mesenchymal and connective tissues (Conget and Minguell, 1999; Caplan, 1991; Pittenger et al., 1999; Alhadlaq and Mao, 2004). In addition, MSCs have been reported to differentiate into hepatic (Petersen et al., 1999), renal (Poulsom et al., 2003), cardiac (Orlic et al., 2001), and neural cells (Brazelton et al., 2000; Mezey et al., 2000).
The first successful isolation of bone marrow MSCs, then called colony-forming fibroblast-like cells, was described almost 4 decades ago (Friedenstein et al., 1970). The isolation method was based on the adherence of marrow-derived MSCs to the plastic substrate of the cell culture plates, and a concomitant lack of adherence of marrow-derived hematopoietic stem cells. To date, this straightforward protocol is widely used for the isolation of MSCs in multiple vertebrate species (Alhadlaq and Mao, 2004).
Previous approaches, utilizing flow cytometry, have investigated the isolation of increasingly homogenous populations of MSCs based on differential cellular features within the bone marrow stroma. Further purification and cloning of MSCs are desirable for stem cell biology, similar to the development of cell lines. Recently, several relatively straightforward MSC culture protocols have been developed. Size-dependent sieving of a cell population from human bone marrow aspirates through a porous membrane resulted in a relatively homogeneous population that had the capacity of self-renewal and multi-lineage differentiation (Hung et al., 2002). Positive selection of MSCs with microbeads, combined with fluorescence-activated cell-sorting (FACS) (Jones et al., 2002) or magnetic-activated cell-sorting (MACS) (Kinnaird et al., 2004), is an effective technique for the increasingly defined isolation and precise characterization of MSCs (Reese et al., 1999; Lee et al., 2001; Arbab et al., 2004).
Whether highly purified or cloned MSC populations are necessarily needed for the engineering of craniofacial structures is not clear. First, stem cell populations that generate native craniofacial structures, such as the mandibular joint, are heterogeneous and likely include both mesenchymal and hematopoietic stem cells. The morphogenesis of the articular condyle requires stem cells, chondrocytes, and osteoblasts in addition to angiogenesis (Mao, 2005a). Second, host cell invasion and stem cell homing are likely inevitable in porous biomimetic scaffolds that are used as carriers for delivering stem cells and/or stem-cell-derived tissue-forming cells (Christopherson et al., 2004; Hidalgo and Frenette, 2005).
(2.2) Dental Pulp Stem Cells
Although the regenerative capacity of the human dentin/pulp complex is not well-understood, it is known that, upon injury, reparative dentin is formed as a protective barrier for the pulp (Murray et al., 2001). Accordingly, one might anticipate that dental pulp contains the dentinogenic progenitors that are responsible for dentin repair. Previous work has shown that dental pulp contains proliferating cells that are analogous to bone cells, because they express osteogenic markers and respond to many growth factors for osteo/odontogenic differentiation (Hanks et al., 1998; Unda et al., 2000; Ueno et al., 2001). In addition, dental pulp cells are capable of forming mineral deposits with distinctive dentin-like crystalline structures (About et al., 2000; Couble et al., 2000; Gronthos et al., 2000). Recently, dental pulp stem cells (DPSCs) have been isolated from extracted human third molars (Gronthos et al., 2000; Shi et al., 2001).
To determine the colony-forming efficiency of the isolated DPSCs, investigators have prepared single-cell suspensions by collagenase/dispase treatment of pulp fragments filtered through a fine mesh to remove cell aggregates, and seeded at low plating densities (Gronthos et al., 2000). Approximately 40 single-colony clusters can be retrieved from 10,000 cells in culture (Gronthos et al., 2000, 2003). The clones from the initial primary culture demonstrated variable capacities for forming dentin-like structures following in vivo implantation (Shi and Gronthos, 2003). DPSCs showed a higher proliferation rate than bone-marrow-derived MSCs under the same culture conditions, potentially attributable to strong expression of cyclin-dependent kinase 6, a cell-cycle activator (Shi et al., 2001). Expression of various perivascular markers—such as STRO-1, VCAM-1, MUC-18, and α-smooth-muscle actin—provides clues that DPSCs are a heterogeneous population of MSCs and likely located in the perivascular niche in the pulp (Gronthos et al., 2000; Shi and Gronthos, 2003).
To elucidate the self-renewal ability, investigators isolated cells from DPSC implants at two months post-subcutaneous implantation, by enzymatic digestion and subsequent expansion in vitro. The isolated cells from the DPSC implants were sorted by fluorescent-activated cell-sorting (FACS) with human beta1-integrin monoclonal antibody. The isolated human cells were re-implanted into immunodeficient mice for two months. The recovered secondary implants yielded the same dentin/pulp-like structures as the primary implants. Human dentin sialophosphoprotein (DSPP) was expressed in dentin-like structures, confirming the human origin of the odontoblast/pulp cells in the secondary DPSC implants (Batouli et al., 2003).
So that their multi-lineage differentiation capacity could be determined, DPSCs were cultured in adipogenic inductive agents. In a few weeks, DPSCs differentiated into adipocyte-like cells that were positive to Oil red O, and expressed PPARγ2 and lipoprotein lipase (LPL) (Batouli et al., 2003). Furthermore, DPSCs were shown to differentiate into neuron-like and glial-like cells by expressing both nestin, an early marker of neural precursor cells, and glial fibrillary acid protein (GFAP), an antigen characteristic of glial cells (Gronthos et al., 2000). These DPSC-derived cells developed long cytoplasmic processes, a departure from their usual bipolar fibroblastic appearance.
(2.3) Stem Cells from Human Exfoliated Deciduous Teeth (SHED)
The exfoliated deciduous tooth houses living pulp remnants consisting of connective tissue, blood vessels, and odontoblasts. We found that from 12 to 20 cells from each exfoliated incisor formed adherent colony clusters with extensive proliferative capacity (Miura et al., 2003). Ex vivo-expanded SHED expressed STRO-1 and CD146 (MUC18), two early cell-surface markers for bone-marrow-derived MSCs (Shi and Gronthos, 2003). In addition, SHED expressed a variety of osteoblast/odontoblastic markers, including Runx2, alkaline phosphatase (ALP), matrix extracellular phosphoglycoprotein (MEPE), bone sialoprotein (BSP), and DSPP. After implantation into immunocompromised mice, with hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier, SHED differentiated into odontoblast-like cells that formed small dentin-like structures (Miura et al., 2003). These results suggest that SHEDs are distinctive from DSPCs with respect to odontogenic differentiation and osteogenic induction (Miura et al., 2003).
(2.4) Periodontal Ligament Stem Cells (PDLSCs)
The periodontal ligament (PDL) connects the cementum to alveolar bone, and functions primarily to support the tooth in the alveolar socket. A recent report identified stem cells in human PDL (PDLSCs) and found that PDLSCs implanted into nude mice generated cementum/PDL-like structures that resemble the native PDL as a thin layer of cementum that interfaced with dense collagen fibers, similar to Sharpey’s fibers (Seo et al., 2004). After a three-week culture with an adipogenic-inductive cocktail, PDLSCs differentiated into Oil-red-O-positive, lipid-laden adipocytes (Seo et al., 2004). Upon four-week osteo/odontogenic inductions, alizarin-red-positive nodules formed in the PDLSC cultures, similar to MSCs and DPSCs (Shi et al., 2002; Seo et al., 2004). Thus, the PDLSCs have the potential for forming periodontal structures, including the cementum and PDL.
(2.5) Challenges in Stem Cell Biology Related to Craniofacial Tissue Engineering
The relationship between bone marrow MSCs and the newly identified stem cells from various craniofacial tissues needs to be defined. In many ways, the newly characterized craniofacial stem cells resemble bone marrow MSCs, especially in terms of their differentiation capacities (Shi et al., 2005).
Whether craniofacial-derived MSCs more effectively regenerate craniofacial structures than do appendicular MSCs needs to be explored. Whether craniofacial-derived MSCs are capable of healing non-craniofacial defects more effectively than are appendicular MSCs also warrants investigation.
How mechanical stress modulates craniofacial morphogenesis and regeneration needs to be further explored (Carter et al., 1998; Mao, 2005b).
The extent to which tissue engineering should mimic or recapitulate the corresponding developmental events needs to be determined (Ferguson et al., 1998).
(3) TISSUE ENGINEERING OF THE TEMPOROMANDIBULAR JOINT FROM STEM CELLS
Temporomandibular disorders (TMD) manifest as pain, myalgia, headaches, and structural destruction, collectively known as degenerative joint disease (Okeson, 1996). The temporomandibular joint (TMJ), like other synovial joints, is also prone to rheumatoid arthritis, injuries, and congenital anomalies (Stohler, 1999). The severe form of TMJ disorders necessitates surgical replacement of the mandibular condyle (Sarnat and Laskin, 1992).
In the past few years, we have reported the tissue engineering of a mandibular condyle exhibiting the shape and dimensions of a human cadaver TMJ. The engineered mandibular condyle had stratified layers of cartilage and bone from a single population of bone-marrow-derived MSCs, and was molded into the shape and dimensions of a human cadaver mandibular condyle at 11 × 7 × 9 mm (l × w × h) (Alhadlaq and Mao, 2003, 2005; Alhadlaq et al., 2004; Mao, 2005b).
(3.1) Cell Survival and Matrix Synthesis in the Tissue-engineered Mandibular Condyle
MSCs were isolated from femoral and tibial bone marrows of adult rats and exposed separately to either chondrogenic or osteogenic supplemented culture medium (Alhadlaq and Mao, 2003; Alhadlaq et al., 2004). Poly(ethylene glycol) diacrylate (PEGDA) was dissolved in PBS with a biocompatible ultraviolet photoinitiator (Alhadlaq and Mao, 2003; Alhadlaq et al., 2004). MSC-derived chondrogenic and osteogenic cells were encapsulated in PEGDA hydrogel into a negative mold of an adult human cadaver mandibular condyle in two stratified and yet integrated layers. The photopolymerized osteochondral construct was implanted into the dorsum of immunodeficient mice for up to 12 wks.
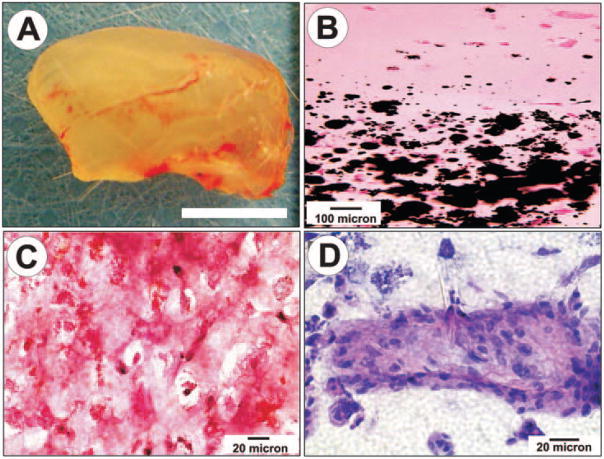
De novo formation of a structure having the same shape and dimensions as the cadaver human mandibular condyle was observed after 4 wks of in vivo implantation (Fig. 1). The tissue-engineered mandibular joint condyles retained the macroscopic shape and dimensions of the cadaver mandibular condyle (Fig. 1A). The interface between the upper-layer PEGDA hydrogel encapsulating MSC-derived chondrogenic cells and the lower-layer PEGDA hydrogel encapsulating MSC-derived osteogenic cells demonstrated distinctive microscopic characteristics (Figs. 1B–1D). The chondrogenic and osteogenic portions remained in their respective layers (cf. interface in Fig. 1B). The chondrogenic layer contained sparse chondrocyte-like cells surrounded by abundant intercellular matrix (Fig. 1C). The intercellular matrix of the chondrogenic layer showed strong, intense staining with safranin O (Fig. 1C), a cationic marker that binds to cartilage-related glycosaminoglycans (GAG), such as chondroitin sulfate and keratan sulfate. Some of the MSC-derived chondrogenic cells were surrounded by pericellular matrix, characteristic of native chondrocytes (Fig. 1C). In contrast, the osteogenic layer contained mineral nodules, as revealed by von Kossa staining (lower portion of Fig. 1B). The osteogenic layer also showed multiple island structures occupied by osteoblast-like cells (Fig. 1D).
Figure 1.
Engineered neogenesis of human-shaped mandibular condyle from mesenchymal stem cells. (A) Harvested osteochondral construct retained the shape and dimension of the cadaver human mandibular condyle after in vivo implantation. Scale bar: 5 mm. (B) Von Kossa-stained section showing the interface between stratified chondral and osseous layers. Multiple mineralization nodules are present in the osseous layer (lower half of the photomicrograph), but absent in the chondral layer. (C) Positive safranin O staining of the chondrogenic layer indicates the synthesis of abundant glycosaminoglycans. (D) H&E-stained section of the osteogenic layer showing a representative osseous island-like structure consisting of MSC-differentiated osteoblast-like cells on the surface and in the center. Reproduced with permission from Biomedical Engineering Society.
(3.2) Cell Density Matters in the Tissue Engineering of Mandibular Condyle
A fundamental question posed above is to what extent the events of development should be recapitulated in tissue engineering. The following is an example that provides some clues to this question. A dissatisfactory feature in our previous work to engineer a human-shaped mandibular joint condyle (Alhadlaq and Mao, 2003; Alhadlaq et al., 2004) was suboptimal tissue maturation. The straight line of osteochondral junction in the engineered synovial joint condyle (Fig. 1B) did not resemble the mutual infiltration of chondral and osseous tissues in the native osteochondral junction. We then attempted to improve tissue maturation and osteochondral integration by increasing the cell encapsulation density from the previous 5 × 10 6 cells/mL to 20 × 106 cells/mL.
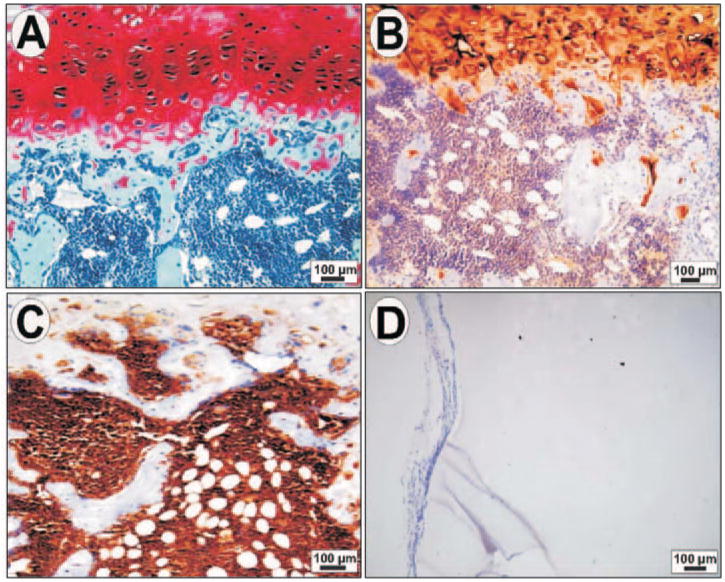
At a density of 20 × 106 cells/mL, the tissue-engineered mandibular joint condyle again retained the pre-defined shape and dimensions of the human cadaver mandibular condyle (Alhadlaq and Mao, 2005). The cartilage layer was positively stained by safranin O, indicating the presence of cartilage-related glycosaminoglycans (Fig. 2A), and contained type II collagen (Fig. 2B). The deep portion of the cartilage layer, near the tissue-engineered osteochondral interface, contained chondrocyte-like cells with a hypertrophic appearance and characterized by the expression of type X collagen (data not shown, but cf. Alhadlaq and Mao, 2005), a marker for hypertrophic and degenerating chondrocytes. In contrast, the osseous layer demonstrated immunolocalization of bone markers such as osteopontin (Fig. 2C) and osteonectin (cf. Alhadlaq and Mao, 2005). The chondrogenic layer lacked immunolocalization of bone markers, whereas the osseous layer lacked immunolocalization of cartilaginous markers (Figs. 2A–2C). There was mutual infiltration of the cartilaginous and osseous components into each other’s territory (Figs. 2A, 2B). This mutual infiltration of chondral and osseous tissues, absent in our previous work, where we used a four-fold-lower cell encapsulation density (Alhadlaq and Mao, 2003; Alhadlaq et al., 2004), resembles the osteochondral interface in age-matched native rat mandibular condyle (Alhadlaq and Mao, 2005). The cell-free PEGDA hydrogel scaffold showed an intact border surrounded by fibrous capsule without host cell invasion (Fig. 2D), further substantiating the conclusion that the engineered mandibular joint condyle is formed solely by MSC-derived chondrogenic cells and osteogenic cells, rather than by host cells.
Figure 2.
Histologic and immunohistochemical characterization of a human-shaped mandibular condyle engineered from mesenchymal stem cells after in vivo implantation. (A) Representative photomicrograph showing positive safranin O staining of the upper cartilage layer, indicating the presence of abundant glycosaminoglycans. In contrast, the osseous portion shows negative safranin O staining. (B) Positive immunohistochemical localization of type II collagen in the cartilage portion. The osseous portion was negative to type II collagen immunolocalization. (C) Positive immunolocalization of osteopontin within the osseous portion. By contrast, the cartilage portion lacks osteopontin expression. (D) Representative micrograph of hydrogel control cell-free construct showing host fibrous-tissue capsule surrounding the construct, but a lack of host cell invasion. Reproduced with permission from Mary Ann Liebert.
(3.3) Functional in vivo Integration of the Engineered Mandibular Condyle
Ongoing research has begun to address the implantation of an engineered mandibular construct into a functional load-bearing model. Since both mass transport and mechanical properties depend upon 3D scaffold architecture, computational design techniques are needed to predict and ultimately optimize a microstructure to achieve the desired balance (Hollister, 2005). Architectural 3D scaffold design with the desired anatomical shape can be produced from image-based (Hollister et al., 2000, 2002) or computer-aided-design (CAD)-based approaches (Hutmacher et al., 2004). Scaffolds from these design approaches can then be built directly or indirectly by Solid Free-Form Fabrication (SFF) (Hutmacher et al., 2004; Yeong et al., 2004; Hollister, 2005), and have been applied in craniofacial reconstruction (Rohner et al., 2003; Hollister et al., 2005).
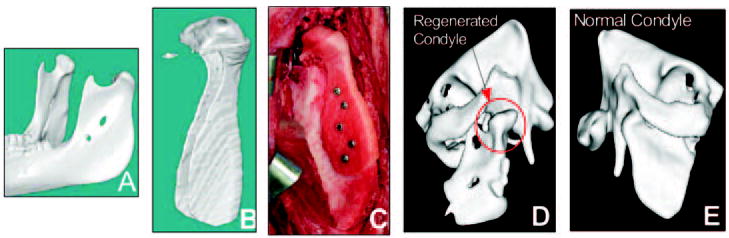
The ultimate goal is to use integrated design/fabricated scaffolds for functional mandibular condyle and other craniofacial reconstruction. Integrated design/fabrication methods not only make functional reconstruction possible, but also permit the testing of design hypotheses concerning scaffold/cell carrier combinations, eventually leading to optimal reconstruction methods. Our group has begun to test anatomically designed scaffolds for mandibular reconstruction in a Yucatan minipig model. We designed and fabricated a mandibular condyle scaffold directly from a minipig mandibular CT scan. The initial design was a shell into which autologous bone marrow was packed at the time of surgery (Figs. 3A–3C). Initial results at 1 and 3 months showed that the minipigs masticated normally, as documented by video, and, furthermore, that bone regenerated in the desired condyle shape, as shown by CT (Figs. 3D, 3E). Specimens will be processed for histology so that cartilage formation can be assessed. These results demonstrate that the shell scaffold design could support functional load-bearing as well as tissue regeneration. Further work will investigate how scaffold design, material, and biologic/carrier combinations affect regeneration.
Figure 3.
Design and engineering of minipig mandibular condyle. (A) Original Computed Tomography (CT) scan of minipig mandible. (B) Image-based design of condyle scaffold. (C) PCL (polycaprolactone) degradable polymer scaffold fabricated with SLS (Selective Laser Sintering) attached to the ramus. (D) Regrowth of condyle following 3 months’ implantation (new condyle shown in red circle). (E) Comparison with normal condyle from contralateral side in Yucatan minipig.
(3.4) Challenges of the Tissue Engineering of the Temporomandibular Joint
To promote matrix synthesis and tissue maturation of stem-cell-derived chondrogenic and osteogenic cells encapsulated in biocompatible and bioactive scaffolds. It may be necessary to incorporate an array of growth factors and/or transcription factors separately for tissue-engineered chondrogenesis and osteogenesis.
To enhance the mechanical properties of a tissue-engineered mandibular condyle for ultimate in situ implantation into the human temporomandibular joint.
To facilitate the remodeling potential of a tissue-engineered mandibular condyle.
(4) PERIODONTAL TISSUE ENGINEERING
A major goal in the reconstruction of periodontal bone defects is the simultaneous restoration of cementum, periodontal ligament, and alveolar bone structures in the face of the microbial assault and altered host immune response. Current periodontal therapies—such as tissue-banked bone allografts, cell-occlusive barriers, or enamel matrix proteins—result in only limited bone regeneration (radiographically < 50% and < 20% for vertical and horizontal bony defects, respectively) (Giannobile and Somerman, 2003; Murphy and Gunsolley, 2003; Reynolds et al., 2003). Despite these constraints, the field of periodontal tissue engineering has witnessed several major incremental advances, as discussed below.
(4.1) Microbial Influences on Periodontal Tissue Engineering
Periodontal infection is initiated by invasive oral pathogens that colonize dental plaque biofilms on the tooth root surface. This chronic challenge of virulent micro-organisms leads to the destruction of tooth-supporting soft and hard tissues, including alveolar bone, tooth root cementum, and the PDL. Thus, tissue-engineered constructs for periodontal reconstruction will likely face constant exposure to the oral bacterial flora, which contains 106–107 colony-forming units per milliliter (CFU/mL) of saliva (Motisuki et al., 2005). Even in the healthy oral cavity, the mucosal surfaces are colonized by approximately 90% anaerobic and 10% aerobic bacteria (Weber, 1997; Callender, 1999). Although the incidence of infection in oral surgical procedures is between 1 and 5% (Loukota, 1991), the consequences will, at least conceptually, compromise the outcome of periodontal tissue engineering.
(4.2) Growth and Amelogenin Factors in Periodontal Regeneration
Over the past decade, numerous polypeptide growth and amelogenin-like factors have been used in the repair of periodontal defects. Pre-clinical investigations have revealed encouraging results with respect to the repair of periodontal defects with growth factors such as platelet-derived growth factor (PDGF) (Lynch et al., 1991; Rutherford et al., 1992; Giannobile et al., 1994; Giannobile, 1996), bone morphogenetic proteins (BMPs) (Sigurdsson et al., 1995; Giannobile et al., 1998), and fibroblast growth factor-2 (Rossa et al., 2000; Takayama et al., 2001; Nakahara et al., 2003). PDGF has demonstrated the promise to regenerate bone in periodontal osseous defects, as shown in human clinical trials when used alone (Camelo et al., 2003; Nevins et al., 2003, 2005) or in combination with IGF-1 (Howell et al., 1997b). BMPs have been shown to induce bone regeneration in peri-implant and maxillary sinus-floor augmentation procedures (Boyne, 1996; Boyne et al., 1997; Hanisch et al., 1997), extraction socket defects (Howell et al., 1997a; Cochran et al., 2000; Fiorellini et al., 2005), and mandibular discontinuity defects (Boyne et al., 1999; Warnke et al., 2004). The use of BMPs for oral and craniofacial reconstruction has recently been reviewed (Nakashima and Reddi, 2003; Wikesjö et al., 2005).
Enamel matrix derivative (EMD) appears to promote periodontal regeneration by mimicking corresponding developmental events. EMD is composed of amelogenins with metallo-endoprotease and serine protease activity. Although their roles in epithelial-mesenchymal interactions remain to be clarified, EMDs have been shown to promote periodontal regeneration in multiple controlled human clinical trials (Heijl et al., 1997; Zetterström et al., 1997; Silvestri et al., 2000; Tonetti et al., 2002). In addition to EMD, a product that utilizes a biomimetic RGD cell-binding peptide fused to anorganic bone matrix has demonstrated efficacy in promoting periodontal bone repair in human clinical trials (Yukna et al., 2000, 2002).
(4.3) Gene Delivery in Periodontal Tissue Engineering
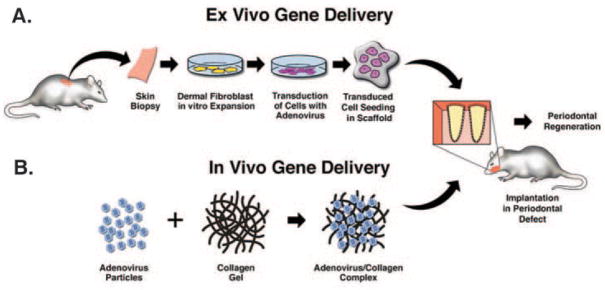
Gene transfer offers significant potential to improve growth factor delivery to tooth-supporting defects (Baum et al., 2002). Gene therapy has demonstrated strong potential to target, deliver, and improve the bioavailability of growth factors (such as BMPs and PDGFs) to stimulate the tissue engineering of periodontal defects (Fig. 4). Delivery of PDGF by gene transfer stimulates remarkable mitogenesis and proliferation of gingival fibroblasts, PDL, and cementoblasts, in comparison with continuous PDGF administration in vitro (Zhu et al., 2001; Chen and Giannobile, 2002; Jin et al., 2004a). Adenovirus-mediated PDGF-B gene transfer accelerated gingival wound healing in an ex vivo wound repair model (Anusaksathien et al., 2003). Surgically created alveolar bone wounds treated with AdPDGF-B revealed significant regeneration of cementum and PDL, along with extended growth factor expression, as demonstrated by in vivo biodistribution (Jin et al., 2004a). Application of a dominant-negative mutant of the PDGF-A gene (PDGF-1308) directly to periodontal osseous defects or to cementoblasts transplanted ex vivo in polymer scaffolds led to impaired periodontal regeneration (Anusaksathien et al., 2004; Jin et al., 2004b). BMP viral gene delivery has showed remarkable potential in the regeneration of both long bones and craniofacial bones (Lieberman et al., 1998, 1999; Alden et al., 2000; Baltzer et al., 2000; Krebsbach et al., 2000; Shen et al., 2004a, b; Dunn et al., 2005). Ex vivo gene delivery of BMP7-transduced fibroblasts has regenerated alveolar bone and cementum, whereas local knock-out of BMP bioactivity by Noggin, a BMP-antagonist, blocks cementogenesis of tissue-engineered cementum (Jin et al., 2003a, 2004b).
Figure 4.
Delivery approaches for periodontal bioengineering. Ex vivo gene therapy involves the harvesting of tissue biopsies, expansion of cell populations, genetic manipulations of cells, and subsequent transplantation to periodontal osseous defects (A), while the in vivo gene transfer approach involves the direct delivery of growth factor transgenes to the periodontal osseous defects (B).
(4.4) Cell-based Therapies in Periodontal Tissue Engineering
The transplantation of periodontal stem cells or their derivatives has been examined for their potential to reconstruct periodontal tissue defects (for reviews, see Thesleff and Tummers, 2003; Risbud and Shapiro, 2005). Transplantation of cells derived from the periodontal ligament has showed the potential to regenerate periodontal attachment structures in vivo (Lekic et al., 2001; Dogan et al., 2003; Nakahara et al., 2004; Akizuki et al., 2005; Hasegawa et al., 2005). Cementoblasts or tooth-lining cells have a marked ability to induce mineralization in an ex vivo model (Jin et al., 2003b) and in vivo in periodontal wounds (Zhao et al., 2004). However, when less-differentiated dental follicle cells are delivered in a similar fashion, these cells inhibit periodontal healing (Zhao et al., 2004). As described above, periodontal stem cells promote the formation of cementum-like mineralized tissue ex vivo (Seo et al., 2004; Trubiani et al., 2005). Allogeneic foreskin fibroblasts have recently been shown to promote new attachment of gingival recession defects (McGuire and Nunn, 2005).
(4.5) Challenges of Periodontal Tissue Engineering
A major unmet challenge is the modulation of the exuberant host response to microbial contamination that plagues the periodontal wound. Dual delivery of host modifiers and anti-infective agents is likely necessary to optimize periodontal regeneration.
The necessary interactions of multiple cell lineages should be clarified. These include cementogenic cells, fibroblasts, and osteogenic cells.
Despite the important role of exogenously delivered cells in the regeneration of severe periodontal defects, it is advantageous to attract endogenous periodontal tissue-forming cells by growth and/or trophic factors.
(5) TISSUE ENGINEERING OF CRANIOFACIAL BONE
Current surgical approaches for reconstructing craniofacial defects include autogenous bone grafts, allogeneic materials, and prosthetic compounds such as metals and plastics (Marchac, 1982; Shenaq, 1988; Goodrich et al., 1992; Nicholson, 1998; Rah, 2000; Bruens et al., 2003; Cowan et al., 2004). Despite certain levels of clinical success, each of these strategies has limitations. For example, autogenous bone grafts, the gold standard for craniofacial bony reconstruction, necessitate donor site morbidity (Silber et al., 2003). Prosthetic materials carry the risk of loosening and infection (Rah, 2000). Distraction osteogenesis, while increasingly utilized as endogenous bone tissue engineering, also has complications in upward of 35% cases, such as pin-tract and soft-tissue infections, scarring, device failure, and non-union (McCarthy et al., 1992; Mofid et al., 2001). Accordingly, stem-cell-based strategies for craniofacial reconstruction may overcome these deficiencies.
(5.1) Adipose-derived Stem Cells Heal Calvarial Defects
Adipose-derived mesenchymal cells (AMCs) are readily obtained via lipo-aspirate, and can be expanded in culture (Zuk et al., 2001, 2002; De Ugarte et al., 2003). Several groups have reported that AMCs are multipotent and capable of differentiation into muscle, bone, and cartilage cells (Zuk et al., 2001, 2002; Gimble and Guilak, 2003; Hicok et al., 2004). Bone tissue engineering is perhaps the most widely investigated application of AMCs. For example, rat AMCs seeded in polyglycolic acid scaffolds and implanted subcutaneously have led to bone formation (Lee et al., 2003). Similar findings have been noted with human AMCs using HA-TCP scaffolds in immunodeficient mice (Hicok et al., 2004). Recently, human AMCs manipulated via BMP-2-mediated gene therapy in collagen I scaffolds induced significant bone formation in the hindlimb of immunodeficient mice (Dragoo et al., 2003, 2005). Finally, AMCs were used as a potential source for cell-based therapies for healing calvarial defects (Fig. 5, from Cowan et al., 2004). The adipose-derived stem cells were seeded in apatite-coated PLGA scaffolds and implanted into the surgically created, critical-size calvarial defects (Cowan et al., 2004). AMCs induced new bone formation similar in amount to that induced by either bone marrow MSCs or osteoblasts (Cowan et al., 2004).
Figure 5.
Bone metabolic activity of animals implanted with control (no cells) or adipose-derived adult stromal (ADS) cell-seeded scaffolds, as determined by radiolabeled methylene diphosphonate incorporation overlaid with micro-CT images. For each time-point, the top row displays the micro-CT scan, the middle row displays the metabolic activity, and the lower row displays the overlaid composite of metabolic activity and micro-CT scan. For all columns at each time-point, the left column is the x axis, the middle column is the y axis, and the right column is the z axis. For orientation, we have marked the defect with a yellow arrow for the three views of the micro-CT image. The location of the defect does not change between 2 and 12 weeks. Bone scan intensity is indicated in color on the left axis of the image, with white and red indicating the highest value and black and blue indicating lowest value. This Fig. originally appeared in Cowan et al. (2004) and is reproduced here with permission from the Nature Publishing Group (http://www.nature.com/).
While these experimental reports from investigations with rodents are exciting, the real question relates to the translation of experimental work into clinical practice. In other words, how close are we to engineering bone using human-derived AMCs? Fortunately, this question has recently been addressed by a case report from Germany. Human-derived AMCs were combined with bone chips from the iliac crest and used to regenerate a large calvarial defect to near-complete continuity in a seven-year-old patient (Lendeckel et al., 2004). This important report provides a proof of principle that AMCs can be utilized for bone tissue engineering. Future strategies will no doubt focus on the enrichment of osteoprogenitors within this heterogeneous population and continued optimization of scaffolds that promote osteogenesis.
(5.2) Special Considerations of Cranial Skeletal Repair
The skeleton of the head has a complex developmental history. Whereas the appendicular skeleton is derived solely from the mesoderm and forms bone through endochondral ossification, the cranial skeleton is a patchwork of bony elements derived from the cranial neural crest and the paraxial mesoderm, which forms bone through both endochondral and intramembranous ossification (Le Lièvre and Douarin, 1975; Noden, 1988; Couly et al., 1993; Chai et al., 2000; Jiang et al., 2002). If adult tissue repair recapitulates fetal development, one might ask: Is cranial repair a different process from skeletal healing in other parts of the body?
Growing evidence indicates that at least one critical cellular player in the repair process—namely, the calvarial osteoblast—exhibits several distinctive characteristics, depending upon cellular derivation (for reviews, see Allen et al., 2004; Barry and Murphy, 2004; Taichman, 2005). The first unique feature among osteoblasts from various parts of the body is their response to molecular signals: Both in vitro and in vivo responses to growth factors are different between calvarial osteoblasts and osteoblasts derived from the appendicular and axial skeletons (Anderson and Danylchuk, 1978; Uddstromer and Ritsila, 1979; Miller et al., 1991; Sheng et al., 1999). A second noticeable difference is the response of osteoblasts from different sites of the body to mechanical stimuli (Hock et al., 1982; Allen et al., 2004; for reviews, see de la Fuente and Helms, 2005; Mao, 2005b). Even within a single skeletal element, periosteal osteoblasts are dissimilar to endosteal osteoblasts, both in their response to molecular signals and in their response to mechanical stress (for reviews, see Epker and Frost, 1965; Jones et al., 1991; Bord et al., 2001; Midura et al., 2003). Another difference is embryonic origin. The appendicular and axial osteoblasts are derived from mesoderm, whereas calvarial osteoblasts originate from the neural crest (Noden, 1988; Couly et al., 1993, 2002; Chai et al., 2000; Brault et al., 2001; Jiang et al., 2002; Ruhin et al., 2003; Brewer et al., 2004; Le Douarin et al., 2004). Does any of these differences affect osteoblast behavior during bone repair? This fundamental question has rarely been systematically addressed. Therefore, we are left with the question: Does the embryonic origin of a bone have an effect on how it heals? Most of our knowledge of skeletal repair comes from the analysis of long-bone fractures. To understand if the repair of cranial defects is equivalent to, or distinctive from, the healing of long bones, one must compare the two healing processes directly. Variables to keep in mind include the dual origins of the cranial skeleton and the process by which individual bones form: i.e., intramembranous vs. endochondral. The mechanical environment plays an indisputable role in long-bone healing (Rodriguez-Merchan and Forriol, 2004; Isaksson et al., 2006) and most likely influences cranial healing as well, but this important aspect remains to be further characterized (Carter et al., 1998; Mao, 2005b). Answers to these kinds of questions will have direct and profound implications for the treatment of skeletal injuries, diseases, and disfigurements.
(5.3) Craniofacial Bone Regeneration after Ablative Cancer Surgery and Radiation Therapy
Oral cancer represents about 5% of all malignancies in the United States (Greenlee et al., 2001). Despite advances in treatment strategies, the morbidity and mortality rates are significant, with a significant number of patients developing recurrence, distant metastasis, and second primary tumors (Denham et al., 2001; Myers et al., 2001). Approximately 50% of all cancer patients receive radiation therapy. Radiation toxicity adversely affects bone development, remodeling, and fracture healing (Mitchell and Logan, 1998; Spear et al., 1999). Radiation therapy also complicates reconstructive surgery, due to increased apoptosis and compromised vascularization (Okunieff et al., 1998a, b). Irradiation of up to 8 Gy did not affect the BMP-2-induced osteoblast differentiation of C2C12 cells in vitro (Ikeda et al., 2000). New bone was formed on BMP-2-treated hydroxyapatite disks implanted into a subperiosteal pocket in rabbits radiated pre-operatively with a fractionated dose of 20 Gy (Howard et al., 1998). Despite these encouraging findings, 3-mm rat calvarial defects pre-operatively treated with a 12-Gy radiation dose, and subsequently with BMP-2, had successful bone regeneration, but incomplete healing of the defect (Wurzler et al., 1998). In rat mandibular defects treated with a fractionated 45-Gy radiation dose, followed by transplantation of demineralized bone powder, only 39% of defects had more than 50% bone fill (Lorente et al., 1992).
Combinatorial therapies appear to improve bone regeneration in irradiated sites. When a pedicle muscle flap was combined with BMP-3 treatment, complete healing of craniofacial defects occurred after a single pre-operative 15-Gy radiation dose (Khouri et al., 1996). Treatment with no implant, with a microvascular muscle flap alone, or with BMP-3 alone did not heal the defect, suggesting the need for a well-vascularized recipient bed and an adequate population of responsive osteogenic cells, in addition to the delivering of an osteoinductive protein (Khouri et al., 1996). The most effective bone regeneration for irradiated cranial sites appears to be a combination of cell transplantation and gene therapy approaches (Nussenbaum et al., 2003, 2005). For example, it has been demonstrated that bone formation by transplanted bone marrow stromal cells could be greatly enhanced by the concurrent administration of anabolic doses of parathyroid hormone (Schneider et al., 2003). However, it has yet to be established if such combinations of therapies would be effective in pre-operatively irradiated sites.
(6) IMPACT OF CRANIOFACIAL TISSUE ENGINEERING ON CLINICAL PRACTICE
Contemporary dental practice is largely based on conventional, non-cell-based therapies that rely on durable materials from outside the patient’s body. Amalgam, composites, metallic implants, synthetic materials, and tissue grafts from human cadavers and other species have been the mainstream choices for the restoration of dental, oral, and craniofacial structures. Despite various levels of clinical success, conventional materials suffer from intrinsic limitations, such as potential immune rejection, transmission of pathogens from the donor, and the general inability of conventional materials to remodel with recipient tissues and organs. An often-preferred approach by surgeons to use autologous grafts, such as bone grafts, necessitates donor site morbidity. In contrast, tissue engineering relies on the principle that mesenchymal stem cells are capable of generating virtually all craniofacial structures, and temporary biomimetic scaffolds are necessary for accommodating cell growth and tissue genesis. Scaffolds provide the temporary structural framework for cells to synthesize extracellular matrices and other functional components in the intended shape and dimensions. Upon neogenesis of tissues or organs derived from stem cells, scaffolds should undergo degradation.
Decades of contemporary biomedical research have focused primarily on the understanding of the mechanisms of biological functions in health and disease. For example, our understanding of the mechanisms of dental caries has advanced tremendously. In contrast, the end goal of tissue engineering is to develop products capable of healing diseased or lost tissues and organs, thus representing a departure from conventional biomedical research, whose primary focus is an understanding of mechanisms. This does not imply that the understanding of mechanisms is unimportant in tissue engineering. Instead, an understanding of the mechanisms of interactions among cells, growth factors, and biomaterials undoubtedly will advance the end goal of developing cell-based therapies and off-the-shelf tissue-engineering products. However, an understanding of mechanisms is the means for tissue engineering, but not the end. A cadre of craniofacial tissue engineers with interdisciplinary skills in stem cell biology, molecular biology and genetics, polymer and materials science, and mechanical engineering, and a clinical knowledge of dental, oral, and craniofacial disorders is needed to advance the field of craniofacial tissue engineering.
Craniofacial tissue engineering is an opportunity that dentistry cannot afford to miss. This notion is based on both biological and strategic reasons. Biologically, mesenchymal cells are primarily responsible for the formation of virtually all dental, oral, and craniofacial structures. Mesenchymal stem cells, the reservoir of mesenchymal cells in the adult, have been demonstrated, in tissue engineering, to generate key dental, oral, and craniofacial structures. Many dental and craniofacial structures are readily accessible, thus presenting a convenient platform for biologists, bioengineers, and clinicians to test tissue-engineered prototypes (Hollinger and Winn, 1999; Alsberg et al., 2001). Strategically, tissue-engineering technologies pioneered outside the dental community may have profound implications on dental practice.
The impact of craniofacial tissue engineering extends beyond dental practice. Several craniofacial structures engineered thus far serve as prototypes for the tissue engineering of non-craniofacial structures. Craniofacial-derived stem cells have potential implications in the tissue engineering of not only craniofacial structures, but also non-craniofacial tissues (Shi et al., 2005; Sonoyama et al., 2005). Tissue-engineered bone with customized shape and dimensions has the potential for the biological replacement of not only craniofacial bones, but also of segmental defects in the appendicular bones (Feinberg et al., 2001; Chu et al., 2002; Moore et al., 2004). Calvarial bone defects are the most frequently used models for bone tissue engineering (Cowan et al., 2004; Meinel et al., 2005). The tissue-engineered mandibular condyle has served as a prototype for the engineering of other joints, such as the knee and hip (Alhadlaq and Mao, 2003, 2005; Alhadlaq et al., 2004; Hollister, 2005; Hollister et al., 2005; Mao, 2005a; Marion et al., 2005). Tissue-engineered cranial sutures and periodontium offer clues for the fabrication of composite tissue constructs with multiple cell types and encapsulated in multiple materials (Giannobile and Somerman, 2003; Hong and Mao, 2004; Nakahara et al., 2004; Rahaman and Mao, 2005; Moioli et al., 2006). Several meritorious studies have led to the in vitro fabrication of a TMJ disc from cell- and growth-factor-based approaches (Almarza and Athanasiou, 2004; Detamore and Athanasiou, 2005). Adipose tissue grafts engineered from stem cells with sustained shape and dimensions may offer applications in both facial reconstructive surgeries as well as augmentative and/or reconstructive procedures elsewhere (Alhadlaq et al., 2005; Stosich and Mao, 2005, 2007). Conversely, craniofacial tissue engineering could not have advanced to the current stage without the incorporation of interdisciplinary skill sets of stem cell biology, bioengineering, polymer chemistry, mechanical engineering, robotics, etc. Thus, craniofacial tissue engineering and regenerative dental medicine are integral components of regenerative medicine.
Acknowledgments
This manuscript is the collective intellectual wisdom of all coauthors. We are indebted to our collaborators, post-doctoral fellows, and graduate students with whom we have had the privilege to work. Specifically, J.J.M. gratefully acknowledges the contributions from the following laboratory members, especially A. Alhadlaq, L. Hong, E. Moioli, and M. Stosich, whose work is cited in this manuscript. J.J.M. also gratefully acknowledges the administrative support of Richard Abbott, Janina Acloque, and Zoila Noguerole. J.A.H. gratefully acknowledges J.-B. Kim and P. Leucht for their intellectual contributions; W.V.G. thanks Mr. Chris Jung for assistance with the figures; S.J.H. gratefully acknowledges the contributions of S. Feinberg, S. Das, M. Smith, and C. Flanagan; M.T.L. gratefully acknowledges the contributions from those laboratory members who have worked on the bone tissue engineering projects: C. Cowan, R. Nacamuli, N. Quarto, D. Wan, Y.Y. Shi, Y. Xu, M. Chiou, P. Malladi, D. Wagner, M. Siedhoff, M. Tataria, K. Sylvester, G. Yang, G. Gurtner, B. Wu, and H.P. Lorenz.
We thank our colleagues whose work has been cited in the manuscript for their inspiration. We are grateful to Dr. Anthony Smith, Editor-in-Chief of the Journal of Dental Research, and Dr. Olav Alvares, Editor of Critical Reviews in Oral Biology & Medicine, for providing us the opportunity to write this review. We appreciate the invaluable comments by two anonymous reviewers.
The following research grants from the National Institutes of Health, especially the National Institute of Dental and Craniofacial Research (NIDCR), are gratefully acknowledged: (J.J.M.) DE13964 and DE15391 from NIDCR, and EB02332 from NIBIB; (W.V.G.) DE13397, DE15384, and DE16619 from NIDCR; (J.A.H.) DE012462 from NIDCR; (S.J.H.) DE013608 and DE016129 from NIDCR; (P.H.K.) DE013835, DE016530, and DE007057 from NIDCR; and (M.T.L.) The Oak Foundation and DE13194, DE13028, and DE14526 from NIDCR.
References
- About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi: 10.1006/excr.2000.4909. [DOI] [PubMed] [Google Scholar]
- Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005;40:245–251. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Alden TD, Beres EJ, Laurent JS, Engh JA, Das S, London SD, et al. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J Craniofac Surg. 2000;11:24–30. doi: 10.1097/00001665-200011010-00005. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Mao JJ. Tissue engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556–566. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787–1795. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001;12:64–75. doi: 10.1177/10454411010120010501. [DOI] [PubMed] [Google Scholar]
- Anderson C, Danylchuk KD. Bone-remodeling rates of the beagle: a comparison between different sites on the same rib. Am J Vet Res. 1978;39:1763–1765. [PubMed] [Google Scholar]
- Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–756. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anusaksathien O, Jin Q, Zhao M, Somerman MJ, Giannobile WV. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J Periodontol. 2004;75:429–440. doi: 10.1902/jop.2004.75.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Jordan EK, Wilson LB, Yocum GT, Lewis BK, Frank JA. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]
- Atala A. Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert Opin Biol Ther. 2005;5:879–892. doi: 10.1517/14712598.5.7.879. [DOI] [PubMed] [Google Scholar]
- Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Kok M, Tran SD, Yamano S. The impact of gene therapy on dentistry: a revisiting after six years. J Am Dent Assoc. 2002;133:35–44. doi: 10.14219/jada.archive.2002.0019. [DOI] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, Compston J. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86:2309–2314. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- Boyne PJ. Animal studies of application of rhBMP-2 in maxillofacial reconstruction. Bone. 1996;19(1 Suppl):83S–92S. doi: 10.1016/s8756-3282(96)00144-5. [DOI] [PubMed] [Google Scholar]
- Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- Boyne PJ, Nakamura A, Shabahang S. Evaluation of the long-term effect of function on rhBMP-2 regenerated hemimandibulectomy defects. Br J Oral Maxillofac Surg. 1999;37:344–352. doi: 10.1054/bjom.1999.0205. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–1352. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Bruens ML, Pieterman H, de Wijn JR, Vaandrager JM. Porous polymethylmethacrylate as bone substitute in the craniofacial area. J Craniofac Surg. 2003;14:63–68. doi: 10.1097/00001665-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Callender DL. Antibiotic prophylaxis in head and neck oncologic surgery: the role of Gram-negative coverage. Int J Antimicrob Agents. 1999;12(Suppl 1):S21–S25. doi: 10.1016/s0924-8579(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23:213–225. [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41–S55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFalphaR and prolongs ERK and Akt/PKB activation. Am J Physiol Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Chu TM, Hollister SJ, Halloran JW, Feinberg SE, Orton DG. Manufacturing and characterization of 3-D hydroxyapatite bone tissue engineering scaffolds. Ann NY Acad Sci. 2002;961:114–117. doi: 10.1111/j.1749-6632.2002.tb03061.x. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Jones AA, Lilly LC, Fiorellini JP, Howell H. Evaluation of recombinant human bone morphogenetic protein-2 in oral applications including the use of endosseous implants: 3-year results of a pilot study in humans. J Periodontol. 2000;71:1241–1257. doi: 10.1902/jop.2000.71.8.1241. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129–138. doi: 10.1007/pl00005833. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- de la Fuente L, Helms JA. Head, shoulders, knees, and toes. Dev Biol. 2005;282:294–306. doi: 10.1016/j.ydbio.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001;50:1105–1106. doi: 10.1016/s0360-3016(01)01556-5. [DOI] [PubMed] [Google Scholar]
- Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann Biomed Eng. 2005;33:383–390. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan A, Ozdemir A, Kubar A, Oygur T. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue Eng. 2003;9:1189–1196. doi: 10.1089/10763270360728099. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Lieberman JR, Lee RS, Deugarte DA, Lee Y, Zuk PA, et al. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673. doi: 10.1097/01.prs.0000161459.90856.ab. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Jin Q, Taba M, Jr, Franceschi RT, Rutherford RB, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11:294–299. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epker BN, Frost HM. A histological study of remodeling at the periosteal, haversian canal, cortical endosteal, and trabecular endosteal surfaces in human rib. Anat Rec. 1965;152:129–135. doi: 10.1002/ar.1091520203. [DOI] [PubMed] [Google Scholar]
- Feinberg SE, Hollister SJ, Halloran JW, Chu TM, Krebsbach PH. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. doi: 10.1159/000047896. [DOI] [PubMed] [Google Scholar]
- Ferguson CM, Miclau T, Hu D, Alpern E, Helms JA. Common molecular pathways in skeletal morphogenesis and repair. Ann NY Acad Sci. 1998;23:33–42. doi: 10.1111/j.1749-6632.1998.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605–613. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19(1 Suppl):23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8:193–204. doi: 10.1902/annals.2003.8.1.193. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I [see comments] J Periodontol. 1994;65:1158–1168. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003;58:137–160. doi: 10.1016/s0070-2153(03)58005-x. [DOI] [PubMed] [Google Scholar]
- Goodrich JT, Argamaso R, Hall CD. Split-thickness bone grafts in complex craniofacial reconstructions. Pediatr Neurosurg. 1992;18:195–201. doi: 10.1159/000120662. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–335. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Hanisch O, Tatakis DN, Rohrer MD, Wohrle PS, Wozney JM, Wikesjö UM. Bone formation and osseointegration stimulated by rhBMP-2 following subantral augmentation procedures in nonhuman primates. Int J Oral Maxillofac Implants. 1997;12:785–792. [PubMed] [Google Scholar]
- Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, et al. Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res. 1998;37:233–249. doi: 10.3109/03008209809002442. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005;11:469–478. doi: 10.1089/ten.2005.11.469. [DOI] [PubMed] [Google Scholar]
- Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24(9 Pt 2):705–714. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Hicok KC, Du Laney TV, Zhou YS, Halvorsen YD, Hitt DC, Cooper LF, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–575. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- Hock JM, Kream BE, Raisz LG. Autoradiographic study of the effect of 1,25 -dihydroxyvitamin D3 on bone matrix synthesis in vitamin D replete rats. Calcif Tissue Int. 1982;34:347–351. doi: 10.1007/BF02411266. [DOI] [PubMed] [Google Scholar]
- Hollinger JO, Winn SR. Tissue engineering of bone in the craniofacial complex. Ann NY Acad Sci. 1999;875:379–385. doi: 10.1111/j.1749-6632.1999.tb08520.x. [DOI] [PubMed] [Google Scholar]
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- Hollister SJ, Levy RA, Chu TM, Halloran JW, Feinberg SE. An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg. 2000;29:67–71. doi: 10.1034/j.1399-0020.2000.290115.x. [DOI] [PubMed] [Google Scholar]
- Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–4103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- Hollister SJ, Lin CY, Saito E, Lin CY, Schek RD, Taboas JM, et al. Engineering craniofacial scaffolds. Orthod Craniofac Res. 2005;8:162–173. doi: 10.1111/j.1601-6343.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Hong L, Mao JJ. Tissue-engineered rabbit cranial suture from autologous fibroblasts and BMP2. J Dent Res. 2004;83:751–756. doi: 10.1177/154405910408301003. [DOI] [PubMed] [Google Scholar]
- Howard BK, Brown KR, Leach JL, Chang CH, Rosenthal DI. Osteoinduction using bone morphogenic protein in irradiated tissue. Arch Otolaryngol Head Neck Surg. 1998;124:985–988. doi: 10.1001/archotol.124.9.985. [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997a;17:124–139. [PubMed] [Google Scholar]
- Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997b;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Hachisu R, Yamaguchi A, Gao YH, Okano T. Radiation retards muscle differentiation but does not affect osteoblastic differentiation induced by bone morphogenetic protein-2 in C2C12 myoblasts. Int J Radiat Biol. 2000;76:403–411. doi: 10.1080/095530000138745. [DOI] [PubMed] [Google Scholar]
- Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K. Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J Biomech. 2006;39:1507–1516. doi: 10.1016/j.jbiomech.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jin QM, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res A. 2003a;67:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003b;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004a;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin QM, Zhao M, Economides AN, Somerman MJ, Giannobile WV. Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res. 2004b;45:50–59. doi: 10.1080/03008200490278142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DB, Nolte H, Scholubbers JG, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- Khouri RK, Brown DM, Koudsi B, Deune EG, Gilula LA, Cooley BC, et al. Repair of calvarial defects with flap tissue: role of bone morphogenetic proteins and competent responding tissues. Plast Reconstr Surg. 1996;98:103–109. doi: 10.1097/00006534-199607000-00017. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- Kyba M. Genesis of hematopoietic stem cells in vitro and in vivo: new insights into developmental maturation. Int J Hematol. 2005;81:275–280. doi: 10.1532/ijh97.04192. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Le Lièvre CS, Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Lee JA, Parrett BM, Conejero JA, Laser J, Chen J, Kogon AJ, et al. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann Plast Surg. 2003;50:610–617. doi: 10.1097/01.SAP.0000069069.23266.35. [DOI] [PubMed] [Google Scholar]
- Lee K, Gerson SL, Maitra B, Koc ON. G156A MGMT-transduced human mesenchymal stem cells can be selectively enriched by O6-benzylguanine and BCNU. J Hematother Stem Cell Res. 2001;10:691–701. doi: 10.1089/152581601753193913. [DOI] [PubMed] [Google Scholar]
- Lekic PC, Rajshankar D, Chen H, Tenenbaum H, McCulloch CA. Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. Anat Rec. 2001;262:193–202. doi: 10.1002/1097-0185(20010201)262:2<193::AID-AR1028>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Lorente CA, Song BZ, Donoff RB. Healing of bony defects in the irradiated and unirradiated rat mandible. J Oral Maxillofac Surg. 1992;50:1305–1309. doi: 10.1016/0278-2391(92)90232-o. [DOI] [PubMed] [Google Scholar]
- Loukota RA. The effect of pre-operative perioral skin preparation with aqueous povidone-iodine on the incidence of infection after third molar removal. Br J Oral Maxillofac Surg. 1991;29:336–337. doi: 10.1016/0266-4356(91)90122-l. [DOI] [PubMed] [Google Scholar]
- Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62:458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Stem-cell-driven regeneration of synovial joints. Biol Cell. 2005a;97:289–301. doi: 10.1042/BC20040100. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Calvarial development: cells and mechanics. Curr Opin Orthop. 2005b;16:331–337. [Google Scholar]
- Marchac D. Split-rib grafts in craniofacial surgery. Plast Reconstr Surg. 1982;69:566–567. doi: 10.1097/00006534-198203000-00044. [DOI] [PubMed] [Google Scholar]
- Marion NW, Liang W, Reilly GC, Day DE, Rahaman MN, Mao JJ. Borate glass supports the in vitro osteogenic differentiation of human mesenchymal stem cells. Mechan Adv Mater Struct. 2005;12:1–8. [Google Scholar]
- McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1–8. discussion 9-10. [PubMed] [Google Scholar]
- McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. J Periodontol. 2005;76:867–880. doi: 10.1902/jop.2005.76.6.867. [DOI] [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Midura RJ, Su X, Morcuende JA, Tammi M, Tammi R. Parathyroid hormone rapidly stimulates hyaluronan synthesis by periosteal osteoblasts in the tibial diaphysis of the growing rat. J Biol Chem. 2003;278:51462–51468. doi: 10.1074/jbc.M307567200. [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM, Miller MA, Bagi CM. Calcium absorption and osseous organ-, tissue-, and envelope-specific changes following ovariectomy in rats. Bone. 1991;12:439–446. doi: 10.1016/8756-3282(91)90033-f. [DOI] [PubMed] [Google Scholar]
- Mitchell MJ, Logan PM. Radiation-induced changes in bone. Radiographics. 1998;18:1125–1136. doi: 10.1148/radiographics.18.5.9747611. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofid MM, Manson PN, Robertson BC, Tufaro AP, Elias JJ, Vander Kolk CA. Craniofacial distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg. 2001;108:1103–1114. doi: 10.1097/00006534-200110000-00001. discussion 1115-1107. [DOI] [PubMed] [Google Scholar]
- Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–546. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Jabbari E, Ritman EL, Lu L, Currier BL, Windebank AJ, et al. Quantitative analysis of interconnectivity of porous biodegradable scaffolds with micro-computed tomography. J Biomed Mater Res A. 2004;71:258–267. doi: 10.1002/jbm.a.30138. [DOI] [PubMed] [Google Scholar]
- Motisuki C, Lima LM, Spolidorio DM, Santos-Pinto L. Influence of sample type and collection method on Streptococcus mutans and Lactobacillus spp. counts in the oral cavity. Arch Oral Biol. 2005;50:341–345. doi: 10.1016/j.archoralbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8:266–302. doi: 10.1902/annals.2003.8.1.266. [DOI] [PubMed] [Google Scholar]
- Murray PE, About I, Franquin JC, Remusat M, Smith AJ. Restorative pulpal and repair responses. J Am Dent Assoc. 2001;132:482–491. doi: 10.14219/jada.archive.2001.0211. [DOI] [PubMed] [Google Scholar]
- Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92:3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Nakamura T, Kobayashi E, Inoue M, Shigeno K, Tabata Y, et al. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003;9:153–162. doi: 10.1089/107632703762687636. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Nakamura T, Kobayashi E, Kuremoto K, Matsuno T, Tabata Y, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10:537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Tissue engineering in the USA. Med Biol Eng Comput. 1992;30:CE8–12. doi: 10.1007/BF02446171. [DOI] [PubMed] [Google Scholar]
- Nevins ML, Camelo M, Lynch SE, Schenk RK, Nevins M. Evaluation of periodontal regeneration following grafting intrabony defects with bio-oss collagen: a human histologic report. Int J Periodontics Restorative Dent. 2003;23:9–17. [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- Nicholson JW. Glass-ionomers in medicine and dentistry. Proc Inst Mech Eng [H] 1998;212:121–126. doi: 10.1243/0954411981533890. [DOI] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Nussenbaum B, Rutherford RB, Teknos TN, Dornfeld KJ, Krebsbach PH. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003;14:1107–1115. doi: 10.1089/104303403322124819. [DOI] [PubMed] [Google Scholar]
- Nussenbaum B, Rutherford RB, Krebsbach PH. Bone regeneration in cranial defects previously treated with radiation. Laryngoscope. 2005;115:1170–1177. doi: 10.1097/01.MLG.0000166513.74247.CC. [DOI] [PubMed] [Google Scholar]
- Okeson JP. Orofacial pain: guidelines for assessment, diagnosis and management. Carol Stream, IL: Quintessence Publishing Co, Inc.; 1996. pp. 1–15. [Google Scholar]
- Okunieff P, Mester M, Wang J, Maddox T, Gong X, Tang D, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998a;150:204–211. [PubMed] [Google Scholar]
- Okunieff P, Wang X, Rubin P, Finkelstein JN, Constine LS, Ding I. Radiation-induced changes in bone perfusion and angiogenesis. Int J Radiat Oncol Biol Phys. 1998b;42:885–889. doi: 10.1016/s0360-3016(98)00339-3. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Parker GC, Anastassova-Kristeva M, Broxmeyer HE, Dodge WH, Eisenberg LM, Gehling UM, et al. Stem cells: shibboleths of development. Stem Cells Dev. 2004;13:579–584. doi: 10.1089/scd.2004.13.579. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15:701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Alison MR, Cook T, Jeffery R, Ryan E, Forbes SJ, et al. Bone marrow stem cells contribute to healing of the kidney. J Am Soc Nephrol. 2003;14:48S–54S. doi: 10.1097/01.asn.0000068162.02174.29. [DOI] [PubMed] [Google Scholar]
- Rah DK. Art of replacing craniofacial bone defects. Yonsei Med J. 2000;41:756–765. doi: 10.3349/ymj.2000.41.6.756. [DOI] [PubMed] [Google Scholar]
- Rahaman MN, Mao JJ. Stem cell based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- Reese JS, Koc ON, Gerson SL. Human mesenchymal stem cells provide stromal support for efficient CD34+ transduction. J Hematother Stem Cell Res. 1999;8:515–523. doi: 10.1089/152581699319966. [DOI] [PubMed] [Google Scholar]
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–265. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Stem cells in craniofacial and dental tissue engineering. Orthod Craniofac Res. 2005;8:54–59. doi: 10.1111/j.1601-6343.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Merchan EC, Forriol F. Nonunion: general principles and experimental data. Clin Orthop Relat Res. 2004;419:4–12. [PubMed] [Google Scholar]
- Rohner D, Hutmacher DW, Cheng TK, Oberholzer M, Hammer B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66:574–580. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- Rossa C, Jr, Marcantonio E, Jr, Cirelli JA, Marcantonio RA, Spolidorio LC, Fogo JC. Regeneration of Class III furcation defects with basic fibroblast growth factor (b-FGF) associated with GTR. A descriptive and histometric study in dogs. J Periodontol. 2000;71:775–784. doi: 10.1902/jop.2000.71.5.775. [DOI] [PubMed] [Google Scholar]
- Ruhin B, Creuzet S, Vincent C, Benouaiche L, Le Douarin NM, Couly G. Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003;228:239–246. doi: 10.1002/dvdy.10380. [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Niekrash CE, Kennedy JE, Charette MF. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J Periodontal Res. 1992;27(4 Pt 1):285–290. doi: 10.1111/j.1600-0765.1992.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Sarnat BG, Laskin DM. The temporomandibular joint: a biological basis for clinical practice. Philadelphia, PA: WB Saunders Publ.; 1992. pp. 43–57. [Google Scholar]
- Schneider A, Taboas JM, McCauley LK, Krebsbach PH. Skeletal homeostasis in tissue-engineered bone. J Orthop Res. 2003;21:859–864. doi: 10.1016/S0736-0266(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shen HC, Peng H, Usas A, Gearhart B, Cummins J, Fu FH, et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004a;34:982–992. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Shen HC, Peng H, Usas A, Gearhart B, Fu FH, Huard J. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. J Gene Med. 2004b;6:984–991. doi: 10.1002/jgm.588. [DOI] [PubMed] [Google Scholar]
- Shenaq SM. Reconstruction of complex cranial and craniofacial defects utilizing iliac crest-internal oblique microsurgical free flap. Microsurgery. 1988;9:154–158. doi: 10.1002/micr.1920090218. [DOI] [PubMed] [Google Scholar]
- Sheng MH, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KH, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25:421–429. doi: 10.1016/s8756-3282(99)00184-2. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter C, Robey PG, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nature Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- Shi S, Bartold P, Miura M, Seo B, Robey P, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Sigurdsson TJ, Lee MB, Kubota K, Turek TJ, Wozney JM, Wikesjö UM. Periodontal repair in dogs: recombinant human bone morphogenic protein-2 significantly enhances periodontal regeneration. J Periodontol. 1995;66:131–138. doi: 10.1902/jop.1995.66.2.131. [DOI] [PubMed] [Google Scholar]
- Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- Silvestri M, Ricci G, Rasperini G, Sartori S, Cattaneo V. Comparison of treatments of infrabony defects with enamel matrix derivative, guided tissue regeneration with a nonresorbable membrane and Widman modified flap. A pilot study. J Clin Periodontol. 2000;27:603–610. doi: 10.1034/j.1600-051x.2000.027008603.x. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Coppe C, Gronthos S, Shi S. Skeletal stem cells in regenerative medicine. Curr Top Dev Biol. 2005;67:305–323. doi: 10.1016/S0070-2153(05)67010-X. [DOI] [PubMed] [Google Scholar]
- Spear MA, Dupuy DE, Park JJ, Halpern EF, Spiro IJ. Tolerance of autologous and allogeneic bone grafts to therapeutic radiation in humans. Int J Radiat Oncol Biol Phys. 1999;45:1275–1280. doi: 10.1016/s0360-3016(99)00339-9. [DOI] [PubMed] [Google Scholar]
- Stohler CS. Muscle-related temporomandibular disorders. J Orofac Pain. 1999;13:273–284. [PubMed] [Google Scholar]
- Stosich MS, Mao JJ. Stem cell based soft tissue grafts for plastic and reconstructive surgeries. Sem Plast Surg. 2006;19:251–260. [Google Scholar]
- Stosich MS, Mao JJ. Adipose tissue engineering from human adult stem cells: clinical implications in plastic and reconstructive surgeries. Plastic Reconstr Surg. 2007 doi: 10.1097/01.prs.0000244840.80661.e7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001;86:2075–2079. doi: 10.1177/00220345010800121001. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Tummers M. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12(Suppl 1):43–50. doi: 10.1159/000069840. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Lang NP, Cortellini P, Suvan JE, Adriaens P, Dubravec D, et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defects. J Clin Periodontol. 2002;29:317–325. doi: 10.1034/j.1600-051x.2002.290407.x. [DOI] [PubMed] [Google Scholar]
- Trubiani O, Di Primio R, Traini T, Pizzicannella J, Scarano A, Piattelli A, et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–221. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- Uddstromer L, Ritsila V. Healing of membranous and long bone defects. An experimental study in growing rabbits. Scand J Plast Reconstr Surg. 1979;13:281–287. doi: 10.3109/02844317909013071. [DOI] [PubMed] [Google Scholar]
- Ueno A, Kitase Y, Moriyama K, Inoue H. MC3T3-E1-conditioned medium-induced mineralization by clonal rat dental pulp cells. Matrix Biol. 2001;20:347–353. doi: 10.1016/s0945-053x(01)00141-x. [DOI] [PubMed] [Google Scholar]
- Unda FJ, Martin A, Hilario E, Bègue-Kirn C, Ruch JV, Arechaga J. Dissection of the odontoblast differentiation process in vitro by a combination of FGF1, FGF2, and TGFbeta1. Dev Dyn. 2000;218:480–489. doi: 10.1002/1097-0177(200007)218:3<480::AID-DVDY1011>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- Weber RS. Wound infection in head and neck surgery: implications for perioperative antibiotic treatment. Ear Nose Throat J. 1997;76:790–791. 795–798. [PubMed] [Google Scholar]
- Wikesjö UM, Polimeni G, Qahash M. Tissue engineering with recombinant human bone morphogenetic protein-2 for alveolar augmentation and oral implant osseointegration: experimental observations and clinical perspectives. Clin Implant Dent Relat Res. 2005;7:112–119. doi: 10.1111/j.1708-8208.2005.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Wurzler KK, DeWeese TL, Sebald W, Reddi AH. Radiation-induced impairment of bone healing can be overcome by recombinant human bone morphogenetic protein-2. J Craniofac Surg. 1998;9:131–137. doi: 10.1097/00001665-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Yeong WY, Chua CK, Leong KF, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004;22:643–652. doi: 10.1016/j.tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. 6-month results. J Periodontol. 2000;71:1671–1679. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Thirty-six month follow-up of 25 patients treated with combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) bone replacement grafts in human infrabony defects. I. Clinical findings. J Periodontol. 2002;73:123–128. doi: 10.1902/jop.2002.73.1.123. [DOI] [PubMed] [Google Scholar]
- Zetterstrom O, Andersson C, Eriksson L, Fredriksson A, Friskopp J, Heden G, et al. Clinical safety of enamel matrix derivative (EMDOGAIN) in the treatment of periodontal defects. J Clin Periodontol. 1997;24(9 Pt 2):697–704. doi: 10.1111/j.1600-051x.1997.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J Dent Res. 2001;80:892–897. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]